Essential Oils and Terpenic Compounds as Potential Hits for Drugs against Amitochondriate Protists
Abstract
:1. Introduction
2. Giardia duodenalis
3. Entamoeba histolytica
4. Trichomonas vaginalis
5. Essential Oils and Terpenic Compounds against Amitochondriate Parasites
5.1. Essential Oils against G. Duodenalis
| Plant Species/ Botanical Family | Parasitic Organism | Active Concentration | Major Components | Results | References |
|---|---|---|---|---|---|
| Amaranthaceae | |||||
| Dysphania ambrosioides | E. histolytica | IC50: 0.7 mg/mL, 8 and 80 mg/kg | Ascaridole epoxide and cis-Ascaridole | Significant amoebicidal activity | [57] |
| Apiaceae | |||||
| Cuminum cyminum | G. duodenalis | LD50: 175 µg/mL | Cuminaldehyde | Significant giardicidal activity | [58] |
| Foeniculum vulgare | T. vaginalis | MLC: 1600 μg/mL | E-anethole, fenchone, and ρ-anisaldehyde | Significant trichomonacidal activity | [59] |
| Pimpinella anisum | G. duodenalis | LD50: 136 µg/mL | trans-Anethole | Significant giardicidal activity | [58] |
| Asteraceae | |||||
| Ageratum conyzoides | G. duodenalis | IC50: 35.00 and 89.33 μg/mL (LW–P and FP fractions) | Precocene I, β-caryophyllene, precocene II, α-caryophyllene | Significant giardicidal activity | [60] |
| Artemisia absinthium | T. vaginalis | GI50: 87.2 μg/mL | cis-epoxycimene, (-)-cis-chrysanthenol, and 3,6-dihydrochamazulene | Significant trichomonacidal activity | [61] |
| Fabaceae | |||||
| Brazilian red propolis (Resinous exudates of Dalbergia ecastophyllum) | T. vaginalis | IC50: 100 μg/mL MIC: 500 μg/mL | Methyl eugenol, (E)-β-farnesene, and δ-amorphene | Significant trichomonacidal activity | [62] |
| Labiatae | |||||
| Dracocephalum kotschyi | T. vaginalis | IC50: 84.07 μg/mL | Copaene, methyl geranate, geranial, and carvone | Significant trichomonacidal activity; induction of an apoptosis-like cell death on trophozoites | [63] |
| Lamiaceae | |||||
| Lavandula angustifolia | T. vaginalis G. duodenalis | IC50: ≤ 1% | Not determined | Significant trichomonacidal and giardicidal activity | [54] |
| Lavandula x intermedia | T. vaginalis G. duodenalis | IC50: ≤ 1% | Not determined | Significant trichomonacidal and giardicidal activity | [54] |
| Ocimum basilicum | T. vaginalis G. duodenalis | MLC: 30 µg/mL IC50: 2 mg/mL | Linalool, eugenol, and α-Trans-bergamotene | Significant trichomonacidal activity; EO was able to kill almost 80% of Giardia trophozoites in 120min | [55,64] |
| Origanum virens | G. duodenalis | IC50: 85 µg/mL | Carvacrol, γ-Terpinene, and p-Cymene | Significant giardicidal activity | [56] |
| Thymbra capitata | G. duodenalis | IC50: 71 µg/mL | Carvacrol, p-Cymene, and γ-Terpinene | Significant giardicidal activity | [56] |
| Thymus vulgaris | E. histolytica | MIC: 0.7 mg/mL | Not determined | Significant amoebicidal activity | [65] |
| Thymus zygis subsp. sylvestris | G. duodenalis | IC50: 185 µg/mL | p-Cymene, γ-Terpinene, and thymol | Significant giardicidal activity | [56] |
| Lauraceae | |||||
| Cinnamomum verum | G. duodenalis | LD50: 108 µg/mL | Cinnamaldehyde | Significant giardicidal activity | [58] |
| Laurus nobilis | G. duodenalis | LD50: 193 µg/mL | Eucalyptol | Significant giardicidal activity | [58] |
| Nectandra megapotamica | T. vaginalis | IC50: 98.7 μg/mL | (+)-α-Bisabolol | Significant trichomonacidal activity | [66] |
| Myrtaceae | |||||
| Eucalyptus globulus | G. duodenalis | Antigiardial activity (73.55%) after exposure to 1000 µL/mL | 1,8-eucalyptol, α-pinene, α-terpineol acetate | Significant giardicidal activity | [67] |
| Eugenia brejoensis | T. vaginalis | MIC: <500 μg/mL | Not determined | Significant trichomonacidal activity | [68] |
| Eugenia flavescens | T. vaginalis | MIC: <500 μg/mL | Not determined | Significant trichomonacidal activity | [68] |
| Eugenia gracillima | T. vaginalis | MIC: 500 μg/mL IC50: 185.6 μg/mL | Not determined | Significant trichomonacidal activity | [68] |
| Eugenia pohliana | T. vaginalis | MIC: 500 μg/mL IC50: 257.8 μg/mL | Delta-cadinene, bicyclogermacrene, and epi-a-muurolol | Significant trichomonacidal activity against ATCC and fresh clinical isolates; synergistic effect when associated with MTZ | [68] |
| Myrciaria floribunda | T. vaginalis | MIC: 500 μg/mL IC50: 162.9 μg/mL | Not determined | Significant trichomonacidal activity | [68] |
| Psidium myrsinites | T. vaginalis | MIC: 500 μg/mL IC50: 179.6 μg/mL | Not determined | Significant trichomonacidal activity against ATCC and fresh clinical isolates; synergistic effect associated with MTZ | [68] |
