Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors
Abstract
1. Introduction
2. Materials and Methods
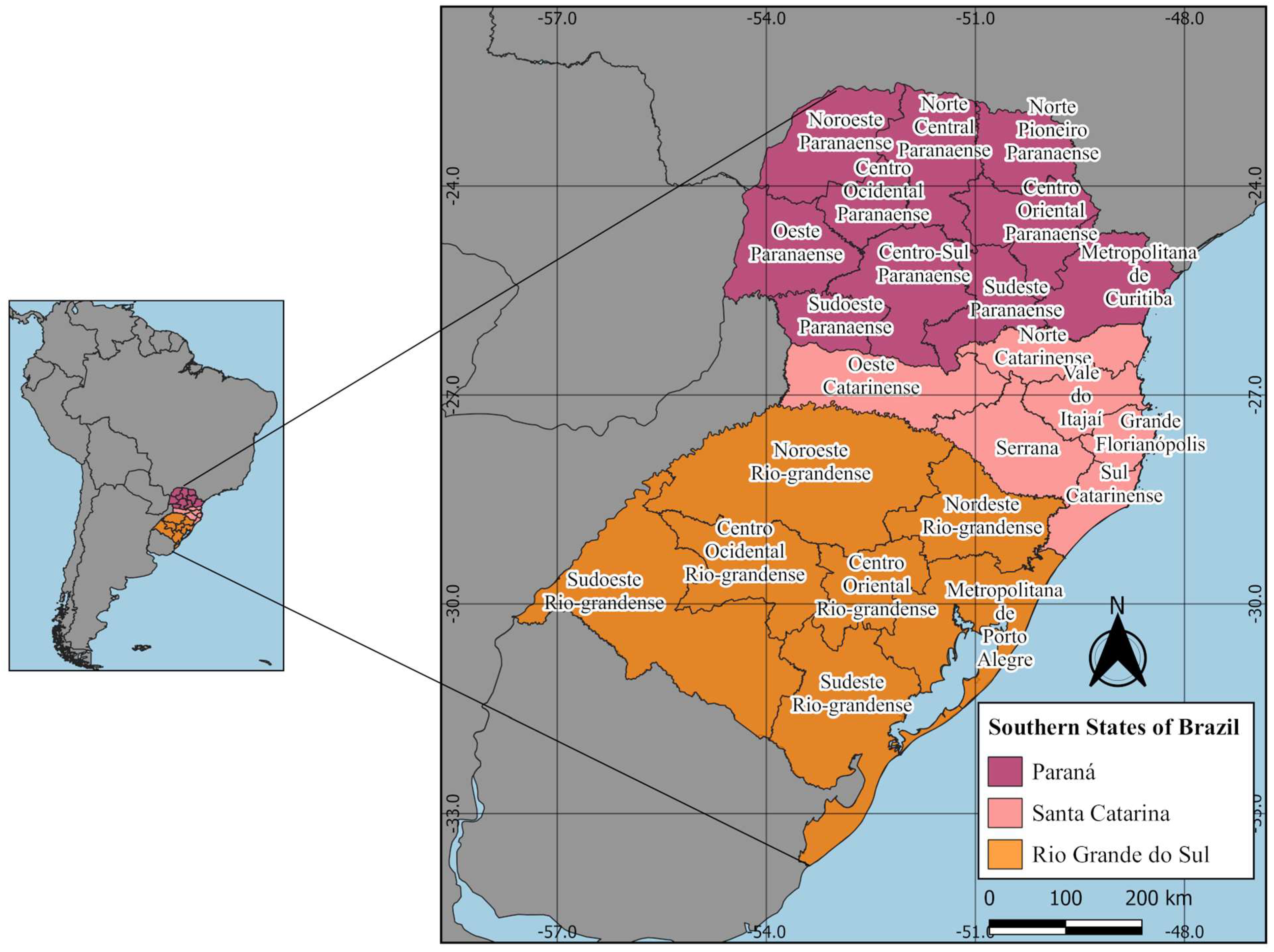
2.1. Study Areas
2.2. Data acquisition
2.2.1. Entomological Data on the Ae. aegypti Vector
2.2.2. Climatic Data
2.2.3. Environmental Data
2.3. Statistical Analysis
3. Results
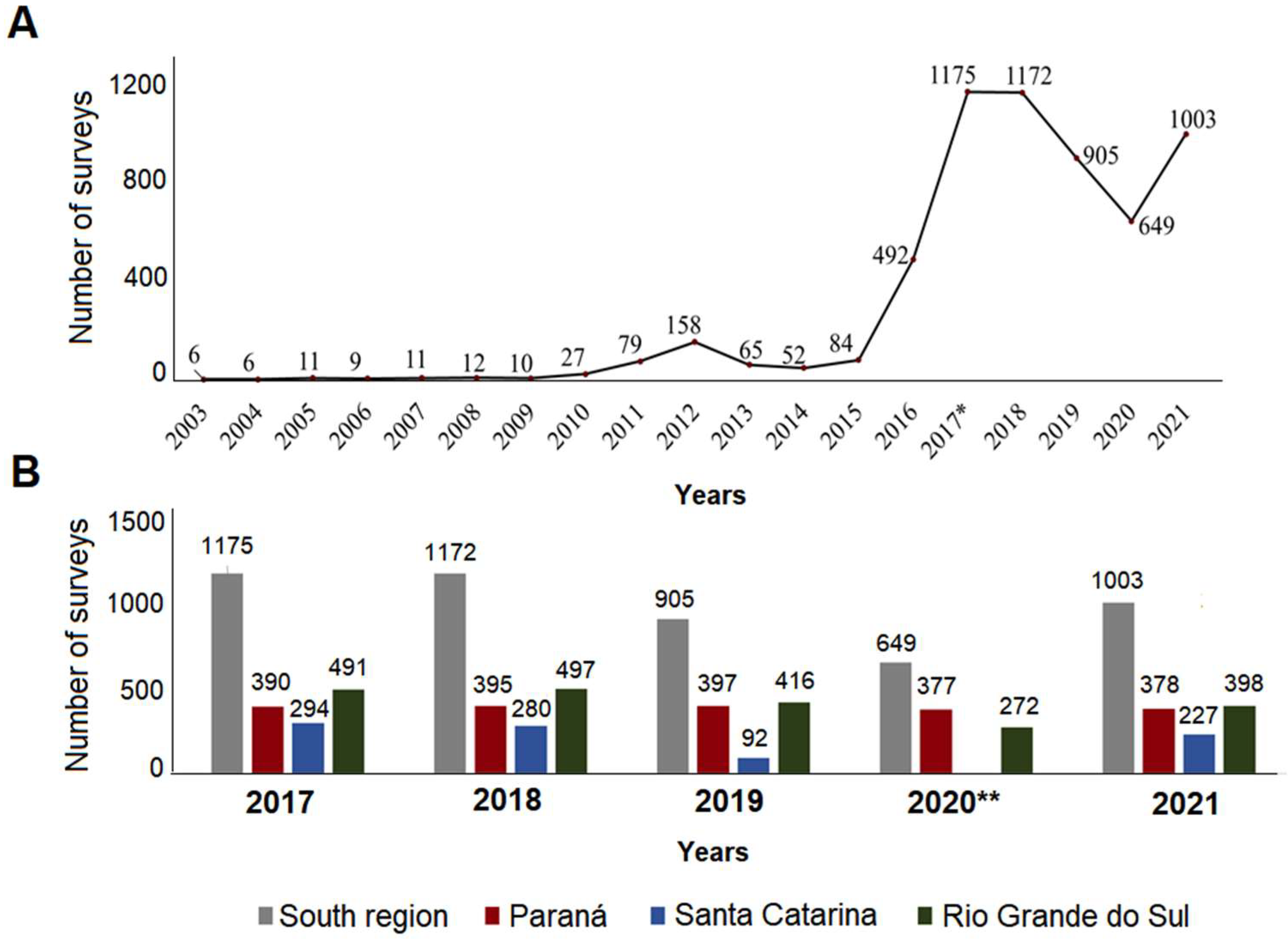
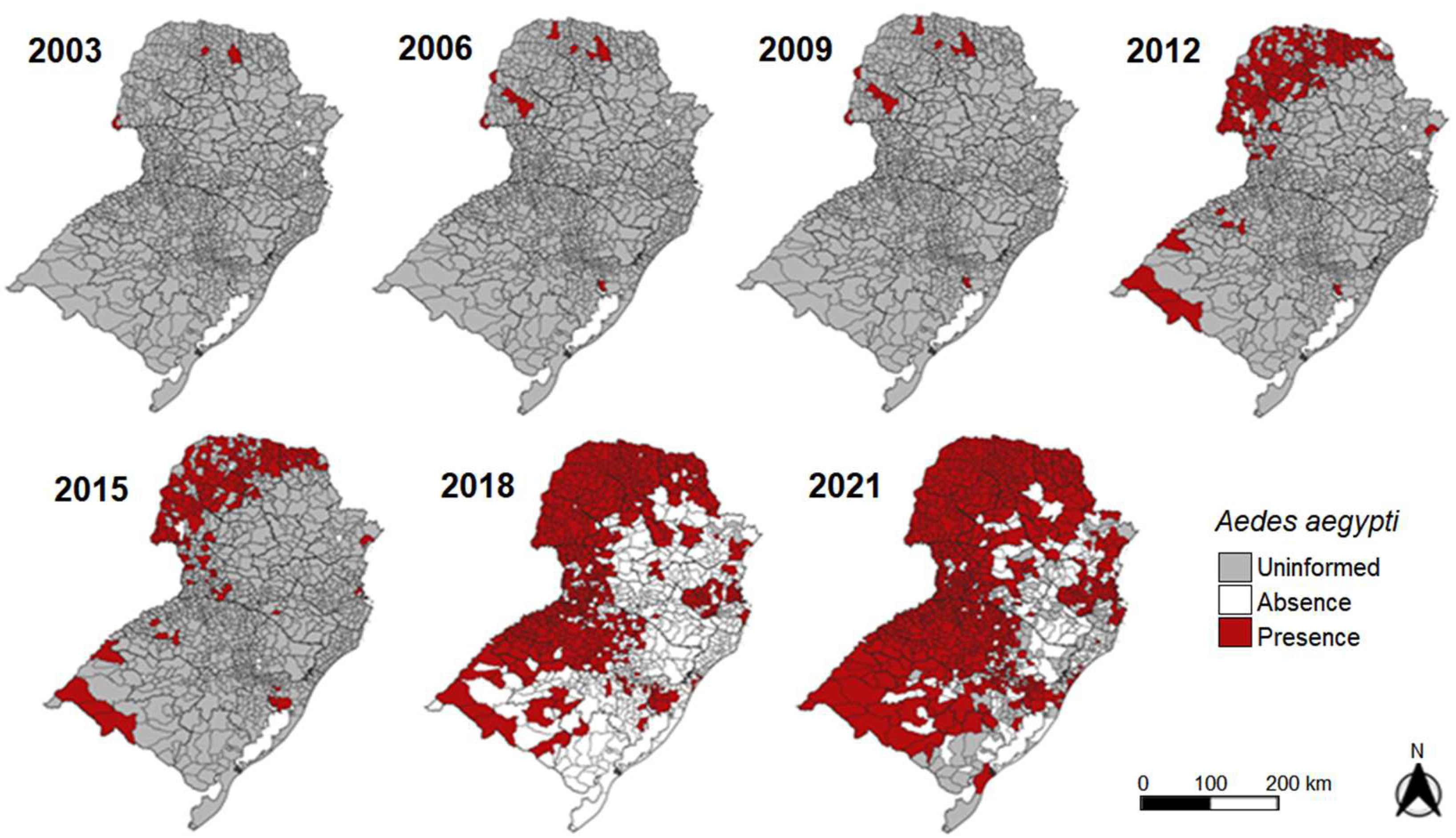
3.1. Ae. aegypti Entomological Survey and Infestation
3.2. Relationship with Climate and Environmental Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donalisio, M.R.; Freitas, A.R.R.; Zuben, A.P.B. Von Arboviruses Emerging in Brazil: Challenges for Clinic and Implications for Public Health. Rev. Saude Publica 2017, 51, 30. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The Global Compendium of Aedes aegypti and Ae. albopictus Occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Kraemer, M.U.G.; Brady, O.J.; Pigott, D.M.; Shearer, F.M.; Weiss, D.J.; Golding, N.; Ruktanonchai, C.W.; Gething, P.W.; Cohn, E.; et al. Mapping Global Environmental Suitability for Zika Virus. Elife 2016, 5, e15272. [Google Scholar] [CrossRef] [PubMed]
