Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Area
2.3. Environmental Factors
2.3.1. Environmental Dominants of Snail Habitus
2.3.2. Data Processing
2.4. Ensemble Ecological Niche Modeling
2.4.1. Standard Ecological Niche Models
2.4.2. Model Evaluation and Validation
2.4.3. Ensemble Model and Risk Classification
- No-risk area: a presence probability ranging from 0 to the minimum presence threshold,
- Low-risk area: a presence probability ranging from the minimum presence threshold to 0.70,
- Medium risk area: a presence probability ranged from 0.70 to 0.80,
- High-risk area: a presence probability of 0.80 to 1.00.
3. Results
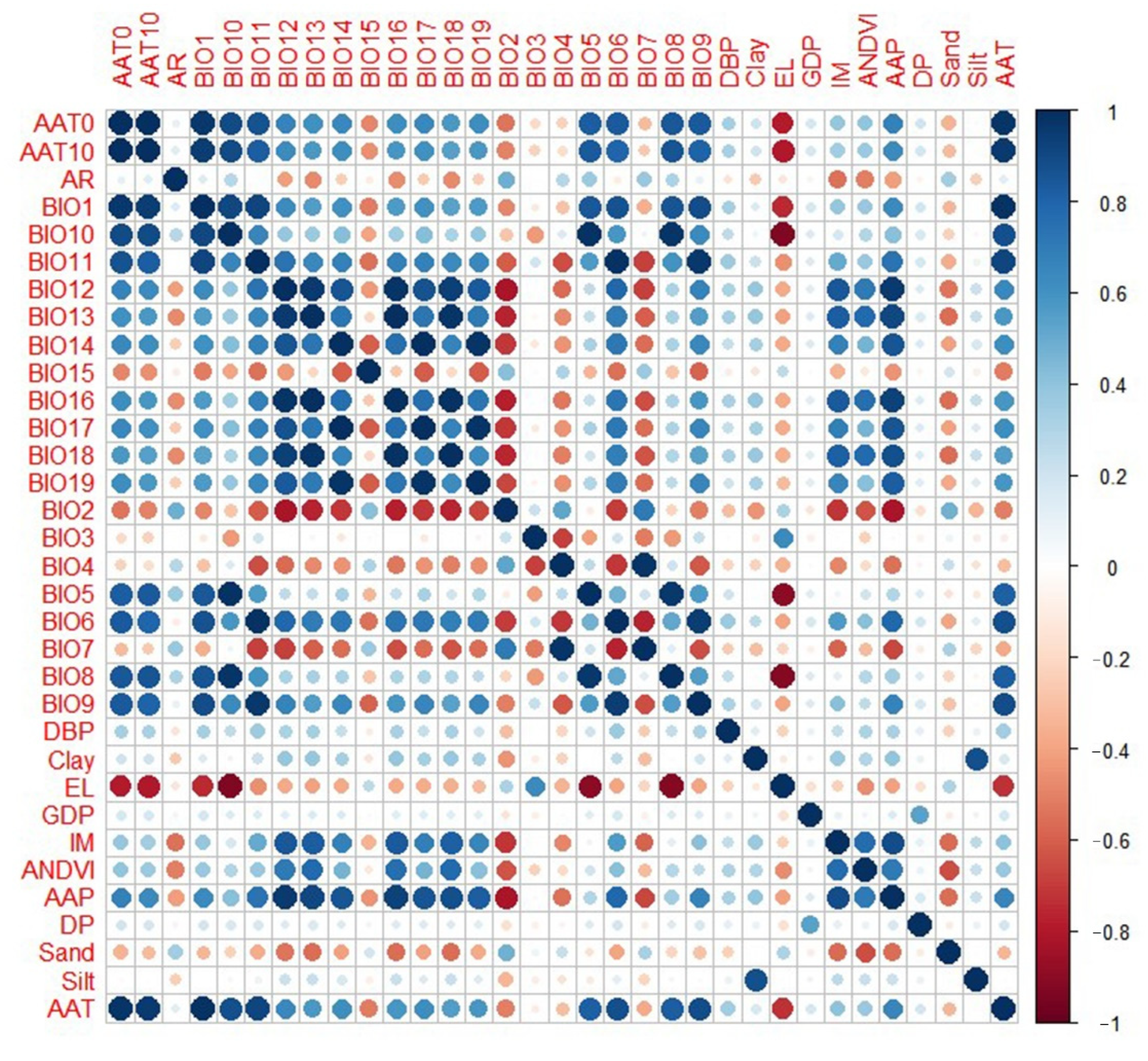
3.1. Results of Collinearity Diagnostics
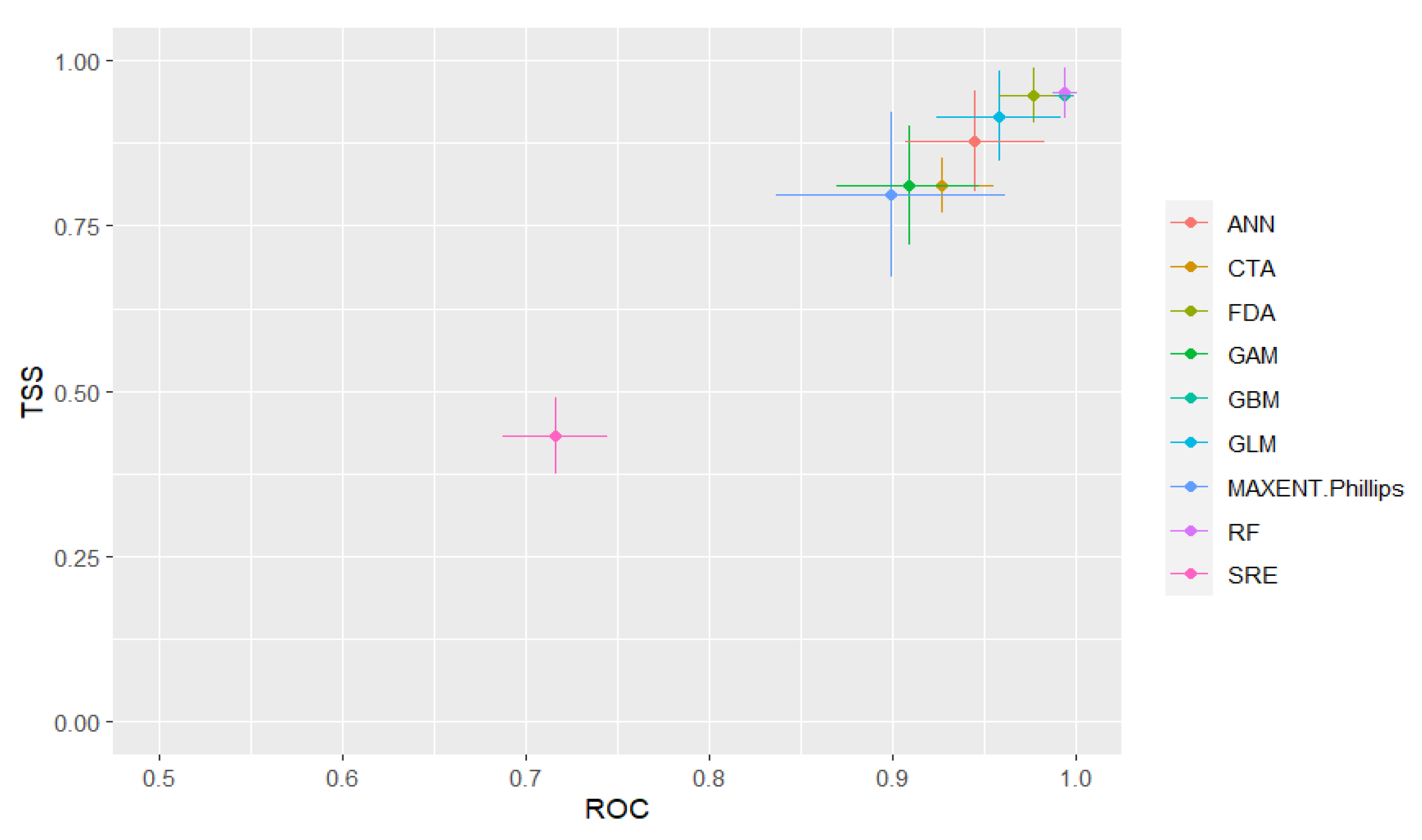
3.2. Performance of Single Ecological Niche Models and the Ensemble Model
3.2.1. Performance of Model-Based Risk Prediction