| Syzygium aromaticum | G. duodenalis | LD50: 139 µg/mL | Eugenol | Significant giardicidal activity | [58] |
| Rutaceae | |||||
| Atalantia sessiflora | T. vaginalis | IC50: 0.016% IC90: 0.03% MLC: 0.06% | Linalool, E-β-caryophyllene, and ledene | Significant trichomonacidal activity | [69] |
| Citrus aurantifolia | G. duodenalis | LD50: 112 µg/mL | Limonene | Significant giardicidal activity | [58] |
| Verbenaceae | |||||
| Lippia berlandieri | G. duodenalis | LD50: 60 µg/mL | Thymol | Significant giardicidal activity | [58] |
| Lippia graveolens | G. duodenalis | IC50: 257 µg/mL | Thymol, p-Cymene, and caryophyllene oxide | Significant giardicidal activity | [56] |
| Zingiberaceae | |||||
| Aframomum sceptrum | T. vaginalis | IC50: 0.12 µL/mL MLC: 1.72 µL/ml | β-pinene, caryophyllene oxide, and cyperene | Significant trichomonacidal activity | [70] |
| Amomum tsao-ko | T. vaginalis | MLC: 44.97µg/mL IC50: 22.49 µg/mL | Geraniol (unpublished) | Significant trichomonacidal activity | [19] |
| Zingiber officinalis | G. duodenalis | Reduction in cysts (61.15%) at 1000 µL/mL | Geraniol, α-zingiberene, (E,E)-α-farnesene | Significant giardicidal activity | [67] |
5.2. Essential Oils against E. histolytica
5.3. Essential Oils against T. vaginalis
5.4. Terpenic Compounds against G. duodenalis
5.5. Terpenic Compounds against E. histolytica
5.6. Terpenic Compounds against T. vaginalis
6. Mechanisms of Action of Essential Oils and Terpenic Compounds against Amitochondriate Parasites
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piperaki, E.T.; Tassios, P.T. Parasitic infections: Their position and impact in the postindustrial world. Clin. Microbiol. Infect. 2016, 22, 469–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parise, M.E.; Hotez, P.J.; Slutsker, L. Neglected parasitic infections in the United States: Needs and opportunities. Am. J. Trop. Med. Hyg. 2014, 90, 783–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez-Maya, S.; Moreno-Herrera, A.; Palos, I.; Rivera, G. Old antiprotozoal drugs: Are they still viable options for parasitic infections or new options for other diseases? Curr. Med. Chem. 2020, 27, 5403–5428. [Google Scholar] [CrossRef]
- Faso, C.; Hehl, A.B. A cytonaut’s guide to protein trafficking in Giardia lamblia. Adv. Parasitol. 2019, 106, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Vanacova, S.; Liston, D.R.; Tachezy, J.; Johnson, P.J. Molecular biology of the amitochondriate parasites, Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. Int. J. Parasitol. 2003, 33, 235–255. [Google Scholar] [CrossRef]
- Santos, H.J.; Nozaki, T. Interorganellar communication and membrane contact sites in protozoan parasites. Parasitol. Int. 2021, 83, 102372. [Google Scholar] [CrossRef]
- Ceruelos, A.H.; Romero-Quezada, L.C.; Ledezma, J.R.; Contreras, L.L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. 2019, 23, 397–401. [Google Scholar] [CrossRef]
- Ehrenkaufer, G.M.; Suresh, S.; Solow-Cordero, D.; Singh, U. High-throughput screening of Entamoeba identifies compounds which target both life cycle stages and which are effective against metronidazole resistant parasites. Front. Cell. Infect. Microbiol. 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Vieira, P.B.; Tasca, T.; Secor, W.E. Challenges and persistent questions in the treatment of Trichomoniasis. Curr. Top. Med. Chem. 2017, 17, 1249–1265. [Google Scholar] [CrossRef]
- Argüello-García, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Barrientes, F.J. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob. Agents Chemother. 2006, 50, 4209–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques-Silva, M.; Lisboa, C.; Gomes, N.; Rodrigues, A.G. Trichomonas vaginalis and growing concern over drug resistance: A systematic review. Eur. Acad. Dermatol. Venereol. 2021, 35, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Kangussu-Marcolino, M.M.; Singh, U. Ponatinib, Lestaurtinib, and mTOR/PI3K Inhibitors Are Promising Repurposing Candidates against Entamoeba histolytica. Antimicrob. Agents Chemother. 2022, 66, e01207-21. [Google Scholar] [CrossRef]
