Prevalence of Human Papillomavirus Infection and Cervical Abnormalities among Women Attending a Tertiary Care Center in Saudi Arabia over 2 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Data Collection
2.3. Ethical Consideration
2.4. Procedures
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hebner, C.M.; Laimins, L.A. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 2006, 16, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D., Jr.; Giuliano, A.R.; Palefsky, J.; Flores, C.A.; Goldstone, S.; Ferris, D.; Hillman, R.J.; Moi, H.; Stoler, M.H.; Marshall, B.; et al. Incidence, Clearance, and Disease Progression of Genital Human Papillomavirus Infection in Heterosexual Men. J. Infect. Dis. 2014, 210, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Lowy, D.R. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008, 113 (Suppl. S2), 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Chan, P.K.; et al. Classification and evolution of human papillomavirus genome variants: Alpha-5 (HPV26, 51, 69, 82), Alpha-6 (HPV30, 53, 56, 66), Alpha-11 (HPV34, 73), Alpha-13 (HPV54) and Alpha-3 (HPV61). Virology 2018, 516, 86–101. [Google Scholar] [CrossRef]
- Lieblong, B.J.; Montgomery, B.E.E.; Su, L.J.; Nakagawa, M. Natural history of human papillomavirus and vaccinations in men: A literature review. Health Sci. Rep. 2019, 2, e118. [Google Scholar] [CrossRef]
- Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Rettig, E.M.; Sethi, R.K. Cancer of the Oropharynx and the Association with Human Papillomavirus. Hematol. Oncol. Clin. North Am. 2021, 35, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chang, M.; Scholl, M.; McKinnon, B.; Berenson, A.B. Trends in Oropharyngeal Cancer Incidence among Adult Men and Women in the United States from 2001 to 2018. Front. Oncol. 2022, 12, 926555. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Saudi Public Health Authority. Saudi Clinical Preventive Guideline Third Edition. 2023. Available online: https://chi.gov.sa/AboutCCHI/CCHIprograms/Documents/Saudi%20Clinical%20Preventive.pdf (accessed on 6 November 2023).
- Alhusayn, K.; Alkhenizan, A.; Abdulkarim, A.; Sultana, H.; Alsulaiman, T.; Alendijani, Y. Attitude and hesitancy of human papillomavirus vaccine among Saudi parents. J. Fam. Med. Prim. Care 2022, 11, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Al-Amri, S.S.; Degnah, A.A.; Tolah, A.M.; Abduljabbar, H.H.; Oraif, A.M.; Abduljabbar, H.S.; Mirza, A.A.; Azhar, E.I.; Hashem, A.M. Prevalence of human papillomavirus in Jeddah, Saudi Arabia. Ann. Saudi Med. 2019, 39, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, E.; Alzamil, L.; Faqih, L.; Dabbagh, D.; Alharbi, S.; Hafiz, T.A.; Alshurafa, H.H.; Altukhais, W.F.; Dabbagh, R. Awareness of Human Papillomavirus among Male and Female University Students in Saudi Arabia. Healthcare 2023, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.-C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef]
- Almehmadi, M.M.; Salih, M.M.; Al-Hazmi, A.S. Awareness of human papillomavirus infection complications, cervical cancer, and vaccine among the Saudi population. A cross-sectional survey. Saudi Med. J. 2019, 40, 555–559. [Google Scholar] [CrossRef]
- Farsi, N.J.; Baharoon, A.H.; Jiffri, A.E.; Marzouki, H.Z.; Merdad, M.A.; Merdad, L.A. Human papillomavirus knowledge and vaccine acceptability among male medical students in Saudi Arabia. Hum. Vaccines Immunother. 2021, 17, 1968–1974. [Google Scholar] [CrossRef]
- Zahid, H.M.; Qarah, A.B.; Alharbi, A.M.; Alomar, A.E.; Almubarak, S.A. Awareness and Practices Related to Cervical Cancer among Females in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 1455. [Google Scholar] [CrossRef]
- Al-Kadri, H.M.; Kamal, M.; Bamuhair, S.S.; Omair, A.A.; Bamefleh, H.S. Prevalence and characteristics of abnormal Papanicolaou smear in Central Saudi Arabia. Saudi Med. J. 2015, 36, 117–122. [Google Scholar] [CrossRef] [PubMed]
- AlBabtain, F.A.; Hussain, A.N.; Alsoghayer, S.A.; Alwahbi, O.A.; Almohaisen, N.; Alkhenizan, A.H. The yield of pap smears and its characteristics in a community based setting in Saudi Arabia. Saudi Med. J. 2020, 41, 661–665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommends DNA Testing as a First-Choice Screening Method for Cervical Cancer Prevention. 2021. Available online: https://www.who.int/europe/news/item/11-09-2021-who-recommends-dna-testing-as-a-first-choice-screening-method-for-cervical-cancer-prevention (accessed on 26 November 2023).