- BRASIL, M.d.S. Levantamento Rápido de Índices Para Aedes aegypti -LIRAa-Para Vigilância Entomólogica do Aedes aegypti No Brasil; Ministério da Saúde: Brasília, Brazil, 2013; ISBN 9788533419995. [Google Scholar]
- Nsoesie, E.O.; Kraemer, M.U.; Golding, N.; Pigott, D.M.; Brady, O.J.; Moyes, C.L.; Johansson, M.A.; Gething, P.W.; Velayudhan, R.; Khan, K.; et al. Global Distribution and Environmental Suitability for Chikungunya Virus, 1952 to 2015. Eurosurveillance 2016, 21, 30234. [Google Scholar] [CrossRef] [PubMed]
- Codeco, C.T.; Oliveira, S.S.; Ferreira, D.A.; Riback, T.I.; Bastos, L.S.; Lana, R.M.; Almeida, I.; Godinho, V.B.; Cruz, O.G.; Coelho, F.C. Fast expansion of dengue in Brazil. Lancet Reg. Health–Am. 2022, 12, 100274. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Lee, S.A.; Economou, T.; Catão, R.d.C.; Barcellos, C.; Lowe, R. The Impact of Climate Suitability, Urbanisation, and Con-nectivity on the Expansion of Dengue in 21st Century Brazil. PLoS Negl. Trop. Dis. 2021, 15, e0009773. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC) Climate Change 2022: Impacts, Adaptation and Vulnerability. Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_FullReport.pdf (accessed on 10 October 2022).
- Wang, X.; Zou, X. Threshold Dynamics of a Temperature-Dependent Stage-Structured Mosquito Population Model with Nested Delays. Bull. Math. Biol. 2018, 80, 1962–1987. [Google Scholar] [CrossRef]
- Terra, M.R.; Da Silva, R.S.; Pereira, M.G.N.; Lima, A.F. Aedes aegypti e as Arboviroses Emergentes No Brasil. Uningá Rev. 2017, 30, 52–60. [Google Scholar]
- Wanderson Batista da Silva Diversida do Mosquito Aedes Spp., (Diptera: Culicidae) Em Área Urbana e de Mata Circunzizinha Em Cuiabá, MT [Dissertação]; Universidade de Cuiabá: Cuiabá, Brazil, 2019.
- Coffey, L.L.; Failloux, A.-B.; Weaver, S.C. Chikungunya Virus-Vector Interactions. Viruses 2014, 6, 4628–4663. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M. Emergent Arboviruses in Brazil. Rev. Soc. Bras. Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Mourão, M.P.G.; Bastos, M.D.S.; Figueiredo, R.M.P.D.; Gimaque, J.B.D.L.; Alves, V.D.C.R.; Saraiva, M.D.G.G.; Figueiredo, M.L.G.; Ramasawmy, R.; Nogueira, M.L.; Figueiredo, L.T.M. Arboviral Diseases in the Western Brazilian Amazon: A Perspective and Analysis from a Tertiary Health & Research Center in Manaus, State of Amazonas. Rev. Soc. Bras. Med. Trop. 2015, 48, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J. Zika: The New Arbovirus Threat for Latin America. J. Infect. Dev. Ctries. 2015, 9, 684–685. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.M.T.; Siqueira, J.B.; Parente, M.P.P.D.; Zara, A.L.d.S.A.; Oliveira, C.S.; Braga, C.; Pimenta, F.G.; Cortes, F.; Lopez, J.G.; Bahia, L.R.; et al. Economic Impact of Dengue: Multicenter Study across Four Brazilian Regions. PLoS Negl. Trop. Dis. 2015, 9, e0004042. [Google Scholar] [CrossRef] [PubMed]
- Organização Mundial da Saúde Países de Las Américas Se Preparan Frente Al Dengue, Chikungunya y Zika. Available online: http://www.paho.org/arg/index.php?option=com_content&view=article&id=9937:2015-11-06-15-02-12&Itemid=268 (accessed on 27 April 2020).