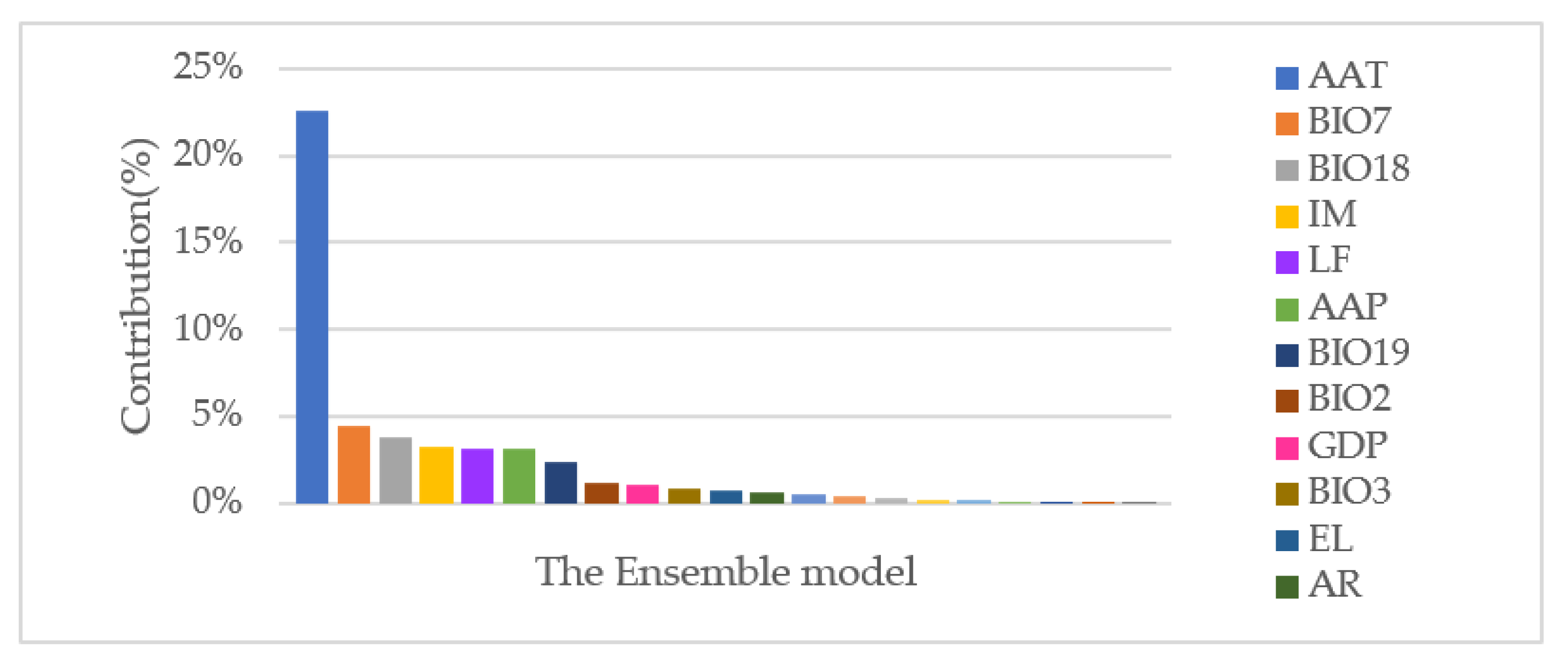
3.2.2. Dominant Predictive Environmental Factors of the Ensemble Model
3.3. Schistosomiasis Risk Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations. Sustainable Development Goals (SDGs). Available online: https://sdgs.un.org/goals (accessed on 2 May 2022).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Schistosomiasis Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 2 March 2020).
