Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Parasites
2.3. Preparation of Antigens
2.4. Formulation of Plain and RA-SLNs
2.5. Nanoparticles’ Profiles
2.5.1. Physical Characterization
2.5.2. Transmission Electron Microscopy (TEM)
2.5.3. Determination of Entrapment Efficiency (EE%)
2.5.4. Release Study and Modeling of the Release Kinetics
2.5.5. Stability Study
2.6. Experimental Setup and Immunization Protocols
2.7. Vaccine Evaluation
2.7.1. Parasitological Studies
2.7.2. Immunological Study
2.7.3. Biochemical Study
2.7.4. Histopathological Analysis
2.8. Statistics
3. Results
3.1. Plain and Adjuvant-Loaded SLN Characterization
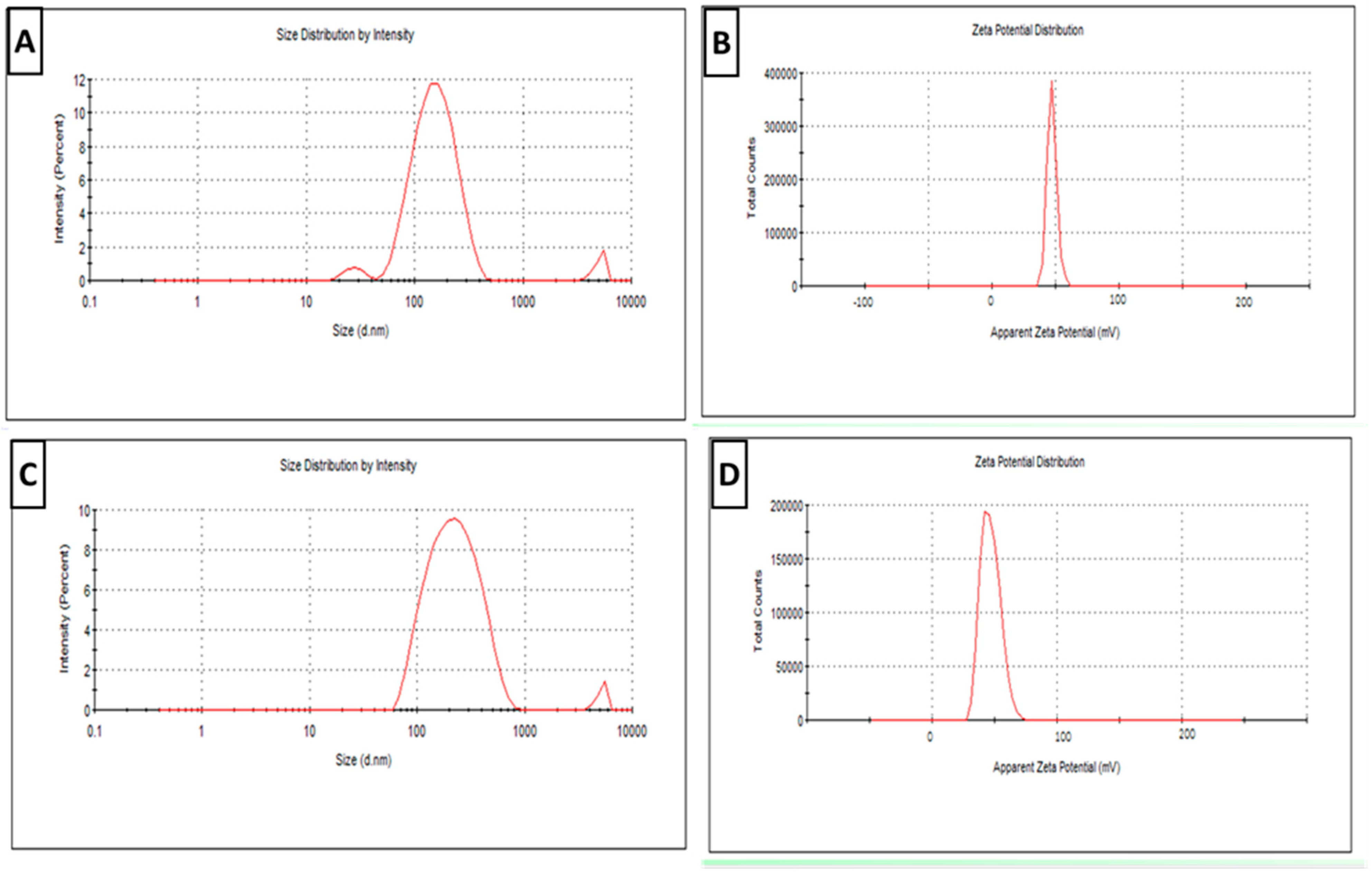
3.1.1. Physical Characterization
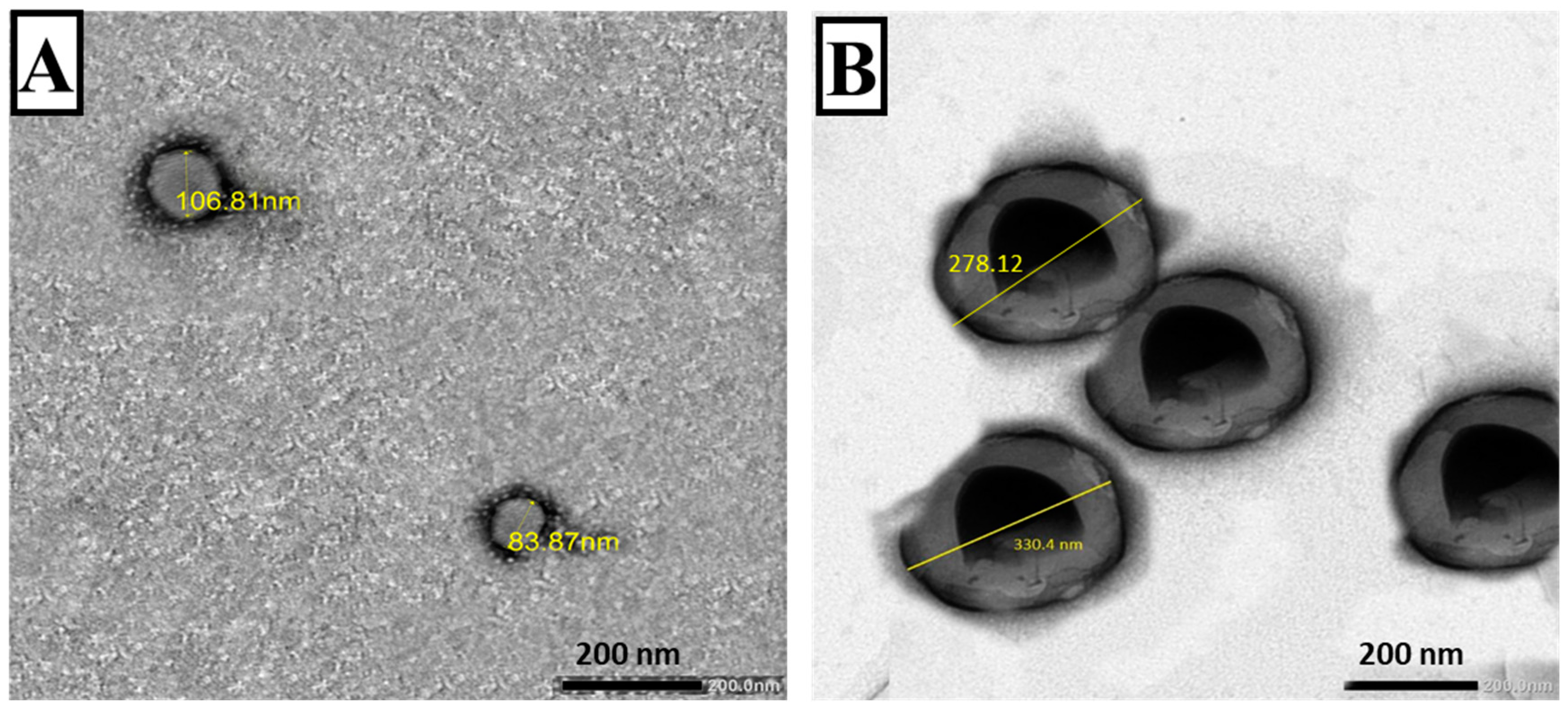
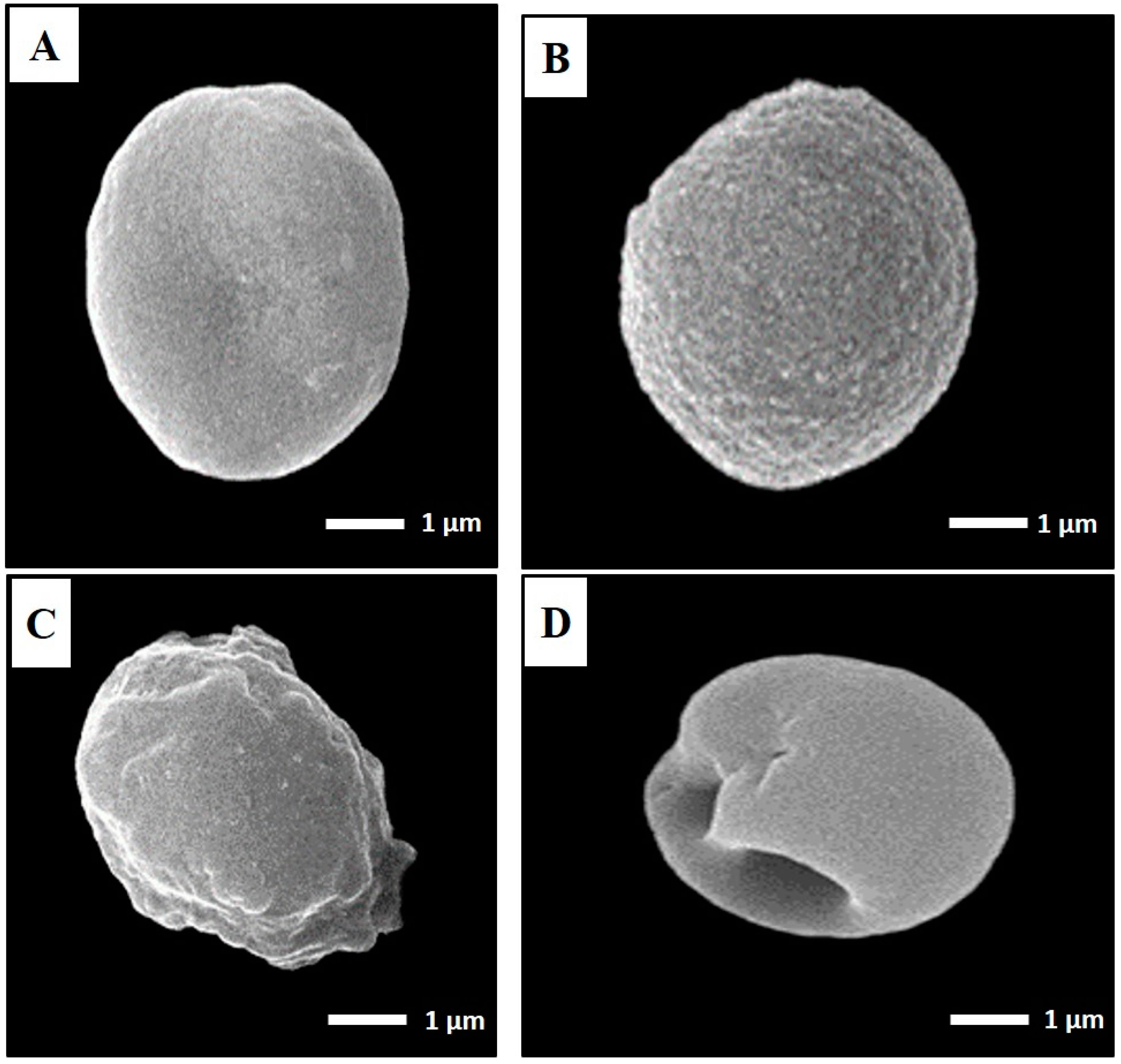
3.1.2. TEM
3.1.3. Entrapment Efficiency and Adjuvant Loading
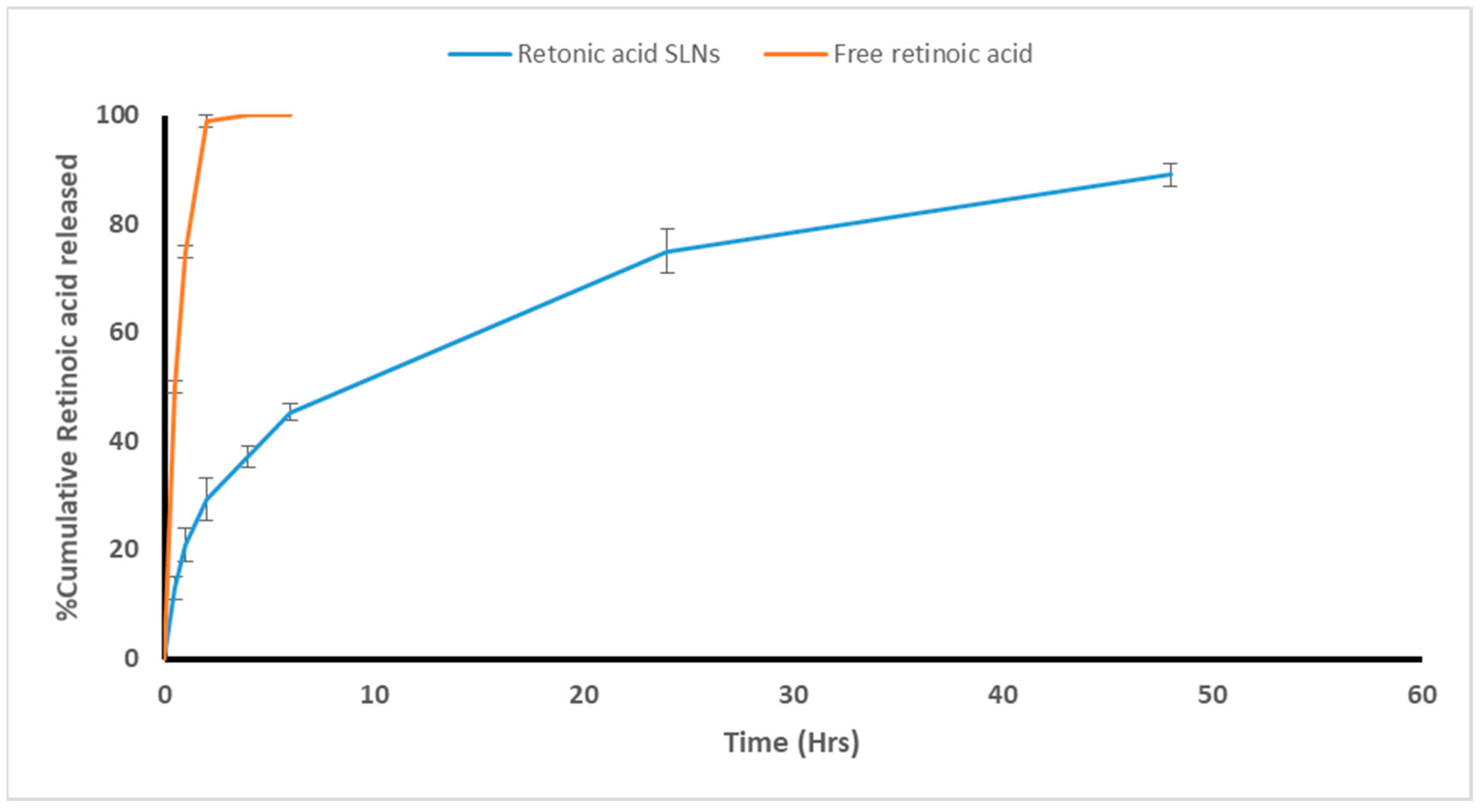
3.1.4. Release Study and Modeling of the Release Kinetics
3.1.5. The RA-Loaded SLNs’ Stability
3.2. Evaluation of the Vaccine
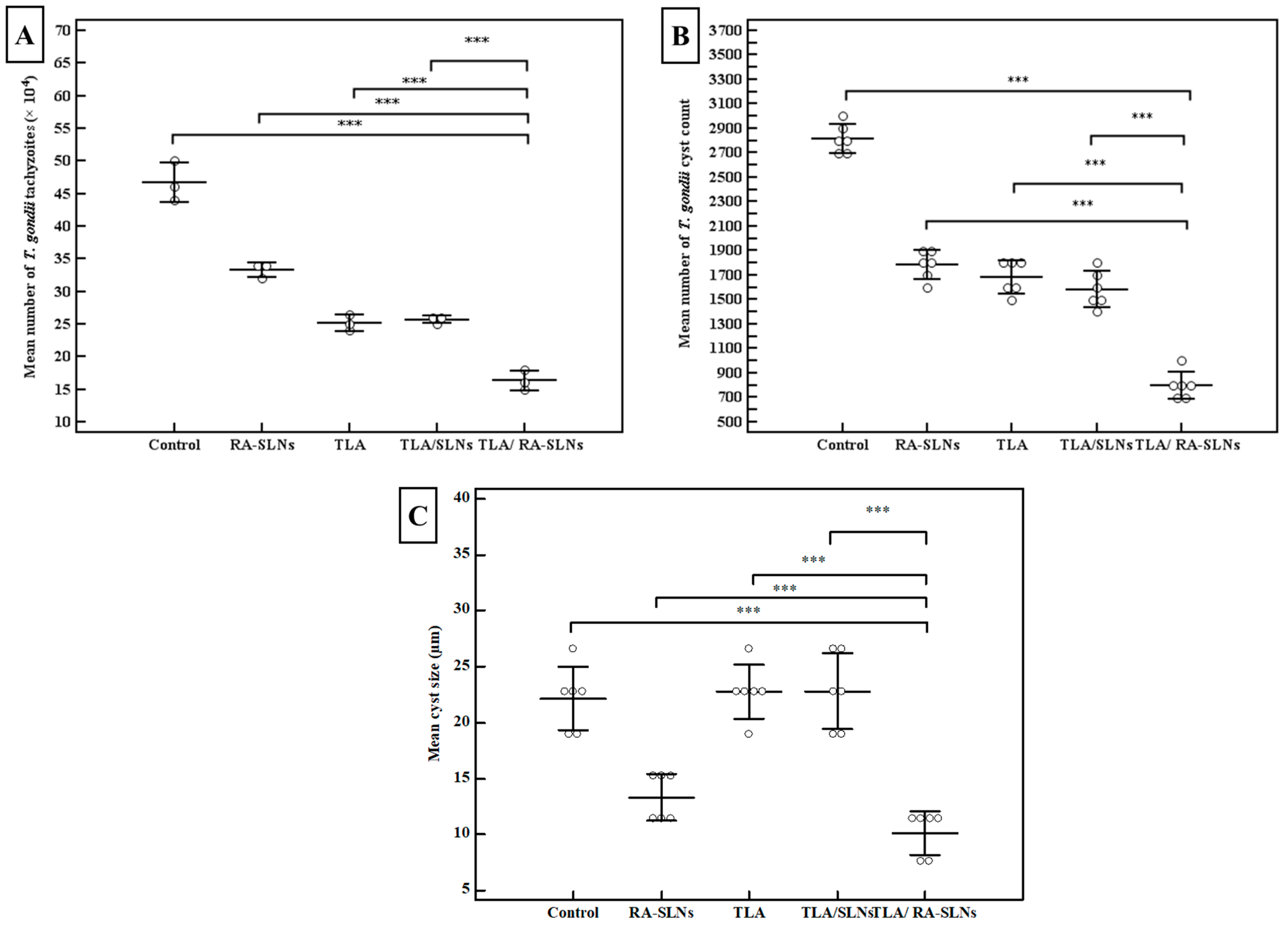
3.2.1. Parasitological Studies
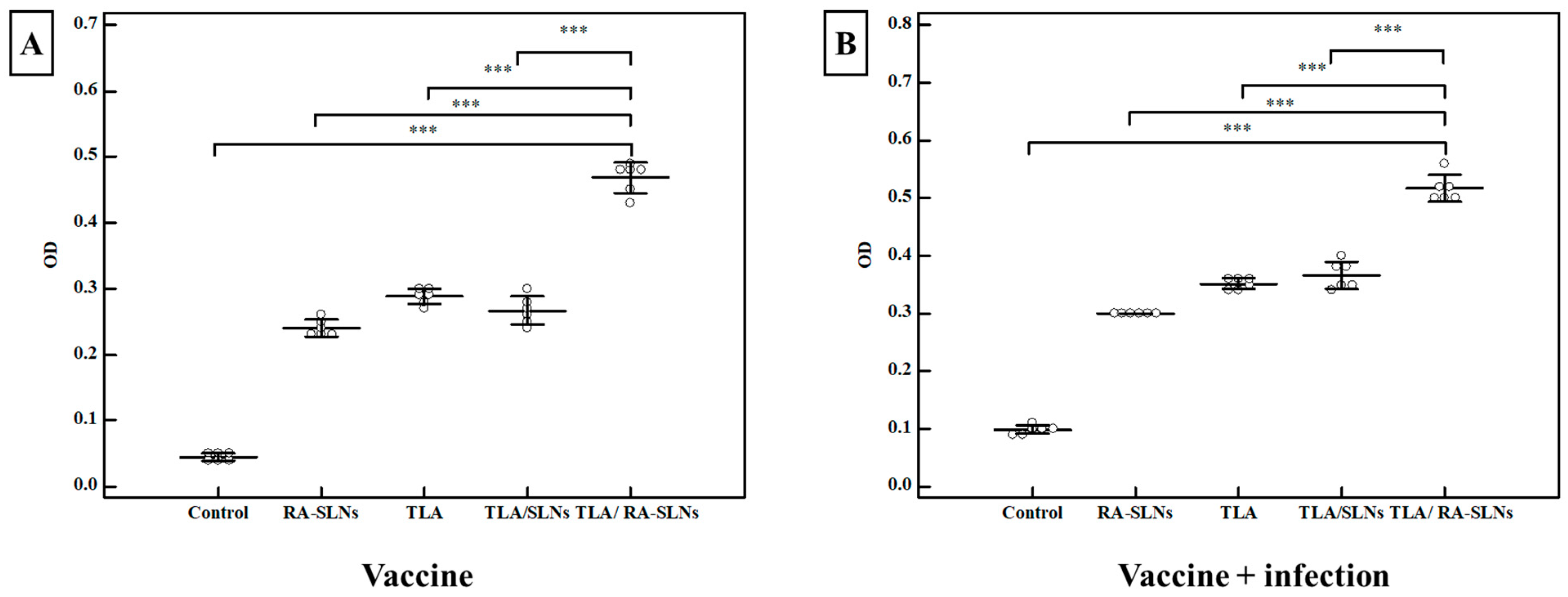
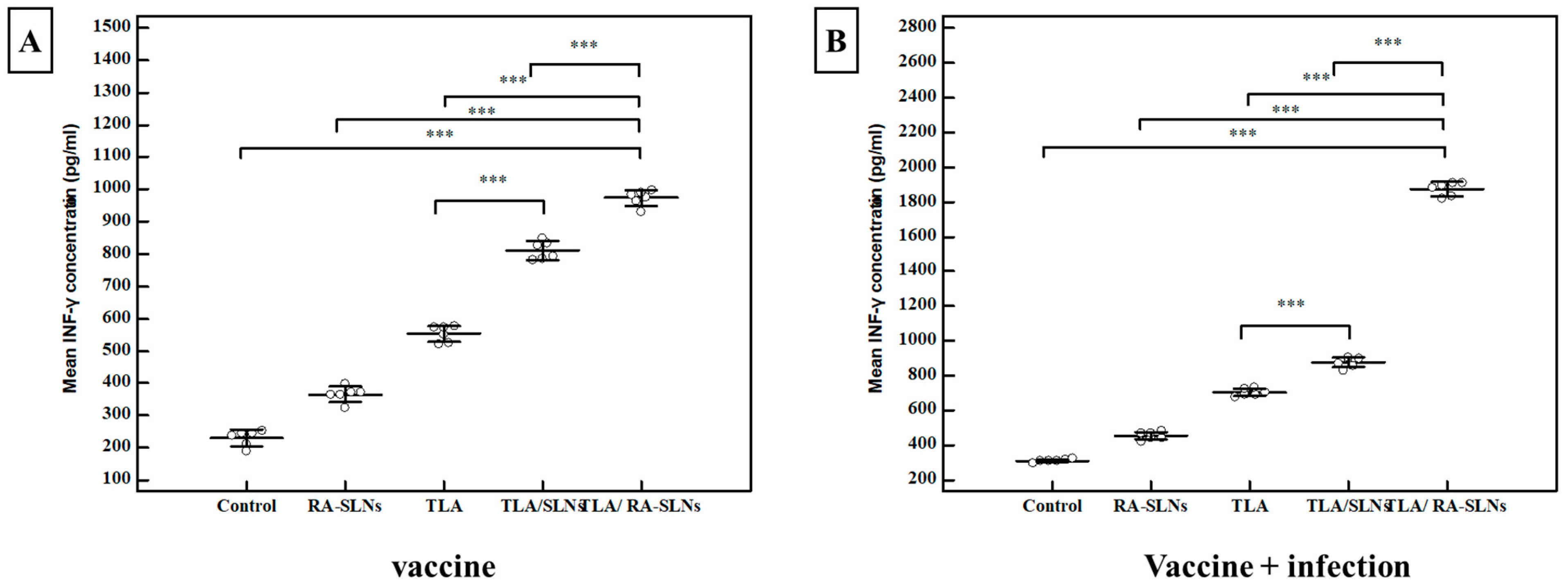
3.2.2. Immunological Results
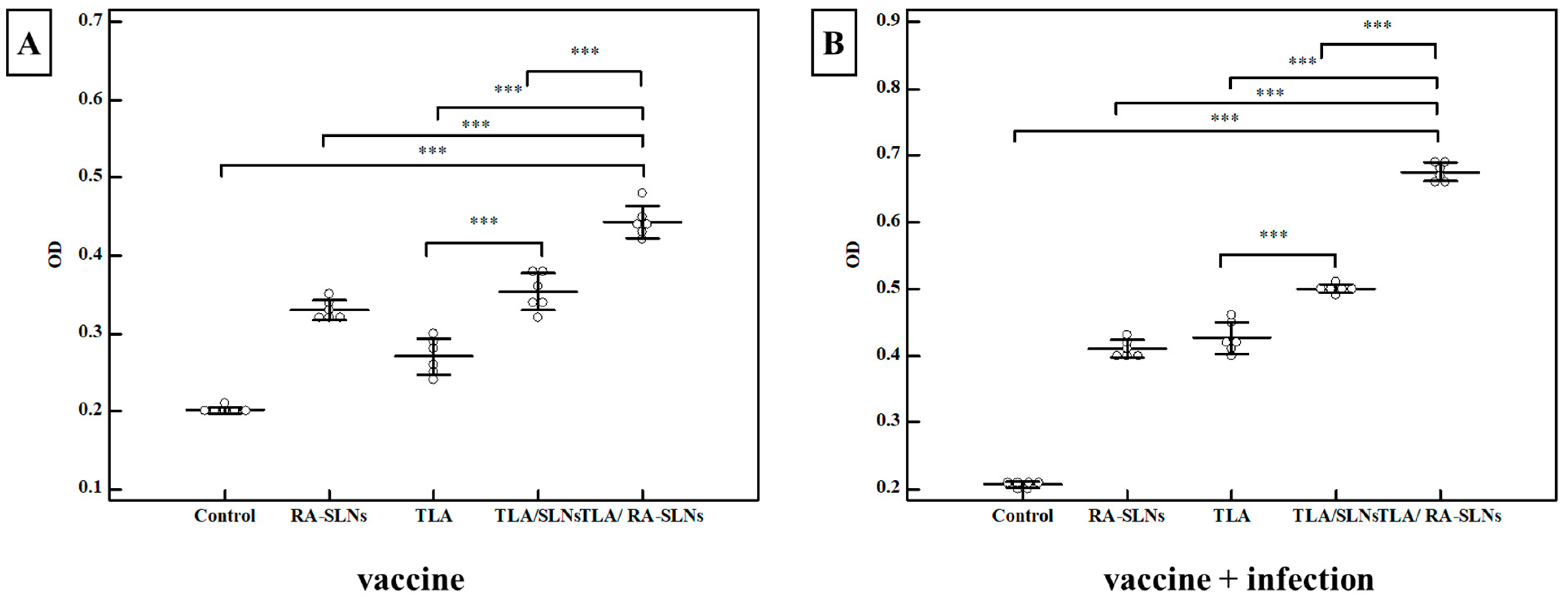
3.2.3. Biochemical Results
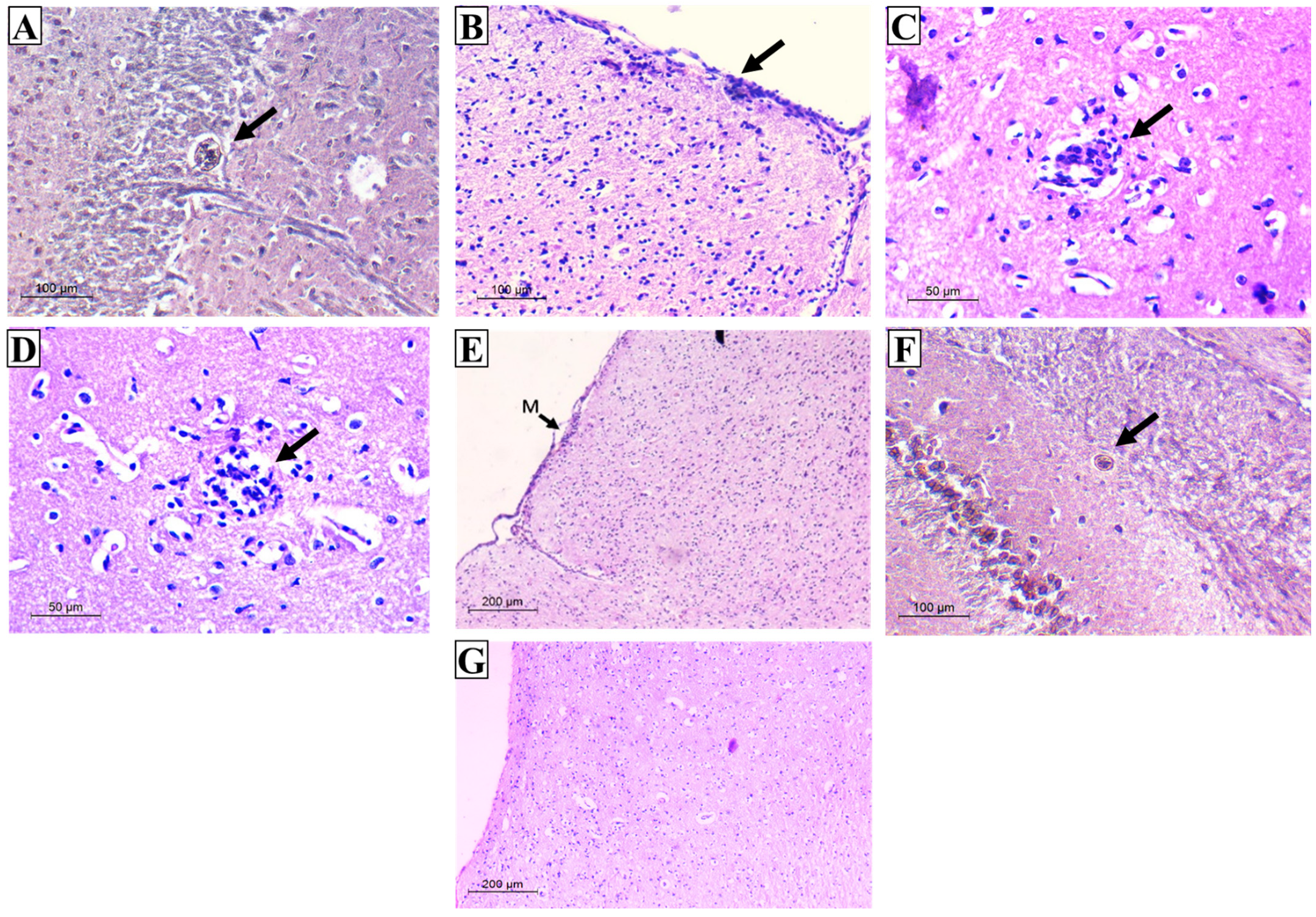
3.2.4. Histopathological Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.L.; Innes, E.A.; Katzer, F. Current progress toward vaccines against Toxoplasma gondii. Vaccine Dev. Ther. 2014, 4, 23–37. [Google Scholar] [CrossRef]
- Liu, Q.; Singla, L.D.; Zhou, H. Vaccines against Toxoplasma gondii: Status, challenges and future directions. Hum. Vaccines Immunother. 2012, 8, 1305–1308. [Google Scholar] [CrossRef]
- Boog, C.J. Principles of vaccination and possible development strategies for rational design. Immunol. Lett. 2009, 122, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef] [PubMed]
- El-Malky, M.; Shaohong, L.; Kumagai, T.; Yabu, Y.; Noureldin, M.S.; Saudy, N.; Maruyama, H.; Ohta, N. Protective effect of vaccination with Toxoplasma lysate antigen and CpG as an adjuvant against Toxoplasma gondii in susceptible C57BL/6 mice. Microbiol. Immunol. 2005, 49, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Schabussova, I.; Ruttkowski, B.; Peschke, R.; Kur, J.; Kundi, M.; Joachim, A.; Wiedermann, U. Prime-boost vaccination with Toxoplasma lysate antigen, but not with a mixture of recombinant protein antigens, leads to reduction of brain cyst formation in BALB/c mice. PLoS ONE 2015, 10, e0126334. [Google Scholar] [CrossRef]