- Smith, M.A.; Sherrah, M.; Sultana, F.; Castle, P.E.; Arbyn, M.; Gertig, D.; Caruana, M.; Wrede, C.D.; Saville, M.; Canfell, K. National experience in the first two years of primary human papillomavirus (HPV) cervical screening in an HPV vaccinated population in Australia: Observational study. BMJ 2022, 376, e068582. [Google Scholar] [CrossRef] [PubMed]
- Maver, P.J.; Poljak, M. Primary HPV-based cervical cancer screening in Europe: Implementation status, challenges, and future plans. Clin. Microbiol. Infect. 2020, 26, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.A.; El-Zein, M.; Ramanakumar, A.V.; Ratnam, S.; Sangwa-Lugoma, G.; Longatto-Filho, A.; Cardoso, M.A.; Coutlée, F.; Franco, E.L.; The PEACHS (Pap Efficacy After Cervical HPV Status) Study Consortium. HPV DNA testing with cytology triage in cervical cancer screening: Influence of revealing HPV infection status. Cancer Cytopathol. 2015, 123, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Vigliotti, J.S.; Vigliotti, V.S.; Jones, W. From Human Papillomavirus (HPV) Detection to Cervical Cancer Prevention in Clinical Practice. Cancers 2014, 6, 2072–2099. [Google Scholar] [CrossRef]
- Turki, R.; Sait, K.; Anfinan, N.; Sohrab, S.S.; Abuzenadah, A.M. Prevalence of Human Papillomavirus in Women from Saudi Arabia. Asian Pac. J. Cancer Prev. 2013, 14, 3177–3181. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 26 November 2023).
- Arslan, E.; Gokdagli, F.; Bozdag, H.; Vatansever, D.; Karsy, M. Abnormal Pap-Smear Frequency and Comparison of Repeat Cytological Follow-up with Colposcopy during Patient Management: The Importance of Pathologist’s Guidance in the Management. North Clin. Istanb. 2018, 6, 69–74. [Google Scholar] [CrossRef]
