West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases
Abstract
:1. Introduction
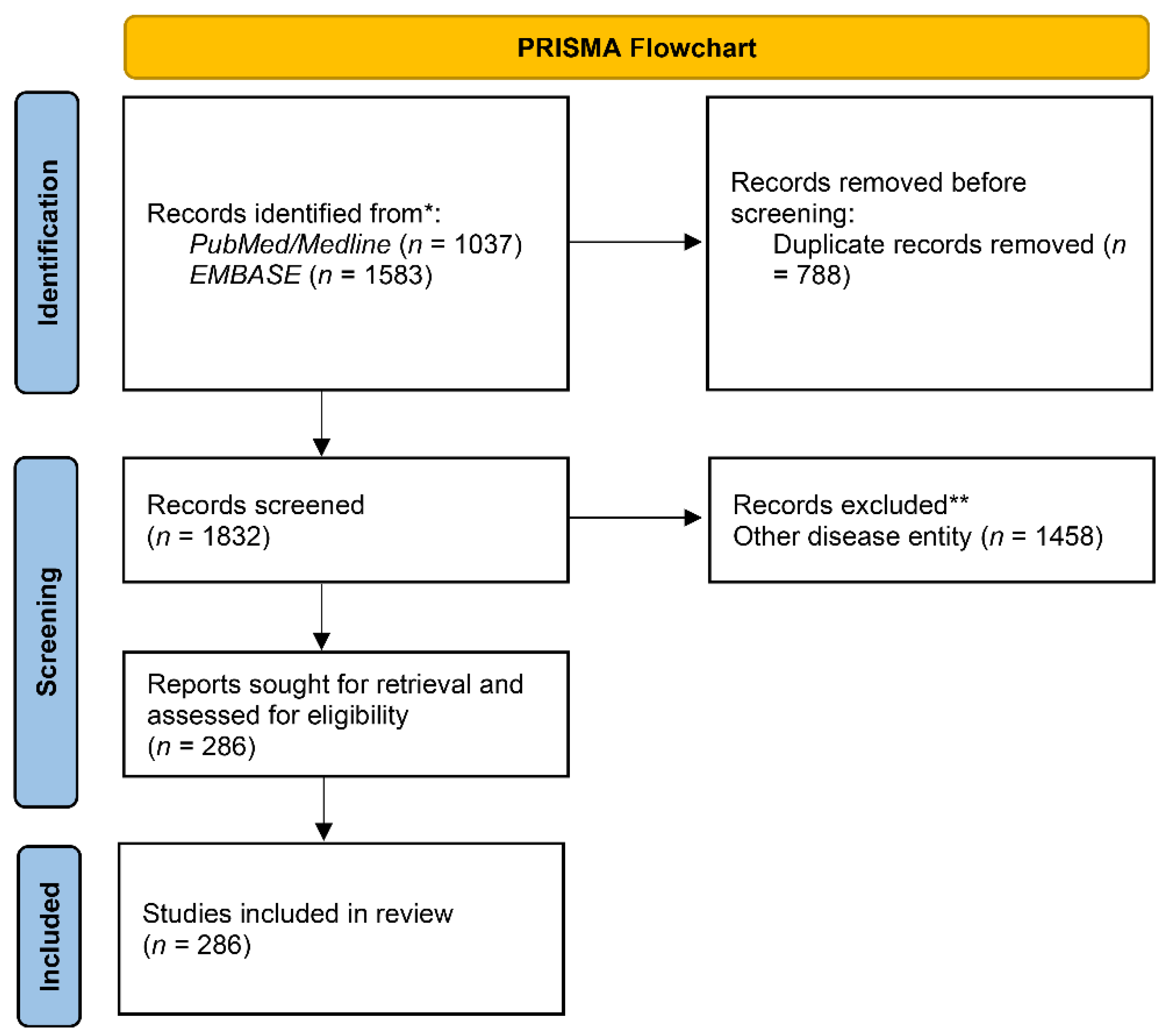
2. Materials and Methods
3. Results
3.1. Symptoms and Cellular and Biochemical Measurements
3.2. Comorbidites
3.3. Treatment and Interventions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Londono, B.; Colpitts, T. A Brief Review of West Nile Virus Biology. Methods Mol. Biol. 2016, 1435, 1–13. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, Chikungunya and Dengue: The Causes and Threats of New and Re-Emerging Arboviral Diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, Pathology, and Clinical Manifestations of West Nile Virus Disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar] [CrossRef]
- Watson, J.T.; Pertel, P.E.; Jones, R.C.; Siston, A.M.; Paul, W.S.; Austin, C.C.; Gerber, S.I. Clinical Characteristics and Functional Outcomes of West Nile Fever. Ann. Intern. Med. 2004, 141, 360–365. [Google Scholar] [CrossRef]
- Ouhoumanne, N.; Lowe, A.-M.; Fortin, A. Morbidity, mortality and long-term sequelae of West Nile virus disease in Québec. Epidemiol. Infect. 2018, 146, 867–874. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version; John Wiley & Sons: Chichester, UK, 2022; Volume 6. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 10, 89. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Di Pol, G.; Crotta, M.; Taylor, R.A. Modelling the Temperature Suitability for the Risk of West Nile Virus Establishment in European Culex Pipiens Populations. Transbound Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Murray, K.; Baraniuk, S.; Resnick, M.; Arafat, R.; Kilborn, C.; Cain, K.; Shallenberger, R.; York, T.L.; Martinez, D.; Hellums, J.S.; et al. Risk Factors for Encephalitis and Death from West Nile Virus Infection. Epidemiol. Infect. 2006, 134, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Haberle, S.; Židovec-Lepej, S.; Savić, V.; Kusulja, M.; Papić, N.; Višković, K.; Župetić, I.; Savini, G.; Barbić, L.; et al. Severe West Nile Virus Neuroinvasive Disease: Clinical Characteristics, Short- and Long-Term Outcomes. Pathogens 2022, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, J.; Fell, D.B.; Sander, B.; Kulkarni, M.A. Comorbid Conditions as Risk Factors for West Nile Neuroinvasive Disease in Ontario, Canada: A Population-Based Cohort Study. Epidemiol. Infect. 2022, 150, E103. [Google Scholar] [CrossRef] [PubMed]
- Samaan, Z.; Vaz, S.M.; Bawor, M.; Potter, T.H.; Eskandarian, S.; Loeb, M. Neuropsychological Impact of West Nile Virus Infection: An Extensive Neuropsychiatric Assessment of 49 Cases in Canada. PLoS ONE 2016, 11, e0158364. [Google Scholar] [CrossRef] [PubMed]