- EL-Malky, M.A.; Al-Harthi, S.A.; Mohamed, R.T.; El Bali, M.A.; Saudy, N.S. Vaccination with Toxoplasma lysate antigen and CpG oligodeoxynucleotides: Comparison of immune responses in intranasal versus intramuscular administrations. Parasitol. Res. 2014, 113, 2277–2284. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Olszewska, W.; Steward, M.W. Nasal delivery of epitope based vaccines. Adv. Drug Deliv. Rev. 2001, 51, 161–171. [Google Scholar] [CrossRef]
- Asanuma, H.; Hirokawa, K.; Uchiyama, M.; Suzuki, Y.; Aizawa, C.; Kurata, T.; Sata, T.; Tamura, S. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine 2001, 19, 3981–3989. [Google Scholar] [CrossRef]
- Thompson, A.L.; Staats, H.F. Cytokines: The future of intranasal vaccine adjuvants. Clin. Dev. Immunol. 2011, 2011, 289597. [Google Scholar] [CrossRef] [PubMed]
- Dincel, G.C.; Atmaca, H.T. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int. J. Immunopathol. Pharmacol. 2016, 29, 226–240. [Google Scholar] [CrossRef]
- Ajith, Y.; Dimri, U.; Dixit, S.K.; Singh, S.K.; Gopalakrishnan, A.; Madhesh, E.; Rajesh, J.B.; Sangeetha, S.G. Immunomodulatory basis of antioxidant therapy and its future prospects: An appraisal. Inflammopharmacology 2017, 25, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef]
- Ourique, A.F.; Melero, A.; de Bona da Silva, C.; Schaefer, U.F.; Pohlmann, A.R.; Guterres, S.S.; Lehr, C.M.; Kostka, K.H.; Beck, R.C. Improved photostability and reduced skin permeation of tretinoin: Development of a semisolid nanomedicine. Eur. J. Pharm. Biopharm. 2011, 79, 95–101. [Google Scholar] [CrossRef]
- Pan, X.Q.; Zheng, X.; Shi, G.; Wang, H.; Ratnam, M.; Lee, R.J. Strategy for the treatment of acute myelogenous leukemia based on folate receptor beta-targeted liposomal doxorubicin combined with receptor induction using all-trans retinoic acid. Blood 2002, 100, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- He, H.; Yao, J.; Zhang, Y.; Chen, Y.; Wang, K.; Lee, R.J.; Yu, B.; Zhang, X. Solid lipid nanoparticles as a drug delivery system to across the blood-brain barrier. Biochem. Biophys. Res. Commun. 2019, 519, 385–390. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A.; Oréfice, R.L.; Vilela, J.M.; Andrade, M.S.; Ferreira, L.A. Development of a new solid lipid nanoparticle formulation containing retinoic acid for topical treatment of acne. J. Microencapsul. 2007, 24, 395–407. [Google Scholar] [CrossRef]