- Rios, J.L. Essential oils: What they are and how the terms are used and defined. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press, EUA: San Diego, CA, USA, 2016; pp. 3–10. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Nieto, G. Biological activities of three essential oils of the Lamiaceae family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Masci, V.L.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crops Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Esquivel, B.; Calzada, F.; Yepez-Mulia, L.; Calderón, J.S.; Porras-Ramirez, J.; Quijano, L. Further thymol derivatives from Ageratina cylindrica. Chem. Biodivers. 2016, 13, 1281–1289. [Google Scholar] [CrossRef]
- Dai, M.; Peng, C.; Peng, F.; Xie, C.; Wang, P.; Sun, F. Anti-Trichomonas vaginalis properties of the oil of Amomum tsao-ko and its major component, geraniol. Pharm. Biol. 2016, 54, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Hübner, D.P.G.; Vieira, P.B.; Frasson, A.P.; Menezes, C.B.; Senger, F.R.; Silva, G.N.S.; Gnoatto, S.C.B.; Tasca, T. Anti-Trichomonas vaginalis activity of betulinic acid derivatives. Biomed. Pharmacother. 2016, 84, 476–484. [Google Scholar] [CrossRef]
- Monzote, L.; Scherbakov, A.M.; Scull, R.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Essential oil from Melaleuca leucadendra: Antimicrobial, antikinetoplastid, antiproliferative and cytotoxic assessment. Molecules 2020, 25, 5514. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Agholi, M.; Ghanbariasad, A.; Ranjbar, A.; Osanloo, M. A nanoemulsion-based nanogel of Citrus limon essential oil with leishmanicidal activity against Leishmania tropica and Leishmania major. J. Parasit. Dis. 2021, 45, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cernikova, L.; Faso, C.; Hehl, A.B. Five facts about Giardia lamblia. PLoS Pathog. 2018, 14, e1007250. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.K.; Leung, A.A.; Wong, A.H.; Sergi, C.M.; Kam, J.K. Giardiasis: An overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef]
- Vivancos, V.; González-Alvarez, I.; Bermejo, M.; Gonzalez-Alvarez, M. Giardiasis: Characteristics, pathogenesis and new insights about treatment. Curr. Top. Med. Chem. 2018, 18, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A.; Bermúdez-Cruz, R.M. Epigenetics in the early divergent eukaryotic Giardia duodenalis: An update. Biochimie 2019, 156, 123–128. [Google Scholar] [CrossRef]
- Barash, N.R.; Nosala, C.; Pham, J.K.; McInally, S.G.; Gourguechon, S.; McCarthy-Sinclair, B.; Dawson, S.C. Giardia colonizes and encysts in high-density foci in the murine small intestine. MSphere 2017, 2, e00343-16. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Mentel, M.; van Hellemond, J.J.; Henze, K.; Woehle, C.; Gould, S.B.; Yu, R.; Giezen, M.V.D.; Tielens, A.G.M.; Martin, W.F. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 2012, 76, 444–495. [Google Scholar] [CrossRef] [Green Version]
- Jedelský, P.L.; Doležal, P.; Rada, P.; Pyrih, J.; Šmíd, O.; Hrdý, I.; Šedinová, M.; Marcinčiková, M.; Voleman, L.; Perry, A.J.; et al. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS ONE 2011, 6, e17285. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in clinical manifestation, pathogenesis, and vaccine development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Huston, C.D.; Hughes, M.; Houpt, E.; Petri Jr, W.A. Amebiasis. N. Engl. J. Med. 2003, 348, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, M.T.; Malik, Z. Revisiting Drug Development Against the Neglected Tropical Disease, Amebiasis. Front. Cell. Infect. Microbiol. 2021, 10, 628257. [Google Scholar] [CrossRef] [PubMed]
- Saidin, S.; Othman, N.; Noordin, R. Update on laboratory diagnosis of amoebiasis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Desure, S.; Mallika, A.; Roy, M.; Jyoti, A.; Kaushik, S.; Srivastava, V.K. The flip side of reactive oxygen species in the tropical disease-Amoebiasis. Chem. Biol. Drug Des. 2021, 98, 930–942. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Yousuf, M.A.; Nakada-Tsukui, K.; Nozaki, T. Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 21731–21736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi-ichi, F.; Makiuchi, T.; Furukawa, A.; Sato, D.; Nozaki, T. Sulfate activation in mitosomes plays an important role in the proliferation of Entamoeba histolytica. PLOS Negl. Trop. Dis. 2011, 5, e1263. [Google Scholar] [CrossRef]
- Mi-ichi, F.; Miyamoto, T.; Takao, S.; Jeelani, G.; Hashimoto, T.; Hara, H.; Nozaki, T.; Yoshida, H. Entamoeba mitosomes play an important role in encystation by association with cholesteryl sulfate synthesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2884–E2890. [Google Scholar] [CrossRef] [Green Version]