- Collins, C.; Xu, J.; Tang, S. Schistosomiasis control and the health system in PR China. Infect. Dis. Poverty 2012, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Z.L.; Zhou, X.N. Eradication of schistosomiasis: A new target and a new task for the National Schistosomiasis Control Programme in the People’s Republic of China. Chin. J. Schistosomiasis Control 2015, 27, 1–4. [Google Scholar]
- Xu, J.; Steinman, P.; Maybe, D.; Zhou, X.N.; Lv, S.; Li, S.Z.; Peeling, R. Evolution of the National Schistosomiasis Control Programs in The People’s Republic of China. Adv. Parasitol. 2016, 92, 1–38. [Google Scholar] [PubMed]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PLoS Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xu, Z.M.; Guo, J.Y.; Dai, S.M.; Dang, H.; Lv, S.; Xu, J.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2018. Chin. J. Schistosomiasis Control 2019, 31, 576–582. [Google Scholar]
- Wang, T.P.; Cao, Z.G. Relationship between environmental changes and schistosomiasis transmission. Chin. J. Epidemiol. 2009, 30, 298–301. [Google Scholar]
- Xu, J.; Lv, S.; Cao, C.L.; Li, S.Z.; Zhou, X.N. Progress and challenges of schistosomiasis elimination in China. Chin. J. Schistosomiasis Control 2018, 30, 605–609. [Google Scholar]
- Zhou, X.N. Intensifying the precision control to facilitate the progress towards schistosomiasis elimination in mountainous and hilly regions of China. Chin. J. Schistosomiasis Control 2019, 31, 229–230. [Google Scholar]
- Lin, D.D.; Liu, Y.M.; Hu, F.; Tao, B.; Wang, X.M.; Zuo, X.X.; Li, J.Y.; Wu, G.L. Evaluation on the application of common diagnosis methods for Schistosomiasis japonica in endemic areas of China II Quantitively analysis of detection results for S. japonicum infection by IHA screening method and Kato-Katz technique. Chin. J. Schistosomiasis Control 2008, 20, 179–183. [Google Scholar]
- Shi, Q.W.; Gong, Y.F.; Zhao, J.; Qin, Z.Q.; Zhang, J.; Wu, J.; Hu, Z.Y.; Li, S.Z. Spatial and Temporal Distribution Pattern of Oncomelania hupensis Caused by Multiple Environmental Factors Using Ecological Niche Models. Front. Environ. Sci. 2022, 10, 888. [Google Scholar] [CrossRef]
- Zhu, G.P.; Liu, G.Q.; Bo, W.J.; Gao, Y.B. Ecological niche modeling and its applications in biodiversity conservation. Biodiv. Sci. 2013, 21, 90–98. [Google Scholar]
- Peterson, A.T. Ecological niche modelling and understanding the geography of disease transmission. Vet. Ital. 2007, 43, 393–400. [Google Scholar] [PubMed]
- Escobar, L.E.; Craft, M.E. Advances and limitations of disease biogeography using ecological niche modeling. Front. Microbiol. 2016, 7, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.L.; Xiao, H.; Tian, H.Y. Applications of ecological niche models in risk predictions of infectious diseases. Chin. J. Prev. Med. 2013, 47, 294–296. [Google Scholar]
- Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Shao, Q.; Cui, Y. Identifying and predicting the geographical distribution patterns of Oncomelania hupensis. Int. J. Environ. Res. Public Health 2019, 16, 2206. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.C.; Hu, Y.; Ward, M.P.; Lynn, H.; Li, S.; Zhang, J.; Hu, J.; Xiao, S.; Lu, C.F.; Li, S.Z.; et al. Identification of high-risk habitats of Oncomelania hupensis, the intermediate host of Schistosoma japonium in the Poyang Lake region, China: A spatial and ecological analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yue, M.; Hu, Y.; Bergquist, R.; Su, C.; Gao, F.; Cao, Z.G.; Zhang, Z. Risk prediction of two types of potential snail habitats in Anhui Province of China: Model-based approaches. PLoS Negl. Trop. Dis. 2020, 14, e0008178. [Google Scholar] [CrossRef]