- Castro, G.A.; Coelho, A.L.L.; Oliveira, C.A.; Mahecha, G.A.; Oréfice, R.L.; Ferreira, L.A. Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles. Int. J. Pharm. 2009, 38, 77–83. [Google Scholar] [CrossRef]
- Eissa, M.M.; El-Moslemany, R.M.; Ramadan, A.A.; Amer, E.I.; El-Azzouni, M.Z.; El-Khordagui, L.K. Miltefosine Lipid Nanocapsules for Single Dose Oral Treatment of Schistosomiasis Mansoni: A Preclinical Study. PLoS ONE 2015, 10, e0141788. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chung, J.F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Dong, Z.; Xie, S.; Chen, X.; Lu, M.; Wang, X.; Li, X.; Zhou, W. Preparation and stability study of norfloxacin-loaded solid lipid nanoparticle suspensions. Colloids Surf. B Biointerfaces 2012, 98, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, I.P.S.; Costa-Souza, B.L.S.; Carneiro, G.; Ferreira, L.A.M.; de Matos Guedes, H.L.; Rossi-Bergmann, B. Nanoencapsulated retinoic acid as a safe tolerogenic adjuvant for intranasal vaccination against cutaneous leishmaniasis. Vaccine 2019, 37, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Kawai, A.; Shibuya, M.; Munakata, L.; Omata, D.; Suzuki, R.; Yoshioka, Y. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines 2020, 8, 433. [Google Scholar] [CrossRef]
- Sanchez, V.R.; Fenoy, I.M.; Picchio, M.S.; Soto, A.S.; Arcon, N.; Goldman, A.; Martin, V. Homologous prime-boost strategy with TgPI-1 improves the immune response and protects highly susceptible mice against chronic Toxoplasma gondii infection. Acta Trop. 2015, 150, 159–165. [Google Scholar] [CrossRef]
- Eissa, M.M.; Barakat, A.M.; Amer, E.I.; Younis, L.K. Could miltefosine be used as a therapy for toxoplasmosis? Exp. Parasitol. 2015, 157, 12–22. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Sarwal, A.; Yangga, P.; Kang, D.; Feinstein, A. New-Onset Cervical Lymphadenopathy in a Patient Undergoing Treatment of Pulmonary Mycobacterium avium Complex Infection: Toxoplasmosis Lymphadenitis. Case Rep. Infect. Dis. 2020, 2020, 8876240. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. Past, present and future: Overview on histology and histopathology. J. Histol. Histopathol. 2014, 1, 5. [Google Scholar] [CrossRef]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Facciola, A.; Visalli, G.; Lagana, P.; La Fauci, V.; Squeri, R.; Pellicano, G.F.; Nunnari, G.; Trovato, M.; Di Pietro, A. The new era of vaccines: The “nanovaccinology”. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7163–7182. [Google Scholar] [CrossRef] [PubMed]