- Jeelani, G.; Nozaki, T. Metabolomic analysis of Entamoeba: Applications and implications. Curr. Opin. Microbiol. 2014, 20, 118–124. [Google Scholar] [CrossRef]
- Andrade, R.M.; Reed, S.L. New drug target in protozoan parasites: The role of thioredoxin reductase. Front. Microbiol. 2015, 6, 975. [Google Scholar] [CrossRef] [Green Version]
- Kusdian, G.; Gould, S.B. The biology of Trichomonas vaginalis in the light of urogenital tract infection. Mol. Biochem. Parasitol. 2014, 198, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Ferrão, A.R.; Pereira, F.M.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Trichomonas vaginalis: An updated overview towards diagnostic improvement. Acta Parasitol. 2016, 61, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. The clinical spectrum of Trichomonas vaginalis infection and challenges to management. Sex. Transm. Infect. 2013, 89, 423–425. [Google Scholar] [CrossRef]
- Seo, M.Y.; Im, S.J.; Gu, N.Y.; Kim, J.H.; Chung, Y.H.; Ahn, M.H.; Ryu, J.S. Inflammatory response of prostate epithelial cells to stimulation by Trichomonas vaginalis. Prostate 2014, 74, 441–449. [Google Scholar] [CrossRef]
- Mercer, F.; Johnson, P.J. Trichomonas vaginalis: Pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol. 2018, 34, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Price, C.M.; Peters, R.P.; Steyn, J.; Mudau, M.; Olivier, D.; De Vos, L.; Morikawa, E.; Kock, M.M.; Medina-Marino, A.; Klausner, J.D. Prevalence and detection of Trichomonas vaginalis in human immunodeficiency virus-infected pregnant women. Sex. Transm. Dis. 2018, 45, 332. [Google Scholar] [CrossRef]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Rotte, C.; Henze, K.; Müller, M.; Martin, W. Origins of hydrogenosomes and mitochondria: Commentary. Curr. Opin. Microbiol. 2000, 3, 481–486. [Google Scholar] [CrossRef]
- Leitsch, D. Recent advances in the molecular biology of the protist parasite Trichomonas vaginalis. Fac. Rev. 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Rigo, G.V.; Frank, L.A.; Galego, G.B.; Santos, A.L.S.D.; Tasca, T. Novel Treatment Approaches to Combat Trichomoniasis, a Neglected and Sexually Transmitted Infection Caused by Trichomonas vaginalis: Translational Perspectives. Venereology 2022, 1, 47–80. [Google Scholar] [CrossRef]
- Moon, T.; Wilkinson, J.M.; Cavanagh, H. Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflata. Parasitol. Res. 2006, 99, 722–728. [Google Scholar] [CrossRef]
- De Almeida, I.; Alviano, D.S.; Vieira, D.P.; Alves, P.B.; Blank, A.F.; Lopes, A.H.; Alviano, C.S.; Rosa, M.D.S.S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Cavaleiro, C.; Custódio, J.; Sousa, M.D.C. Anti-Giardia activity of phenolic-rich essential oils: Effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010, 106, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Blanco, M.E.; Rodríguez, M.G.; Moreno Duque, J.L.; Muñoz-Ortega, M.; Ventura-Juárez, J. Amoebicidal activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an amoebic liver abscess hamster model. Evid. Based Complement. Alternat. Med. 2014, 2014, 930208. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef]
- Karami, F.; Dastan, D.; Fallah, M.; Matini, M. In vitro activity of Foeniculum vulgare and its main essential oil component trans-anethole on Trichomonas vaginalis. Iran. J. Parasitol. 2019, 14, 631–638. [Google Scholar] [CrossRef]
- Pintong, A.R.; Ruangsittichai, J.; Ampawong, S.; Thima, K.; Sriwichai, P.; Komalamisra, N.; Popruk, S. Efficacy of Ageratum conyzoides extracts against Giardia duodenalis trophozoites: An experimental study. BMC Complement. Altern. Med. 2020, 20, 63. [Google Scholar] [CrossRef]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; Heras, L.D.L.; Prado, G.D.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. Inst. Oswaldo Cruz 2015, 110, 693–699. [Google Scholar] [CrossRef]
- Sena-Lopes, A.; Bezerra, F.S.B.; das Neves, R.N.; de Pinho, R.B.; Silva, M.T.D.O.; Savegnago, L.; Collares, T.; Seixas, F.; Begnini, K.; Henriques, J.A.P.; et al. Chemical composition, immunostimulatory, cytotoxic and antiparasitic activities of the essential oil from Brazilian red propolis. PLoS ONE 2018, 13, e0191797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehkordi, B.C.; Esmaeilifallah, M.; Kalantari, R.; Benchimol, M.; Khamesipour, F. Induction of apoptosis by essential oil of Dracocephalum kotschyi on Trichomonas vaginalis. Vet. Med. Sci. 2022, 1–9. [Google Scholar] [CrossRef]