- Focks, D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- MacCormack-Gelles, B.; Lima Neto, A.S.; Sousa, G.S.; do Nascimento, O.J.; Castro, M.C. Evaluation of the Usefulness of Aedes aegypti Rapid Larval Surveys to Anticipate Seasonal Dengue Transmission between 2012–2015 in Fortaleza, Brazil. Acta Trop. 2020, 205, 105391. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.S.; Ferreira, D.F.; Azevedo, R.C.; Santos, G.B.G.D.; Medronho, R.D.A. Aedes aegypti Larval Indices and Dengue Incidence: An Ecological Study in the State of Rio de Janeiro, Brazil. Cad. Saúde Pública 2021, 37, 1–13. [Google Scholar] [CrossRef]
- Azevedo, T.S.; Lorenz, C.; Chiaravalloti-Neto, F. Spatiotemporal Evolution of Dengue Outbreaks in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 593–602. [Google Scholar] [CrossRef]
- Ministério da Saúde (Brasil) Monitoramento Dos Casos de Arboviroses Urbanas Transmitidas Pelo Aedes aegypti (Dengue, Chikungunya e Zika), Semanas Epidemiológicas 1 a 50. 2020. Available online: https://www.gov.br/saude/pt-br/assuntos/media/pdf/2020/dezembro/28/boletim_epidemiologico_svs_51.pdf (accessed on 25 June 2021).
- Secretaria de Vigilância em Saúde; Ministério da Saúde Monitoramento Dos Casos de Arboviroses Urbanas Causados Por Vírus Transmitidos Pelo Mosquito Aedes (Dengue, Chikungunya e Zika), Semanas Epidemiológicas 1 a 51. 2021. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/edicoes/2021 (accessed on 20 January 2022).
- Secretaria de Vigilância em Saúde; Ministério da Saúde Monitoramento Dos Casos de Arboviroses Até a Semana Epidemiológica 22 de 2022. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no22.pdf/view (accessed on 7 July 2022).
- Instituto Brasileiro de Geografia e Estatística—IBGE Brasil|Cidades e Estados|IBGE. Available online: https://www.ibge.gov.br/cidades-e-estados.html?view=municipio (accessed on 3 April 2021).
- Governo do Estado de Santa Geografia Do Estado de Santa Catarina. Available online: https://www.sc.gov.br/conhecasc/geografia (accessed on 30 August 2021).
- Governo do Estado do Paraná História|VIAJE PARANÁ. Available online: http://www.viajeparana.com/Historia (accessed on 3 April 2021).
- Governo do Rio Grande do Sul Geografia—Portal Do Estado Do Rio Grande Do Sul. Available online: https://www.estado.rs.gov.br/geografia (accessed on 3 April 2021).
- Wrege, M.; Steinmetz, S.; Junior, C.R. Atlas Climático da Região Sul do Brasil: Estados do Paraná, Santa Catarina e Rio Grande do Sul; Embrapa, Ed.; Livro técnico (CPACT): Brasília, Brazil, 2012; ISBN 978-85-7035-013-8. [Google Scholar]
- Brasil Resolução No 12, de Janeiro de 2017—Imprensa Nacional. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/20596177/do1-2017-01-27-resolucao-n-12-de-26-de-janeiro-de-2017-20596073 (accessed on 2 February 2022).
- National Aeronautics and Space Administration (NASA) Giovanni. Available online: https://giovanni.gsfc.nasa.gov/giovanni/ (accessed on 4 July 2021).
- MapBiomas Projeto de Mapeamento Anual do Uso e Cobertura da Terra No Brasil. Available online: https://mapbiomas.org/o-projeto (accessed on 4 July 2021).
- Anderson, M.J.; Gorley, R.N.; Clarke, R.K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- The Jamovi Project Jamovi 2021. Available online: https://www.jamovi.org (accessed on 10 August 2021).
- QGIS Development Team, 2016. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 10 March 2020).
- Instituto Brasileiro de Geografia e Estatística—IBGE Malha Municipal|IBGE. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais/15774-malhas.html?=&t=downloads (accessed on 6 September 2022).