- Gowder, S. Antioxidants and Vaccines. Int. J. Vaccines Vaccin. 2016, 2, 8. [Google Scholar] [CrossRef]
- Mwanza-Lisulo, M.; Kelly, P. Potential for use of retinoic acid as an oral vaccine adjuvant. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140145. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yang, G.H.; Zhang, L.L.; Wang, J.; Wang, J.F. Melatonin as Immune Potentiator for Enhancing Subunit Vaccine Efficacy against Bovine Viral Diarrhea Virus. Vaccines 2021, 9, 1039. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, S.; Narang, R.K.; Budhiraja, R.D. Amoxicillin loaded chitosan-alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for h. Pylori. Sci. Pharm. 2011, 79, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Mohiti, H.; Hamishehkar, H.; Varshosaz, J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res. Pharm. Sci. 2015, 10, 17–33. [Google Scholar] [PubMed]
- Abdelbary, G.; Fahmy, R.H. Diazepam-loaded solid lipid nanoparticles: Design and characterization. AAPS PharmSciTech 2009, 10, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.K.; Mishra, S.; Bajpai, M.; Mishra, A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: In vitro drug release and pharmacokinetics studies. BioMed Res. Int. 2014, 2014, 363404. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Exp. Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Hosseini, A.Z.; Dalimi, A. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet. Parasitol. 2003, 113, 123–134. [Google Scholar] [CrossRef]
- Schijns, V.E.; Lavelle, E.C. Trends in vaccine adjuvants. Exp. Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef]
- Chen, K.; Cerutti, A. Vaccination strategies to promote mucosal antibody responses. Immunity 2010, 33, 479–491. [Google Scholar] [CrossRef]
- Mack, D.G.; McLeod, R. Human Toxoplasma gondii-specific secretory immunoglobulin A reduces T. gondii infection of enterocytes in vitro. J. Clin. Investig. 1992, 90, 2585–2592. [Google Scholar] [CrossRef]