- Sundström, K.; Lu, D.; Elfström, K.M.; Wang, J.; Andrae, B.; Dillner, J.; Sparén, P. Follow-up of women with cervical cytological abnormalities showing atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion: A nationwide cohort study. Am. J. Obstet. Gynecol. 2017, 216, 48.e1–48.e15. [Google Scholar] [CrossRef][Green Version]
- Regauer, S.; Reich, O.; Kashofer, K. HPV-negative Squamous Cell Carcinomas of the Cervix with Special Focus on Intraepithelial Precursor Lesions. Am. J. Surg. Pathol. 2022, 46, 147–158. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2020, 10, 606335. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Estrada, M.; Juárez-Torres, E.; Román-Bassaure, E.; Medina-Martinez, I.; Alfaro, A.; Benuto, R.E.; Dean, M.; Villegas-Sepulveda, N.; Berumen, J. The Distribution of High-Risk Human Papillomaviruses Is Different in Young and Old Patients with Cervical Cancer. PLoS ONE 2014, 9, e109406. [Google Scholar] [CrossRef] [PubMed]

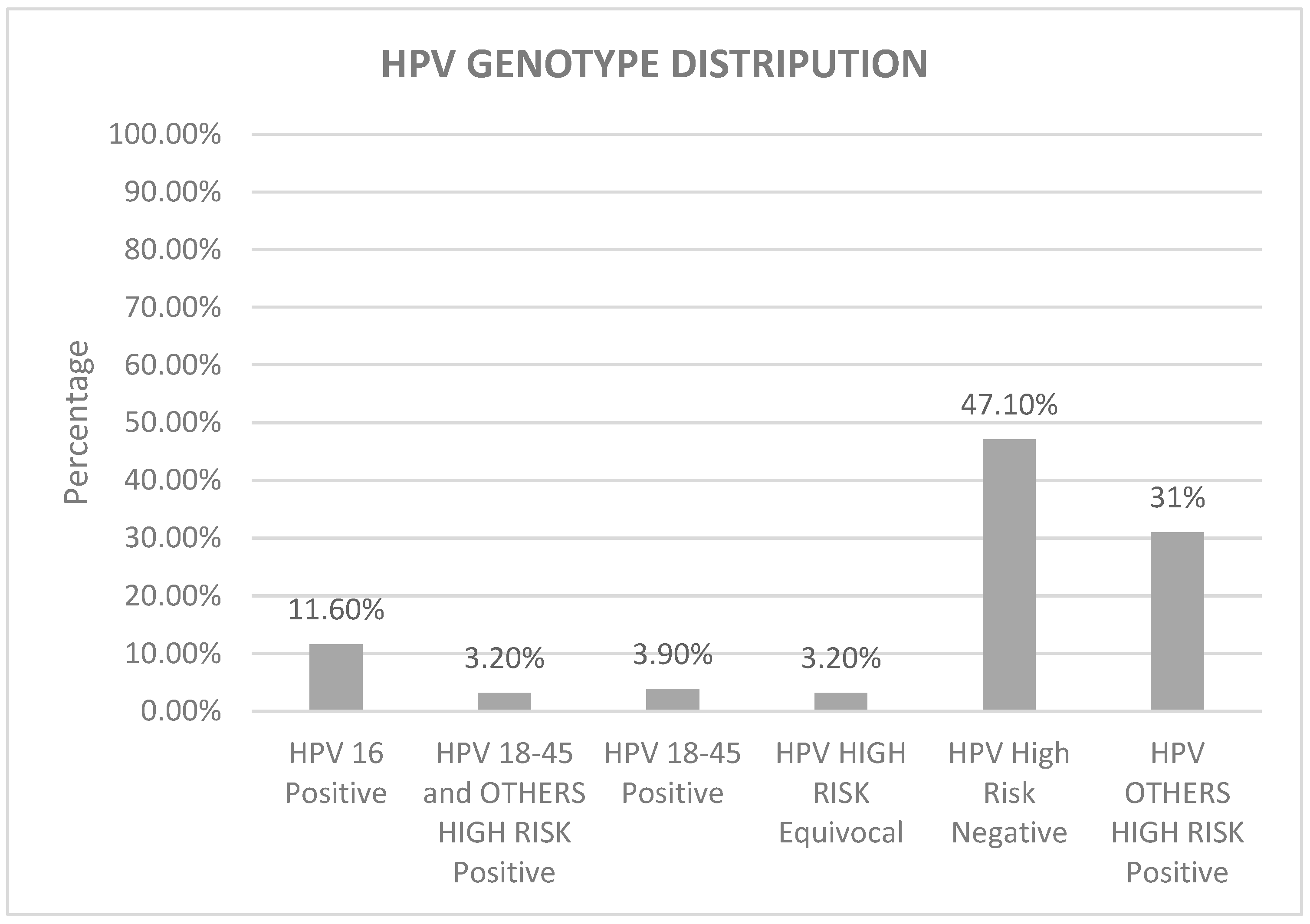
| HPV PCR N (%) | HPV 16 Positive | HPV 18-45 Positive | HPV 18-45 + Other HR Positive | HPV Other HR Positive | HPV HR Equivocal | HPV HR Negative | Total |
|---|---|---|---|---|---|---|---|
| 20–29 | 4 (2.6%) | 2 (1.3%) | 1 (0.6%) | 9 (5.8%) | 2 (1.3%) | 5 (3.2%) | 23 (14.8%) |
| 30–39 | 10 (6.5%) | 1 (0.6%) | 2 (1.3%) | 23 (15%) | 2 (1.3%) | 37 (24%) | 75 (48.7%) |
| 40–49 | 4 (2.6%) | 3 (1.9%) | 2 (1.3%) | 15 (9.7%) | 1 (0.6%) | 20 (12.9%) | 45 (29%) |