- Eldin, H.M.E.; Badawy, A.F. In vitro anti-Trichomonas vaginalis activity of Pistacia lentiscus mastic and Ocimum basilicum essential oil. J. Parasit. Dis. 2015, 39, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnia, M.; Haghighi, A.; Komeylizadeh, H.; Tabaei, S.J.S.; Abadi, A. Inhibitory effects of Iranian Thymus vulgaris extracts on in vitro growth of Entamoeba histolytica. Korean J. Parasitol. 2008, 46, 153–156. [Google Scholar] [CrossRef]
- Farias, K.S.; Kato, N.N.; Boaretto, A.G.; Weber, J.I.; Brust, F.R.; Alves, F.M.; Tasca, T.; Macedo, A.J.; Silva, D.B.; Carollo, C.A. Nectandra as a renewable source for (+)-α-bisabolol, an antibiofilm and anti-Trichomonas vaginalis compound. Fitoterapia 2019, 136, 104179. [Google Scholar] [CrossRef]
- Dehghani-Samani, A.; Madreseh-Ghahfarokhi, S.; Pirali, Y. In-vitro antigiardial activity and GC-MS analysis of Eucalyptus globulus and Zingiber officinalis essential oils against Giardia lamblia cysts in simulated condition to human’s body. Ann. Parasitol. 2019, 65, 129–138. [Google Scholar] [CrossRef]
- Menezes, S.A.; Galego, G.B.; Rigo, G.D.V.; de Aguiar, J.C.R.D.O.F.; Veras, B.D.O.; Vandesmet, L.C.S.; dos Santos, C.R.B.; Sampaio, M.G.V.; Marques, C.C.; Lermen, V.L.; et al. Anti-Trichomonas vaginalis activity of essential oils extracted from Caatinga Myrtaceae species and chemical composition of Eugenia pohliana DC. Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Le, N.T.; Donadu, M.G.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Marchetti, M.; Usai, M.; et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J. Infect. Dev. Ctries. 2020, 14, 1054–1064. [Google Scholar] [CrossRef]
- Cheikh-Ali, Z.; Adiko, M.; Bouttier, S.; Bories, C.; Okpekon, T.; Poupon, E.; Champy, P. Composition, and antimicrobial and remarkable antiprotozoal activities of the essential oil of rhizomes of Aframomum sceptrum K. Schum.(Zingiberaceae). Chem. Biodivers. 2011, 8, 658–667. [Google Scholar] [CrossRef]
- Mena-Rejón, G.J.; Pérez-Espadas, A.R.; Moo-Puc, R.E.; Cedillo-Rivera, R.; Bazzocchi, I.L.; Jiménez-Diaz, I.A.; Quijano, L. Antigiardial activity of triterpenoids from root bark of Hippocratea excelsa. J. Nat. Prod. 2007, 70, 863–865. [Google Scholar] [CrossRef]
- Calzada, F.; Bautista, E.; Yépez-Mulia, L.; García-Hernandez, N.; Ortega, A. Antiamoebic and antigiardial activity of clerodane diterpenes from Mexican Salvia species used for the treatment of diarrhea. Phytother. Res. 2015, 29, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Sánchez-Castellanos, M.; Esquivel, B.; Calderón, J.S.; Calzada, F.; Yepez-Mulia, L.; Joseph-Nathan, P.; Cuevas, G.; Quijano, L. ent-Kaurene Glycosides from Ageratina cylindrica. J. Nat. Prod. 2015, 78, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Sánchez-Castellanos, M.; Esquivel, B.; Calderón, J.S.; Calzada, F.; Yepez-Mulia, L.; Hernández-Barragán, A.; Joseph-Nathan, P.; Cuevas, G.; Quijano, L. Structure, absolute configuration, and antidiarrheal activity of a thymol derivative from Ageratina cylindrica. J. Nat. Prod. 2014, 77, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Rufino-González, Y.; Ponce-Macotela, M.; Jiménez-Estrada, M.; Jiménez-Fragoso, C.N.; Palencia, G.; Sansón-Romero, G.; Anzo-Osorio, A.; Martínez-Gordillo, M.N. Piqueria trinervia as a source of metabolites against Giardia duodenalis. Pharm. Biol. 2017, 55, 1787–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.F.; Shen, H.E.; Li, J.; Chen, Y.; Yang, Z.H.; Lu, S.Q. The effects of dihydroartemisinin on Giardia lamblia morphology and cell cycle in vitro. Parasitol. Res. 2010, 107, 369–375. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Rufino-Gonzalez, Y.; Ponce-Macotela, M.; Delgado, G. In vitro activity of ‘Mexican Arnica’ Heterotheca inuloides Cass natural products and some derivatives against Giardia duodenalis. Parasitology 2015, 142, 576–584. [Google Scholar] [CrossRef]
- Bautista, E.; Calzada, F.; López-Huerta, F.A.; Yépez-Mulia, L.; Ortega, A. Antiprotozoal activity of 8-acyl and 8-alkyl incomptine A analogs. Bioorganic Med. Chem. Lett. 2014, 24, 3260–3262. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; Araya, J.; González, J.; Neira, I.; Sagua, H.; San-Martín, A. Diterpenoids from Azorella yareta and their trichomonicidal activities. Phytochemistry 2001, 56, 177–180. [Google Scholar] [CrossRef]
- Innocente, A.M.; Vieira, P.B.; Frasson, A.P.; Casanova, B.B.; Gosmann, G.; Gnoatto, S.C.B.; Tasca, T. Anti-Trichomonas vaginalis activity from triterpenoid derivatives. Parasitol. Res. 2014, 113, 2933–2940. [Google Scholar] [CrossRef]