- Mineo, J.R.; McLeod, R.; Mack, D.; Smith, J.; Khan, I.A.; Ely, K.H.; Kasper, L.H. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J. Immunol. 1993, 150, 3951–3964. [Google Scholar] [CrossRef]
- Sayles, P.C.; Gibson, G.W.; Johnson, L.L. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 2000, 68, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Q.; Ross, A.C. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J. Immunol. 2005, 174, 7961–7969. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, T.; Segawa, R.; Mizuno, N.; Eguchi, S.; Akamatsu, H.; Fukuda, M.; Nakata, F.; Leonard, W.J.; Hiratsuka, M.; Hirasawa, N. All-Trans Retinoic Acid Enhances Antibody Production by Inducing the Expression of Thymic Stromal Lymphopoietin Protein. J. Immunol. 2018, 200, 2670–2676. [Google Scholar] [CrossRef]
- Chen, X.; Esplin, B.L.; Garrett, K.P.; Welner, R.S.; Webb, C.F.; Kincade, P.W. Retinoids accelerate B lineage lymphoid differentiation. J. Immunol. 2008, 180, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Goodnow, C.C.; Vinuesa, C.G.; Randall, K.L.; Mackay, F.; Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 2010, 11, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Indrevær, R.L.; Moskaug, J.Ø.; Paur, I.; Bøhn, S.K.; Jørgensen, S.F.; Blomhoff, R.; Aukrust, P.; Fevang, B.; Blomhoff, H.K. IRF4 is a Critical Gene in Retinoic Acid-Mediated Plasma Cell Formation and is Deregulated in Common Variable Immunodeficiency-Derived B Cells. J. Immunol. 2015, 195, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Gao, Q.; Wang, M.; Hou, J.L.; Zhang, F.K.; Hu, L.Y.; Zhu, X.Q. Protective Efficacy against Acute and Chronic Toxoplasma gondii Infection Induced by Immunization with the DNA Vaccine TgDOC2C. Front. Microbiol. 2018, 9, 2965. [Google Scholar] [CrossRef]
- Ju, C.H.; Chockalingam, A.; Leifer, C.A. Early response of mucosal epithelial cells during Toxoplasma gondii infection. J. Immunol. 2009, 183, 7420–7427. [Google Scholar] [CrossRef]
- Cerutti, A.; Cols, M.; Gentile, M.; Cassis, L.; Barra, C.M.; He, B.; Puga, I.; Chen, K. Regulation of mucosal IgA responses: Lessons from primary immunodeficiencies. Ann. N. Y. Acad. Sci. 2011, 1238, 132–144. [Google Scholar] [CrossRef]