- Haaland, K.Y.; Sadek, J.; Pergam, S.; Echevarria, L.A.; Davis, L.E.; Goade, D.; Harnar, J.; Nofchissey, R.A.; Sewel, C.M.; Ettestad, P. Mental Status after West Nile Virus Infection. Emerg. Infect. Dis. 2006, 12, 1260–1262. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Shrikanth, V.; Salazar, L.; Khoury, N.; Wootton, S.; Hasbun, R. Hypoglycorrhachia in Adults with Community-Acquired Meningitis: Etiologies and Prognostic Significance. Int. J. Infect. Dis. 2015, 39, 39–43. [Google Scholar] [CrossRef]
- Tyler, K.L.; Pape, J.; Goody, R.J.; Corkill, M.; Kleinschmidt-DeMasters, B.K. CSF Findings in 250 Patients with Serologically Confirmed West Nile Virus Meningitis and Encephalitis. Neurology 2006, 66, 361–365. [Google Scholar] [CrossRef]
- Diamond, M.S.; Shrestha, B.; Mehlhop, E.; Sitati, E.; Engle, M. Innate and Adaptive Immune Responses Determine Protection against Disseminated Infection by West Nile Encephalitis Virus. Viral. Immunol. 2003, 16, 259–278. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kokatnur, L.; Chernyshev, O. Neurological Respiratory Failure. Diseases 2018, 6, 7. [Google Scholar] [CrossRef]
- Betensley, A.D.; Jaffery, S.H.; Collins, H.; Sripathi, N.; Alabi, F. Bilateral Diaphragmatic Paralysis and Related Respiratory Complications in a Patient with West Nile Virus Infection. Thorax 2004, 59, 268–269. [Google Scholar] [CrossRef] [Green Version]
- Morrey, J.D.; Siddharthan, V.; Wang, H.; Hall, J.O. Respiratory Insufficiency Correlated Strongly with Mortality of Rodents Infected with West Nile Virus. PLoS ONE 2012, 7, e38672. [Google Scholar] [CrossRef] [PubMed]
- Jean, C.M.; Honarmand, S.; Louie, J.K.; Glaser, C.A. Risk Factors for West Nile Virus Neuroinvasive Disease, California, 2005. Emerg. Infect. Dis. 2007, 13, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Medical Risk Factors for Severe West Nile Virus Disease, United States, 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 179–184. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Viviani, F. Cardiovascular Comorbidities in Patients with West Nile Disease Infection: An Unexplored Issue. Eur. J. Intern. Med. 2019, 63, e17–e18. [Google Scholar] [CrossRef]
- Badawi, A.; Velummailum, R.; Ryoo, S.G.; Senthinathan, A.; Yaghoubi, S.; Vasileva, D.; Ostermeier, E.; Plishka, M.; Soosaipillai, M.; Arora, P. Prevalence of Chronic Comorbidities in Dengue Fever and West Nile Virus: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0200200. [Google Scholar] [CrossRef]
- Piquet, A.L.; Lyons, J.L. Infectious Meningitis and Encephalitis. Semin. Neurol. 2016, 36, 367–372. [Google Scholar] [CrossRef]