| 50–59 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.6%) | 0 (0%) | 7 (4.5%) | 8 (5.1%) |
| ≥60 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (2.6%) | 4 (2.6%) |
| Total | 18 (11.6%) | 6 (3.9%) | 5 (3.2%) | 48 (31%) | 5 (3.2%) | 73 (47.1%) | 155 (100%) |
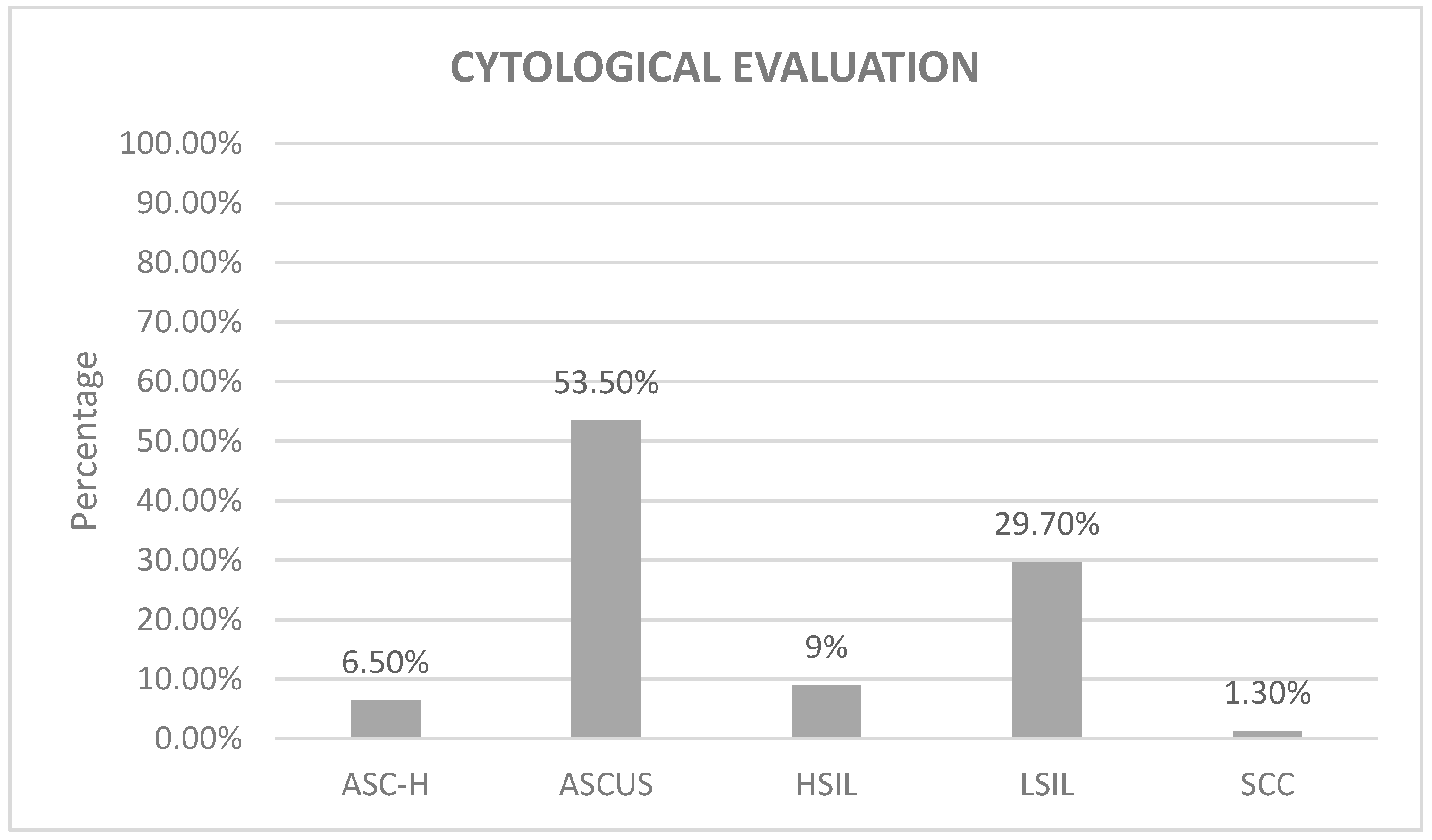
| GYN Interp N (%) | 20–29 | 30–39 | 40–49 | 50–59 | ≥60 | Total |
|---|---|---|---|---|---|---|
| ASC-H | 2 (1.3%) | 5 (3.2%) | 2 (1.3%) | 0 (0%) | 1 (0.6%) | 10 (6.5%) |
| ASC-US | 12 (7.7%) | 40 (26%) | 22 (14.2%) | 8 (5.2%) | 1 (0.6%) | 83 (53.5%) |
| HSIL | 1 (0.6%) | 7 (4.5%) | 4 (2.6%) | 1 (0.6%) | 1 (0.6%) | 14 (9%) |
| LSIL | 6 (4%) | 24 (15.5%) | 14 (9%) | 2 (1.3%) | 0 (0%) | 46 (29.7%) |
| SCC | 0 (0%) | 0 (0%) | 1 (0.6%) | 0 (0%) | 1 (0.6%) | 2 (1.3%) |
| Total | 21 (13.6%) | 76 (49.2%) | 43 (27.7%) | 11 (7.1%) | 4 (2.4%) | 155 (100%) |
| GYN Interp N (%) p-Value | ASC-H | ASC-US | HSIL | LSIL | SCC | Total |
|---|---|---|---|---|---|---|
| HPV 16 | 4 (2.6%) | 3 (2%) | 6 (3.9%) | 5 (3.2%) | 0 (0%) | 18 (11.7%) |
| HPV 18-45 | 1 (0.6%) | 1 (0.6%) | 1 (0.6%) | 3 (2%) | 0 (0%) | 6 (3.8%) |
| HPV 18-45 + Other HR | 0 (0%) | 2 (1.3%) | 0 (0%) | 3 (2%) | 0 (0%) | 5 (3.3%) |
| HPV Other HR | 1 (0.6%) | 25 (16.1%) | 2 (1.3%) | 20 (12.9%) | 0 (0%) | 48 (30.9%) |
| HPV HR Equivocal | 1 (0.6%) | 3 (2%) | 0 (0%) | 0 (0%) | 1 (0.6%) | 5 (3.2%) |
| HPV HR Negative | 3 (2%) | 49 (31.6%) | 5 (3.2%) | 15 (9.7%) | 1 (0.6%) | 73 (47.1%) |