- Asanuma, H.; Zamri, N.B.; Sekine, S.; Fukuyama, Y.; Tokuhara, D.; Gilbert, R.S.; Fukuiwa, T.; Fujihashi, K.; Sata, T.; Tashiro, M.; et al. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine 2012, 30, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.I.; Pedron, T.; Tournebize, R.; Olivo-Marin, J.C.; Sansonetti, P.J.; Phalipon, A. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity 2003, 18, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal. Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dimier-Poisson, I.; Aline, F.; Mevelec, M.N.; Beauvillain, C.; Buzoni-Gatel, D.; Bout, D. Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells. Infect. Immun. 2003, 71, 5254–5265. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Bhatia, E.; Sharma, S.; Ahamad, N.; Banerjee, R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020, 108, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, M.M.; Noor, Z.; Tomai, M.A.; Elvecrog, J.; Fox, C.B.; Petri, W.A., Jr. Nanoformulation of synergistic TLR ligands to enhance vaccination against Entamoeba. histolytica. Vaccine 2017, 35, 916–922. [Google Scholar] [CrossRef]
- Riccomi, A.; Piccaro, G.; Christensen, D.; Palma, C.; Andersen, P.; Vendetti, S. Parenteral Vaccination with a Tuberculosis Subunit Vaccine in Presence of Retinoic Acid Provides Early but Transient Protection to M. Tuberculosis Infection. Front. Immunol. 2019, 10, 934. [Google Scholar] [CrossRef]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.A.; Olias, P.; Sibley, L.D. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef]
- Martens, S.; Parvanova, I.; Zerrahn, J.; Griffiths, G.; Schell, G.; Reichmann, G.; Howard, J.C. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005, 1, e24. [Google Scholar] [CrossRef]
- Gigley, J.P.; Bhadra, R.; Khan, I.A. CD8 T Cells and Toxoplasma gondii: A New Paradigm. J. Parasitol. Res. 2011, 2011, 243796. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Hannah, R.; Lutshumba, J.; Ochiai, E.; Weiss, L.M.; Suzuki, Y. Penetration of CD8+ Cytotoxic T Cells into Large Target, Tissue Cysts of Toxoplasma gondii, Leads to Its Elimination. Am. J. Pathol. 2019, 189, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Shahriari, A.; Tavalla, M.; Azadmanesh, S.; Hamidinejat, H. Blood Levels of Oxidant/Antioxidant Parameters in Rats Infected with Toxoplasma gondii. Oxid. Med. Cell. Longev. 2016, 2016, 8045969. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; O, W.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Contreras, M.; Peres Rubio, C.; de la Fuente, J.; Villar, M.; Merino, O.; Mosqueda, J.; Ceron, J.J. Changes in Serum Biomarkers of Oxidative Stress in Cattle Vaccinated with Tick Recombinant Antigens: A Pilot Study. Vaccines 2020, 9, 5. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Bauerbach, E.; Plath, M.; Steuber, M.; Heers, C.; Tegtmeier, F.; Krieglstein, J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic. Biol. Med. 2001, 30, 1067–1077. [Google Scholar] [CrossRef]