- Pyrgos, V.; Younus, F. High-Dose Steroids in the Management of Acute Flaccid Paralysis Due to West Nile Virus Infection. Scand. J. Infect. Dis. 2004, 36, 509–512. [Google Scholar] [CrossRef]
- Leis, A.A.; Sinclair, D.J. Lazarus Effect of High Dose Corticosteroids in a Patient With West Nile Virus Encephalitis: A Coincidence or a Clue? Front. Med. 2019, 6, 81. [Google Scholar] [CrossRef]
- Bai, F.; Thompson, E.A.; Vig, P.J.S.; Leis, A.A. Current Understanding of West Nile Virus Clinical Manifestations, Immune Responses, Neuroinvasion, and Immunotherapeutic Implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef]
- Iwamoto, M.; Jernigan, D.B.; Guasch, A.; Trepka, M.J.; Blackmore, C.G.; Hellinger, W.C.; Pham, S.M.; Zaki, S.; Lanciotti, R.S.; Lance-Parker, S.E.; et al. Transmission of West Nile Virus from an Organ Donor to Four Transplant Recipients. N. Engl. J. Med. 2003, 348, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Pepperell, C.; Rau, N.; Krajden, S.; Kern, R.; Humar, A.; Mederski, B.; Simor, A.; Low, D.E.; McGeer, A.; Mazzulli, T.; et al. West Nile Virus Infection in 2002: Morbidity and Mortality among Patients Admitted to Hospital in Southcentral Ontario. CMAJ 2003, 168, 1399–1405. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bampali, M.; Konstantinidis, K.; Kellis, E.E.; Pouni, T.; Mitroulis, I.; Kottaridi, C.; Mathioudakis, A.G.; Beloukas, A.; Karakasiliotis, I. West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases. Trop. Med. Infect. Dis. 2022, 7, 236. https://doi.org/10.3390/tropicalmed7090236
Bampali M, Konstantinidis K, Kellis EE, Pouni T, Mitroulis I, Kottaridi C, Mathioudakis AG, Beloukas A, Karakasiliotis I. West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases. Tropical Medicine and Infectious Disease. 2022; 7(9):236. https://doi.org/10.3390/tropicalmed7090236
Chicago/Turabian StyleBampali, Maria, Konstantinos Konstantinidis, Emmanouil E. Kellis, Theodoti Pouni, Ioannis Mitroulis, Christine Kottaridi, Alexander G. Mathioudakis, Apostolos Beloukas, and Ioannis Karakasiliotis. 2022. "West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases" Tropical Medicine and Infectious Disease 7, no. 9: 236. https://doi.org/10.3390/tropicalmed7090236
APA StyleBampali, M., Konstantinidis, K., Kellis, E. E., Pouni, T., Mitroulis, I., Kottaridi, C., Mathioudakis, A. G., Beloukas, A., & Karakasiliotis, I. (2022). West Nile Disease Symptoms and Comorbidities: A Systematic Review and Analysis of Cases. Tropical Medicine and Infectious Disease, 7(9), 236. https://doi.org/10.3390/tropicalmed7090236