| Total | 10 (6.4%) | 83 (53.6%) | 14 (9%) | 46 (29.8%) | 2 (1.2%) | 155 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faqih, L.; Alzamil, L.; Aldawood, E.; Alharbi, S.; Muzzaffar, M.; Moqnas, A.; Almajed, H.; Alghamdi, A.; Alotaibi, M.; Alhammadi, S.; et al. Prevalence of Human Papillomavirus Infection and Cervical Abnormalities among Women Attending a Tertiary Care Center in Saudi Arabia over 2 Years. Trop. Med. Infect. Dis. 2023, 8, 511. https://doi.org/10.3390/tropicalmed8120511
Faqih L, Alzamil L, Aldawood E, Alharbi S, Muzzaffar M, Moqnas A, Almajed H, Alghamdi A, Alotaibi M, Alhammadi S, et al. Prevalence of Human Papillomavirus Infection and Cervical Abnormalities among Women Attending a Tertiary Care Center in Saudi Arabia over 2 Years. Tropical Medicine and Infectious Disease. 2023; 8(12):511. https://doi.org/10.3390/tropicalmed8120511
Chicago/Turabian StyleFaqih, Layla, Lama Alzamil, Esraa Aldawood, Sarah Alharbi, Moammer Muzzaffar, Amani Moqnas, Heba Almajed, Ahmed Alghamdi, Mohammed Alotaibi, Sultan Alhammadi, and et al. 2023. "Prevalence of Human Papillomavirus Infection and Cervical Abnormalities among Women Attending a Tertiary Care Center in Saudi Arabia over 2 Years" Tropical Medicine and Infectious Disease 8, no. 12: 511. https://doi.org/10.3390/tropicalmed8120511
APA StyleFaqih, L., Alzamil, L., Aldawood, E., Alharbi, S., Muzzaffar, M., Moqnas, A., Almajed, H., Alghamdi, A., Alotaibi, M., Alhammadi, S., & Alwelaie, Y. (2023). Prevalence of Human Papillomavirus Infection and Cervical Abnormalities among Women Attending a Tertiary Care Center in Saudi Arabia over 2 Years. Tropical Medicine and Infectious Disease, 8(12), 511. https://doi.org/10.3390/tropicalmed8120511