- Elomda, A.M.; Saad, M.F.; Saeed, A.M.; Elsayed, A.; Abass, A.O.; Safaa, H.M.; Mehaisen, G.M.K. Antioxidant and developmental capacity of retinol on the in vitro culture of rabbit embryos. Zygote 2018, 26, 326–332. [Google Scholar] [CrossRef]
- Watson, B.D. Evaluation of the concomitance of lipid peroxidation in experimental models of cerebral ischemia and stroke. Prog. Brain Res. 1993, 96, 69–95. [Google Scholar] [CrossRef]
- Ondrejkova, A.; Suli, J.; Harvanova, J.; Ondrejka, R.; Prokes, M. Antioxidative Protection of Squalene Adjuvant and Rabies Vaccine with Adjuvant. Biol. Pharm. Bull. 2017, 40, 1029–1034. [Google Scholar] [CrossRef]
- Kayalar, O.; Bayrak, B.B.; Yanardag, R. Effects of all-trans retinoic acid on reducing hyperoxia-induced oxidative stress in mice brain. Nobel Med. 2013, 9, 22–26. [Google Scholar]
- Karaman, U.; Celik, T.; Kiran, T.R.; Colak, C.; Daldal, N.U. Malondialdehyde, glutathione, and nitric oxide levels in Toxoplasma gondii seropositive patients. Korean J. Parasitol. 2008, 46, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Fatollahzadeh, M.; Eskandarian, A.; Darani, H.Y.; Pagheh, A.S.; Ahmadpour, E. Evaluation of Th17 immune responses of recombinant DNA vaccine encoding GRA14 and ROP13 genes against Toxoplasma gondii in BALB/c mice. Infect. Genet. Evol. 2021, 96, 105150. [Google Scholar] [CrossRef]
- Ramani, M.; van Groen, T.; Kadish, I.; Ambalavanan, N.; McMahon, L.L. Vitamin A and retinoic acid combination attenuates neonatal hyperoxia-induced neurobehavioral impairment in adult mice. Neurobiol. Learn. Mem. 2017, 141, 209–216. [Google Scholar] [CrossRef]
- Jiang, H.; Dan, Z.; Wang, H.; Lin, J. Effect of ATRA on contents of liver retinoids, oxidative stress and hepatic injury in rat model of extrahepatic cholestasis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2007, 27, 491–494. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Greenlund, L.J.; Deckwerth, T.L.; Johnson, E.M., Jr. Superoxide dismutase delays neuronal apoptosis: A role for reactive oxygen species in programmed neuronal death. Neuron 1995, 14, 303–315. [Google Scholar] [CrossRef] [Green Version]