- Bitencourt, F.G.; Vieira, P.B.; Meirelles, L.C.; Rigo, G.V.; da Silva, E.F.; Gnoatto, S.C.B.; Tasca, T. Anti-Trichomonas vaginalis activity of ursolic acid derivative: A promising alternative. Parasitol. Res. 2018, 117, 1573–1580. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.; Cavaleiro, C.; Sousa, M.C. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Van Puyvelde, L.; Maes, L.; Bosselaers, J.; De Kimpe, N. Antitrichomonas in vitro activity of Cussonia holstii. Engl. Nat. Prod. Res. 2003, 17, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Domínguez, J.; Marchat, L.A.; López-Camarillo, C.; Mendoza-Hernández, G.; Sánchez-Espíndola, E.; Calzada, F.; Ortega-Hernández, A.; Sánchez-Monroy, V.; Ramírez-Moreno, E. Effect of the sesquiterpene lactone incomptine A in the energy metabolism of Entamoeba histolytica. Exp. Parasitol. 2013, 135, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; Pérez-Hernández, N.; Rivera, G.; Marchat, L.A.; Ramírez-Moreno, E. Antiamoebic activity of Petiveria alliacea leaves and their main component, isoarborinol. J. Microbiol. Biotechnol. 2017, 27, 1401–1408. [Google Scholar] [CrossRef]
- Argüello-García, R.; Calzada, F.; Chávez-Munguía, B.; Matus-Meza, A.S.; Bautista, E.; Barbosa, E.; Velazquez, C.; Hernández-Caballero, M.E.; Ordoñez-Razo, R.M.; Velázquez-Domínguez, J.A. Linearolactone Induces Necrotic-like Death in Giardia intestinalis Trophozoites: Prediction of a Likely Target. Pharmaceuticals 2022, 15, 809. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Domínguez, J.A.; Hernández-Ramírez, V.I.; Calzada, F.; Varela-Rodríguez, L.; Pichardo-Hernández, D.L.; Bautista, E.; Herrera-Martínez, M.; Castellanos-Mijangos, R.D.; Matus-Meza, A.S.; Chávez-Munguía, B.; et al. Linearolactone and kaempferol disrupt the actin cytoskeleton in Entamoeba histolytica: Inhibition of amoebic liver abscess development. J. Nat. Prod. 2020, 83, 3671–3680. [Google Scholar] [CrossRef]
- Samie, A.; Housein, A.; Lall, N.; Meyer, J.J.M. Crude extracts of, and purified compounds from, Pterocarpus angolensis, and the essential oil of Lippia javanica: Their in-vitro cytotoxicities and activities against selected bacteria and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 2009, 103, 427–439. [Google Scholar] [CrossRef]
- Lima, A.A.; Soares, A.M.; Lima, N.L.; Mota, R.M.; Maciel, B.L.; Kvalsund, M.P.; Barrett, L.J.; Fitzgerald, R.P.; Blaner, W.S.; Guerrant, R.L. Vitamin A supplementation effects on intestinal barrier function, growth, total parasitic and specific Giardia spp. infections in Brazilian children: A prospective randomized, double-blind, placebo-controlled trial. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Wani, M.Y.; Athar, F.; Salauddin, A.; Agarwal, S.M.; Azam, A.; Choi, I.; Bhat, A.R. Novel terpene based 1, 4, 2-dioxazoles: Synthesis, characterization, molecular properties and screening against Entamoeba histolytica. Eur. J. Med. Chem. 2011, 46, 4742–4752. [Google Scholar] [CrossRef]
- Vieira, P.B.; Silva, N.L.F.; da Silva, G.N.S.; Silva, D.B.; Lopes, N.P.; Gnoatto, S.C.B.; Silva, M.V.; Macedo, A.J.; Bastida, J.; Tasca, T. Caatinga plants: Natural and semi-synthetic compounds potentially active against Trichomonas vaginalis. Bioorg. Med. Chem. Lett. 2016, 26, 2229–2236. [Google Scholar] [CrossRef]
- Tachezy, J.; Makki, A.; Hrdý, I. The hydrogenosomes of Trichomonas vaginalis. J. Eukaryot. Microbiol. 2022, 69, e12922. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.B.; Giordani, R.B.; Macedo, A.J.; Tasca, T. Natural and synthetic compound anti-Trichomonas vaginalis: An update review. Parasitol. Res. 2015, 114, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Barros, F.J.; Costa, R.J.O.; Cesário, F.R.A.S.; Rodrigues, L.B.; da Costa, J.G.M.; Coutinho, H.D.M.; Galvao, H.B.F.; de Menezes, I.R.A. Activity of essential oils of Piper aduncum anf and Cinnamomum zeylanicum by evaluating osmotic and morphologic fragility of erythrocytes. Eur. J. Integr. Med. 2016, 8, 505–512. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Hardin, W.R.; Alas, G.C.; Taparia, N.; Thomas, E.B.; Steele-Ogus, M.C.; Hvorecny, K.L.; Halpern, A.R.; Tumová, P.; Kollman, J.M.; Vaughan, J.C.; et al. The Giardia ventrolateral flange is a lamellar membrane protrusion that supports attachment. PLoS Pathog. 2022, 18, e1010496. [Google Scholar] [CrossRef]