- Barcellos, C.; Lowe, R. Expansion of the Dengue Transmission Area in Brazil: The Role of Climate and Cities. Trop. Med. Int. Health 2014, 19, 159–168. [Google Scholar] [CrossRef]
- Lins, R.; Júnior, R.; Daniel, F.; Silva, S.; Duarte, D.; Pinto, C.; Lisboa Costa, R.; Gomes, H.B.; Luís Herdies, D.; Silva, T.; et al. Analysis of Temperature Extremes in the South of Brazil. Rev. Bras. Climatol. 2022, 30, 445–460. [Google Scholar] [CrossRef]
- Portela Câmara, F.; Lúcia Gonçalves Theophilo, R.; Teixeira dos Santos, G.; Regina Ferreira Gonçalves Pereira, S.; Cardoso Câmara, D.P.; Rodrigues de Matos, R.C. Regional and Dynamics Characteristics of Dengue in Brazil: A Retrospective Study. Rev. Soc. Bras. Med. Trop. 2007, 40, 192–196. [Google Scholar]
- Fonseca Júnior, D.P.; da Serpa, L.L.N.; Barbosa, G.L.; Pereira, M.; Holcmam, M.M.; Voltolini, J.C.; Marques, G.R.A.M. Vectors of Arboviruses in the State of São Paulo: 30 Years of Aedes aegypti and Aedes Albopictus. Rev. Saude Publica 2019, 53, 84. [Google Scholar] [CrossRef] [PubMed]
- Heinisch, M.R.S.; Diaz-Quijano, F.A.; Chiaravalloti-Neto, F.; Menezes Pancetti, F.G.; Rocha Coelho, R.; dos Santos Andrade, P.; Urbinatti, P.R.; de Almeida, R.M.M.S.; Lima-Camara, T.N. Seasonal and Spatial Distribution of Aedes aegypti and Aedes albopictus in a Municipal Urban Park in São Paulo, SP, Brazil. Acta Trop. 2019, 189, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Castro, M.G.; de Barros, F.S.M.; Magalhães, M.d.A.F.M.; Sabroza, P.C. The Spatial Distribution of Aedes aegypti and Aedes albopictus in a Transition Zone, Rio de Janeiro, Brazil. Cad. Saude Publica 2009, 25, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Lubinda, J.; Walsh, M.R.; Moore, A.J.; Hanafi-Bojd, A.A.; Akgun, S.; Zhao, B.; Barro, A.S.; Begum, M.M.; Jamal, H.; Angulo-Molina, A.; et al. Environmental Suitability for Aedes aegypti and Aedes albopictus and the Spatial Distribution of Major Arboviral Infections in Mexico. Parasite Epidemiol. Control 2019, 6, e00116. [Google Scholar] [CrossRef]
- Simoy, M.I.; Simoy, M.V.; Canziani, G.A. The Effect of Temperature on the Population Dynamics of Aedes aegypti. Ecol. Modell. 2015, 314, 100–110. [Google Scholar] [CrossRef]
- Stewart Ibarra, A.M.; Ryan, S.J.; Beltrán, E.; Mejía, R.; Silva, M.; Muñoz, Á. Dengue Vector Dynamics (Aedes aegypti) Influenced by Climate and Social Factors in Ecuador: Implications for Targeted Control. PLoS ONE 2013, 8, e78263. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M.; Harrington, L.C. Flushing Effect of Rain on Container-Inhabiting Mosquitoes Aedes aegypti and Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 28–35. [Google Scholar] [CrossRef]
- Pontes, R.J.S.; Freeman, J.; Oliveira-Lima, J.W.; Hodgson, J.C.; Spielman, A. Vector Densities That Potentiate Dengue Outbreaks in a Brazilian City. Am. J. Trop. Med. Hyg. 2000, 62, 378–383. [Google Scholar] [CrossRef]
- Mohammed, A.; Chadee, D.D. Effects of Different Temperature Regimens on the Development of Aedes aegypti (L.) (Diptera: Culicidae) Mosquitoes. Acta Trop. 2011, 119, 38–43. [Google Scholar] [CrossRef]