| RA-SLNs at 4 °C | Zero (n = 3) | 1 Month (n = 3) | 2 Months (n = 3) | 3 Months (n = 3) | 6 Months (n = 3) | F | p |
|---|---|---|---|---|---|---|---|
| Size (nm) | 326 ± 6 | 327 ± 3 | 328 ± 4 | 328 ± 5 | 330 ± 2 | 0.367 | 0.827 |
| PDI | 0.27 ± 0.3 | 0.27 ± 0.1 | 0.27 ± 0.03 | 0.27 ± 0.1 | 0.27 ± 0.07 | 0.003 | 1.000 |
| ZP (mV) | 47.0 ± 0.6 | 46.0 ± 0.5 | 47.0 ± 0.5 | 47.0 ± 0.7 | 46.0 ± 0.6 | 2.632 | 0.098 * |
| EE (%) | 89.1 ± 0.6 | 89 ± 0.03 | 89.3 ± 0.1 | 88.9 ± 0.1 | 88.8 ± 0.7 | 0.637 | 0.648 |
| Group I (n = 6) | Group II (n = 6) | Group III (n = 6) | Group IV (n = 6) | Group V (n = 6) | Fp | |
|---|---|---|---|---|---|---|
| SOD | ||||||
| Pre-infection | 1415 ± 9.7 | 1424 ± 5.8 | 1426 a ± 4.5 | 1428 a ± 3.4 | 1430 a ± 6.1 | 0.006 * |
| Post-infection | 1541 ± 16.4 | 1739 a ± 26.2 | 1753 a ± 9.4 | 1658 abc ± 23.2 | 2018 abcd ± 18.7 | <0.001 * |
| MDA | ||||||
| Pre-infection | 16.92 ± 0.70 | 17.33 ± 0.64 | 16.98 ± 0.55 | 17.13 ± 0.35 | 15.08 abcd ± 0.21 | <0.001 * |
| Post-infection | 29.70 ± 0.59 | 11.03 a ± 0.34 | 22.88 ab ± 0.81 | 22.67 ab ± 0.30 | 13.85 abcd ± 0.39 | <0.001 * |
| TAC | ||||||
| Pre-infection | 0.99 ± 0.03 | 1.32 a ± 0.04 | 1.45 ab ± 0.03 | 1.42 ab ± 0.02 | 1.47 abd ± 0.02 | <0.001 * |
| Post-infection | 1.26 ± 0.02 | 1.45 a ± 0.03 | 1.52 ab ± 0.02 | 1.53 ab ± 0.02 | 1.59 abcd ± 0.01 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, D.E.; Amer, E.I.; Sheta, E.; Makled, S.; Arafa, F.M.; Diab, H.E. Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis. Trop. Med. Infect. Dis. 2023, 8, 106. https://doi.org/10.3390/tropicalmed8020106
Said DE, Amer EI, Sheta E, Makled S, Arafa FM, Diab HE. Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis. Tropical Medicine and Infectious Disease. 2023; 8(2):106. https://doi.org/10.3390/tropicalmed8020106
Chicago/Turabian StyleSaid, Doaa E., Eglal I. Amer, Eman Sheta, Shaimaa Makled, Fadwa M. Arafa, and Hala E. Diab. 2023. "Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis" Tropical Medicine and Infectious Disease 8, no. 2: 106. https://doi.org/10.3390/tropicalmed8020106
APA StyleSaid, D. E., Amer, E. I., Sheta, E., Makled, S., Arafa, F. M., & Diab, H. E. (2023). Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis. Tropical Medicine and Infectious Disease, 8(2), 106. https://doi.org/10.3390/tropicalmed8020106