- Chulanetra, M.; Chaicumpa, W. Revisiting the Mechanisms of Immune Evasion Employed by Human Parasites. Front. Cell. Infect. Microbiol. 2021, 29, 639. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasite immune evasion: A momentous molecular war. Trends Ecol. Evol. 2008, 23, 318–326. [Google Scholar] [CrossRef]
- Hernández, H.M.; Marcet, R.; Sarracent, J. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 2014, 21, 54. [Google Scholar] [CrossRef]
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine proteases: Modes of activation and future prospects as pharmacological targets. Front. Pharmacol. 2016, 7, 107. [Google Scholar] [CrossRef]
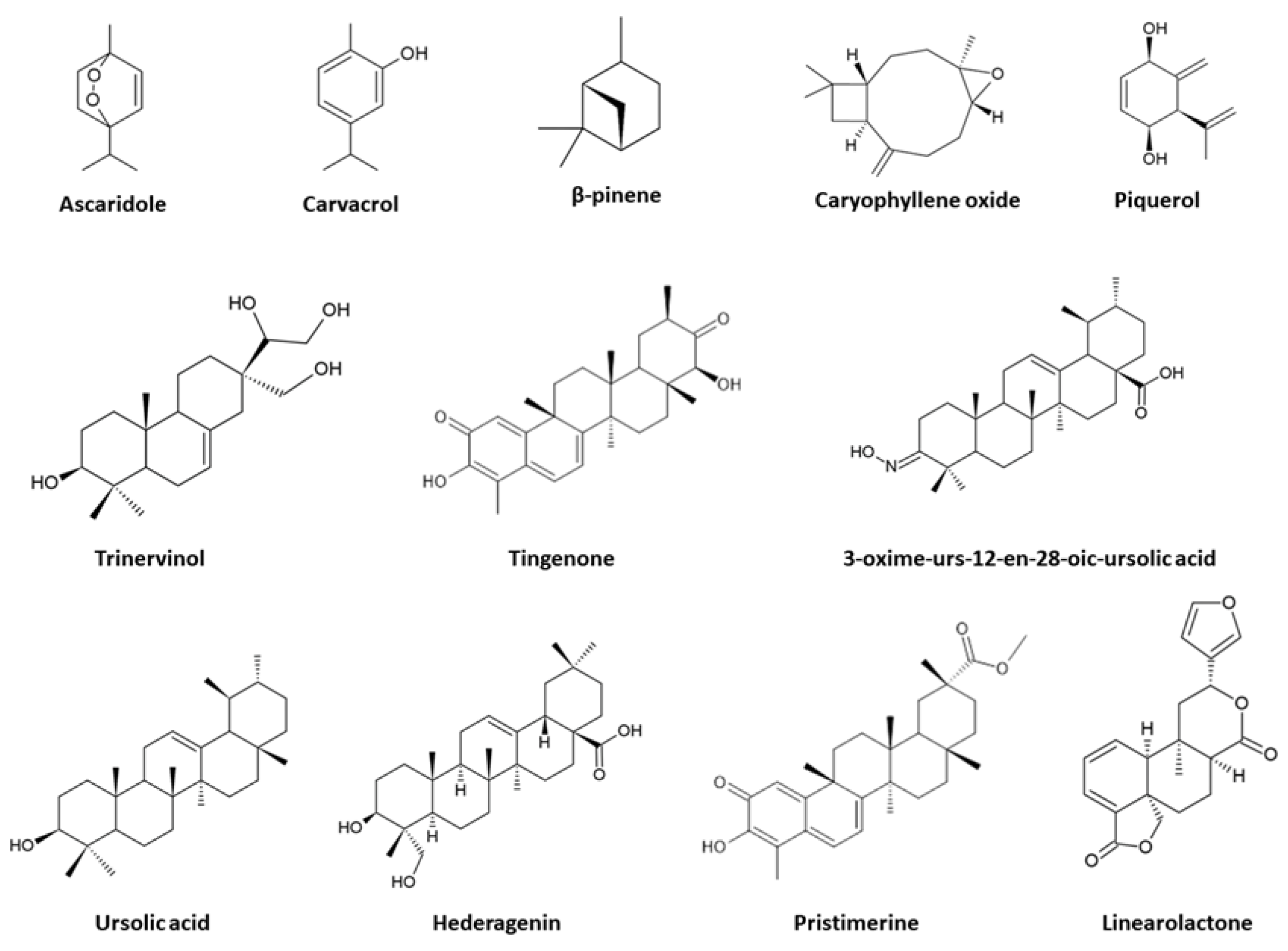

| Terpene/Terpenoids | Protist | Active Concentration | Effect/ Mechanism of Action (MoA) | References |
|---|---|---|---|---|
| (8S)-8,9-epoxy-6-hydroxy-l0-benzoyloxy-7-oxothymol isobutyrate | G. duodenalis E. histolytica | IC50: 167.4 μM IC50: 184.9 μM | Growth inhibition/ unknown MoA | [74] |
| [2-(2-[(2-methylpropanoyl)oxy]-4-{[(2-methylpropanoyl)oxy]methyl}phenyl)oxiran-2-yl]methyl benzoate | G. duodenalis | IC50: 164.5 μM | Antiprotozoal properties associated with the presence of 2 methylpropanoate groups at C(7) and/or C(3) | [18] |
| [2-(5-hydroxy2-[(2-methylpropanoyl)oxy]-4-{[(2-methylpropanoyl)oxy]methyl}phenyl)oxiran-2-yl]methyl benzoate | G. duodenalis | IC50: 151.1 μM | Antiprotozoal properties associated with the presence of 2 methylpropanoate groups at C(7) and/or C(3) | [18] |
| 13b-hydroxyazorellane | T. vaginalis | LD50: 100 μM | Growth inhibition/ unknown MoA | [79] |
| 13α-hydroxyazorellane | T. vaginalis | LD50: 119 μM | Growth inhibition/ unknown MoA | [79] |
| Piperazine derivative from betulinic acid | T. vaginalis | MIC: 91.2 μM | Growth inhibition/ unknown MoA | [80] |
| 7-hydroxy-3,4-dihydrocadalene | G. duodenalis | IC50: 15.3 μg/mL IC90: 23.69 μg/mL | Growth inhibition/morphological and ultrastructural changes (dense material accumulation around nuclei, vacuolization of the cytoplasm, lateral membrane disruption, and ventral disc fragmentation) | [77] |
| 7-hydroxycalamenene | G. duodenalis | IC50: 13.5 μg/mL IC90: 24.21 μg/mL | Growth inhibition/ unknown MoA | [77] |
| (E)-3-oximeurs-12-en-28-oic acid | T. vaginalis (MTZ sensitive and resistant isolate) | MIC: 25 µM (MTZ-sensitive) and 12.5 µM (MTZ-resistant) | Synergic effect with MTZ against the resistant isolate; growth inhibition/unknown MoA | [81] |
| Anethole | G. duodenalis | LD50: 134.99 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Ascaridole | E. histolytica | 80mg of Ascaridole decreased significantly the number of trophozoites | Growth inhibition/ unknown MoA | [57] |
| Azorellanol | T. vaginalis | LD50: 40.5 µM | Growth inhibition/ unknown MoA | [79] |
| N-{3-[4-(3-Aminopropyl)piperazinopropyl]terbutylcarbamate}-3-O-hexanoylbetulinamide | T. vaginalis | MIC: 50 µM | ROS production by neutrophils was reduced and showed anti-inflammatory effect | [20] |
| Betulinic acid derivative | T. vaginalis | MIC: 50 µM | ROS production by neutrophils was reduced | [20] |
| Carvacrol | G. duodenalis | LD50: 31.92 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Caryophyllene oxide | T. vaginalis | IC50: 0.16 mg/mL; MLC: 0.625 mg/ml | Growth inhibition/ unknown MoA | [70] |
| Cinnamaldehyde | G. duodenalis | LD50: 76.42 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Cuminaldehyde | G. duodenalis | LD50: 141.16 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Dihydroartemisinin | G. duodenalis | LD50: 200 μg/mL | Morphological and ultrastructural changes; damages to the cytoskeleton-impaired parasites to complete cell division at different stages, resulting in suppression of growth and differentiation | [76] |
| ent-15β-(β-L-Fucosyloxy)kaur-16-en-19-oic acid β-D-glucopyranosyl ester | G. duodenalis E. histolytica | IC50: 41.9 μM IC50: 43.3 μM | Growth inhibition/ unknown MoA | [73] |
| ent-15β-(4-Acetoxy-β-L-fucosyloxy)kaur-16-en-19-oic acid β-Dglucopyranosyl ester | E. histolytica | IC50: 49.5 μM | Growth inhibition/ unknown MoA | [73] |
| ent-15β-(3-Acetoxy-β-L-fucosyloxy)kaur-16-en-19-oic acid β-Dglucopyranosyl ester | G. duodenalis | IC50: 48.9 μM | Growth inhibition/ unknown MoA | [73] |
| Eucalyptol | G. duodenalis | LD50: 265.43 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Eugenol | G. duodenalis | LD50: 104.04 µg/mL IC50: 101 µg/mL | Morphological and ultrastructural changes (membrane blebs, precipitates in the cytoplasm, fragmentation of ventral disc, autophagic vacuoles, and swelling of peripheral vacuoles) | [55,58,82] |