- Zhu, G.; Fan, J.; Peterson, A.T. Schistosoma japonicum transmission risk maps at present and under climate change in mainland China. PLoS Negl. Trop. Dis. 2017, 11, e0006021. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2007, 17, 145–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.R.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Shi, Q.W.; Duan, L.P.; Qin, Z.Q.; Wang, W.S.; Shen, L.; Hua, X.T.; Shen, L.E.; Cao, J.Q.; Zhu, F.K.; Wu, J.Z.; et al. The Biosafety Evaluation for Crustaceans: A Novel Molluscicide PBQ Using against Oncomelania hupensis, the Intermediate Host of Schistosoma japonica. Trop. Med. Infect. Dis. 2022, 7, 294. [Google Scholar] [CrossRef]
- Xue, J.B.; Wang, X.Y.; Zhang, L.J.; Hao, Y.W.; Chen, Z.; Lin, D.D.; Xu, J.; Xia, S.; Li, S.Z. Potential impact of flooding on schistosomiasis in Poyang Lake regions based on multi-source remote sensing images. Parasites Vectors 2021, 14, 116. [Google Scholar] [CrossRef]
- Hao, Y.; Guan, W.; Wu, H.; Li, L.; Abe, E.M.; Xue, J.; Qin, Z.; Wang, Q.; Lv, S.; Xu, J. Intestinal microbiome profiles in Oncomelania hupensis in mainland China. Acta Trop. 2020, 201, 105202. [Google Scholar] [CrossRef]
- Gong, Y.F.; Hu, X.K.; Hao, Y.W.; Luo, Z.W.; Feng, J.X.; Xue, J.B.; Guo, Z.Y.; Li, Y.L.; Zhang, L.J.; Xia, S. Projecting the proliferation risk of Oncomelania hupensis in China driven by SSPs: A multi-scenario comparison and integrated modeling study. Adv. Clim. Chang. Res. 2022, 13, 258–265. [Google Scholar] [CrossRef]
- Gong, Y.F.; Li, Y.L.; Zhang, L.J.; Lv, S.; Xu, J.; Li, S.Z. The Potential Distribution Prediction of Oncomelania hupensis Based on Newly Emerging and Reemergent Habitats—China, 2015−2019. China CDC Wkly. 2021, 3, 90. [Google Scholar] [CrossRef]
- Peterson, A.T. Mapping Disease Transmission Risk: Enriching Models Using Biogeography and Ecology; JHU Press: Baltimore, MD, USA, 2014. [Google Scholar]
- Gong, Y.F.; Feng, J.X.; Luo, Z.W.; Xue, J.B.; Guo, Z.Y.; Zhang, L.J.; Xia, S.; Lv, S.; Xu, J.; Li, S.Z. Spatiotemporal heterogeneity of schistosomiasis in mainland China: Evidence from a multi-stage continuous downscaling sentinel monitoring. Asian Pac. J. Trop. Med. 2022, 15, 26. [Google Scholar]
- Gong, Y.F.; Zhu, L.Q.; Li, Y.L.; Zhang, L.J.; Xue, J.B.; Xia, S.; Lv, S.; Xu, J.; Li, S.Z. Identification of the high-risk area for schistosomiasis transmission in China based on information value and machine learning: A newly data-driven modeling attempt. Infect. Dis. Poverty 2021, 10, 88. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Soberón, J.; Peterson, A.T. No silver bullets in correlative ecological niche modelling: Insights from testing among many potential algorithms for niche estimation. Method. Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef] [Green Version]
- Muscarella, R.T.; Galante, P.J.; Soley, G.M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Method. Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]


| Variable Categories | Variable | Definition |
|---|---|---|
| Meteorological factors | AR | Aridity |
| IM | Index of moisture | |
| AAP | Average annual precipitation | |
| AAT | Average annual temperature | |
| AAT0 | ≥0 °C annual accumulated temperature | |