| Geraniol | T. vaginalis | MLC: 342.96 µg/mL IC50: 171.48 µg/ml | Morphological and ultrastructural changes (autophagic vacuoles formation, organelles disintegration, partial cell membrane damaging, and cytoplasmic leakage) | [19] |
| Hederagenin | T. vaginalis | IC50: 2.8 μM | Growth inhibition/ unknown MoA | [83] |
| Incomptine A | G. lamblia E. histolytica | IC50: 11.8 μg/mL IC50: 2.6 μg/mL | The proteomic profile evidenced a down-regulation of enolase, pyruvate:ferredoxin oxidoreductase (PFOR), and fructose-1,6-biphosphate aldolase; ultrastructural alterations (increase in cytoplasmic glycogen granules) | [78,84] |
| Isoarborinol | E. histolytica | 85.2% of growth inhibition at 0.3 mg/ml | Growth inhibition/ unknown MoA | [85] |
| Limonene | G. duodenalis | LD50: 127.59 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Linalool | G. duodenalis | MIC: 300 μg/ml | Cysteine proteases inhibition | [55] |
| Linearolactone | G. duodenalis E. histolytica | IC50: 28.2 μM IC50: 22.9 μM | Induction of a necrotic-like death with ultrastructural alterations and the prediction of GdAldRed as a likely target in G. duodenalis trophozoites; induction of an apoptosis-like death with the intracellular production of ROS and alteration of the actin cytoskeleton in E. histolytica trophozoites; reduction in the development of amoebic liver abscesses (ALA) in vivo | [72,86,87] |
| Piperitone | E. histolytica | IC50: 25 µg/mL | Growth inhibition/ unknown MoA | [88] |
| Piquerol | G. duodenalis | IC50: 2.42 μg/mL IC90: 8.74 μg/mL | Growth inhibition/ unknown MoA | [75] |
| Pristimerine | G. duodenalis | IC50: 0.11 μM | Growth inhibition/ unknown MoA | [71] |
| Retinol/vitamin A | Giardia spp. | Retinol at 100,000–200,000 IU | Giardia spp. infections were significantly reduced in the group treated with retinol when compared with the placebo group, suggesting an improvement of the host defenses against Giardia infections. | [89] |
| 2-(3-(benzo[d][1,3]dioxol-5-yl)-5-methyl-1,4,2-dioxazol-5-yl) pyridine | E. histolytica | IC50: 1.00 μM | Growth inhibition/ unknown MoA | [90] |
| 2-(3-(benzo[d][1,3]dioxol-5-yl)-1,4,2-dioxazol-5-yl) pyridine | E. histolytica | IC50: 1.03 μM | Growth inhibition/ unknown MoA | [90] |
| 3-(benzo[d][1,3]dioxol-5-yl)-5-(furan-2-yl)-5-methyl-1,4,2-dioxazole | E. histolytica | IC50: 1.10 μM | Growth inhibition/ unknown MoA | [90] |
| 3-(benzo[d][1,3]dioxol-5-yl)-5-(furan-2-yl)-1,4,2-dioxazole | E. histolytica | IC50: 1.09 μM | Growth inhibition/ unknown MoA | [90] |
| 2-(5-(benzo[d][1,3] dioxol-5-yl)-1,4,2-dioxazol-3-yl)pyridine | E. histolytica | IC50: 1.06 μM | Growth inhibition/ unknown MoA | [90] |
| 5-(benzo[d][1,3]dioxol-5-yl)-3-(furan-2-yl)-1,4,2-dioxazole | E. histolytica | IC50: 1.05 μM | Growth inhibition/ unknown MoA | [90] |
| Thymol | G. duodenalis | LD50: 21.44 µg/mL | Growth inhibition/ unknown MoA | [58] |
| Tingenone | G. duodenalis | IC50: 0.74 μM | Growth inhibition/ unknown MoA | [71] |
| Trans-anethole | T. vaginalis | MLC: 1600 μg/mL | Growth inhibition/ unknown MoA | [59] |
| Trinervinol | G. duodenalis | IC50: 2.03 μg/mL IC90: 13.03 μg/mL | Growth inhibition/ unknown MoA | [75] |
| Ursolic acid | T. vaginalis | MIC: 50 μM IC50: 35.3 μM | Morphological and ultrastructural changes (disruption of shape with membrane projections and holes, and undulating membrane and flagella was displayed) | [91] |
| β-pinene | T. vaginalis | IC50: 0.44 mg/mL; MLC: 1.25 mg/ml | Growth inhibition/ unknown MoA | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, S.A.; Tasca, T. Essential Oils and Terpenic Compounds as Potential Hits for Drugs against Amitochondriate Protists. Trop. Med. Infect. Dis. 2023, 8, 37. https://doi.org/10.3390/tropicalmed8010037
Menezes SA, Tasca T. Essential Oils and Terpenic Compounds as Potential Hits for Drugs against Amitochondriate Protists. Tropical Medicine and Infectious Disease. 2023; 8(1):37. https://doi.org/10.3390/tropicalmed8010037
Chicago/Turabian StyleMenezes, Saulo Almeida, and Tiana Tasca. 2023. "Essential Oils and Terpenic Compounds as Potential Hits for Drugs against Amitochondriate Protists" Tropical Medicine and Infectious Disease 8, no. 1: 37. https://doi.org/10.3390/tropicalmed8010037
APA StyleMenezes, S. A., & Tasca, T. (2023). Essential Oils and Terpenic Compounds as Potential Hits for Drugs against Amitochondriate Protists. Tropical Medicine and Infectious Disease, 8(1), 37. https://doi.org/10.3390/tropicalmed8010037