| AAT10 | ≥10 °C annual accumulated temperature | |
| Bioclimatic factors | BIO1 | Annual mean air temperature |
| BIO2 | Monthly mean diurnal temperature range | |
| BIO3 | Isothermality | |
| BIO4 | Temperature seasonality | |
| BIO5 | Maximum air temperature in the warmest month | |
| BIO6 | Minimum air temperature in the coldest month | |
| BIO7 | Temperature annual range | |
| BIO8 | Mean air temperature in the wettest quarter | |
| BIO9 | Mean air temperature in the driest quarter | |
| BIO10 | Mean air temperature in the warmest quarter | |
| BIO11 | Mean air temperature in the coldest quarter | |
| BIO12 | Annual precipitation | |
| BIO13 | Precipitation in the wettest month | |
| BIO14 | Precipitation in the driest month | |
| BIO15 | Precipitation seasonality | |
| BIO16 | Precipitation in the wettest quarter | |
| BIO17 | Precipitation in the driest quarter | |
| BIO18 | Precipitation in the warmest quarter | |
| BIO19 | Precipitation in the coldest quarter | |
| Geographical factors | EL | Elevation |
| LF | Type of landform | |
| LD | Type of land use | |
| Sand | Soil texture classified as sand | |
| Silt | Soil texture classified as silt | |
| Clay | Soil texture classified as clay | |
| ANDVI | Annual normalized difference vegetation index | |
| Socioeconomic factors | DBP | The density of bovine populations |
| GDP | Gross domestic product | |
| DP | Population density |
| Categories | Model | Definition |
|---|---|---|
| Environmental envelope algorithm | SRE | Surface range envelope |
| Statistical regression algorithm | GLM | Generalized linear models |
| GAM | Generalized additive models | |
| MARS | Multivariate adaptive regression spline | |
| Classification algorithm | GBM | Generalized boosted models model |
| CTA | Classification tree analysis model | |
| FDA | Flexible discriminant analysis model | |
| Machine learning algorithm | ANN | Artificial neural network model |
| RF | Random forest model | |
| MaxEnt | Maximum entropy model |
| Parameter | Failure | Poor | Fair | Good | Very Good |
|---|---|---|---|---|---|
| ROC | 0.00–0.49 | 0.50–0.69 | 0.70–0.79 | 0.80–0.89 | 0.90–1.00 |
| Kappa | −1.00–0.39 | 0.40–0.54 | 0.55–0.69 | 0.70–0.84 | 0.85–1.00 |
| TSS | −1.00–0.39 | 0.40–0.54 | 0.55–0.69 | 0.70–0.84 | 0.85–1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Hu, X.; Hao, Y.; Gong, Y.; Wang, X.; Huang, L.; Lv, S.; Xu, J.; Li, S.; Xia, S. Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling. Trop. Med. Infect. Dis. 2023, 8, 24. https://doi.org/10.3390/tropicalmed8010024
Xue J, Hu X, Hao Y, Gong Y, Wang X, Huang L, Lv S, Xu J, Li S, Xia S. Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling. Tropical Medicine and Infectious Disease. 2023; 8(1):24. https://doi.org/10.3390/tropicalmed8010024
Chicago/Turabian StyleXue, Jingbo, Xiaokang Hu, Yuwan Hao, Yanfeng Gong, Xinyi Wang, Liangyu Huang, Shan Lv, Jing Xu, Shizhu Li, and Shang Xia. 2023. "Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling" Tropical Medicine and Infectious Disease 8, no. 1: 24. https://doi.org/10.3390/tropicalmed8010024
APA StyleXue, J., Hu, X., Hao, Y., Gong, Y., Wang, X., Huang, L., Lv, S., Xu, J., Li, S., & Xia, S. (2023). Transmission Risk Predicting for Schistosomiasis in Mainland China by Exploring Ensemble Ecological Niche Modeling. Tropical Medicine and Infectious Disease, 8(1), 24. https://doi.org/10.3390/tropicalmed8010024