- Grech, M.G.; Sartor, P.D.; Almirón, W.R.; Ludueña-Almeida, F.F. Effect of Temperature on Life History Traits during Immature Development of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) from Córdoba City, Argentina. Acta Trop. 2015, 146, 1–6. [Google Scholar] [CrossRef]
- Guzmán, C.; Calderón, A.; Mattar, S.; Tadeu-Figuereido, L.; Salazar-Bravo, J.; Alvis-Guzmán, N.; Martinez, E.Z.; González, M. Ecoepidemiology of Alphaviruses and Flaviviruses. In Emerging and Reemerging Viral Pathogens: Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–125. ISBN 9780128194003. [Google Scholar]
- Couret, J.; Dotson, E.; Benedict, M.Q. Temperature, Larval Diet, and Density Effects on Development Rate and Survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE 2014, 9, e87468. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(St) Century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.S.; Cota, A.L.S.; Rodrigues, D.F. Sanitation, Arboviruses, and Environmental Determinants of Disease: Impacts on Urban Health. Cien. Saude Colet. 2020, 25, 3857–3868. [Google Scholar] [CrossRef] [PubMed]

| Source | df | SS | MS | Pseudo-F | p (perm) |
|---|---|---|---|---|---|
| State | 2 | 3.5726 | 1.7863 | 4.7848 | 0.019 |
| Year | 4 | 2.1326 | 0.53314 | 7.8968 | 0.001 |
| Mesoregion (State) | 20 | 7.8791 | 0.39395 | 5.8352 | 0.001 |
| State X Year | 7 | 1.1697 | 0.1671 | 2.4751 | 0.025 |
| Res | 107 | 19.382 |
| Groups (Term State) | t | p (perm) |
|---|---|---|
| Paraná, Santa Catarina | 2.8503 | 0.015 |
| Paraná, Rio Grande do Sul | 2.4083 | 0.03 |
| Groups (Terms Year) | t | p(perm) |
| 2017, 2019 | 3.0173 | 0.004 |
| 2017, 2020 | 5.4357 | 0.001 |
| 2017, 2021 | 6.0309 | 0.001 |
| 2018, 2020 | 6.2661 | 0.001 |
| 2018, 2021 | 3.3379 | 0.005 |
| 2019, 2020 | 4.8183 | 0.001 |
| 2020, 2021 | 3.8327 | 0.004 |
| Term ‘State × Year’ for pairs of levels of factor ‘State’ | ||
| Groups | t | p(perm) |
| 2017 | ||
| Paraná, Santa Catarina | 5.0024 | 0.002 |
| Paraná, Rio Grande do Sul | 2.2776 | 0.031 |
| 2018 | ||
| Paraná, Santa Catarina | 3.5714 | 0.007 |
| Paraná, Rio Grande do Sul | 2.7738 | 0.017 |
| 2020 | ||
| Paraná, Rio Grande do Sul | 2.463 | 0.031 |
| 2021 | ||
| Paraná, Rio Grande do Sul | 2.1969 | 0.013 |
| Paraná, Santa Catarina | 2.8811 | 0.009 |
| Term ‘State × Year’ for pairs of levels of factor ‘Year’ | ||
| Groups | t | p (perm) |
| Paraná | ||
| 2017, 2018 | 2.2718 | 0.05 |
| 2017, 2020 | 7.6971 | 0.001 |
| 2017, 2021 | 7.8195 | 0.001 |
| 2018, 2020 | 6.8357 | 0.001 |
| 2019, 2020 | 3.7084 | 0.009 |
| 2020, 2021 | 4.9221 | 0.002 |
| Santa Catarina | ||
| 2017, 2021 | 3.2204 | 0.026 |
| 2018, 2021 | 7.9261 | 0.004 |
| Rio Grande do Sul | ||
| 2017,2019 | 2.5486 | 0.033 |
| 2017, 2020 | 4.4384 | 0.002 |
| 2017,2021 | 2.5116 | 0.038 |
| 2018,2019 | 3.2216 | 0.023 |
| 2018, 2020 | 5.0224 | 0.002 |
| 2019, 2020 | 4.0522 | 0.007 |
| Aedes aegypti | ||||||
|---|---|---|---|---|---|---|
| Region/State | R | R² | F | df1 | df2 | p |
| Southern region | 0.85 | 0.72 | 27.46 | 9 | 98 | <0.001 |
| Paraná | 0.87 | 0.76 | 13.82 | 9 | 40 | < 0.001 |
| Santa Catarina * | 0.88 | 0.77 | 4.74 | 9 | 13 | 0.006 |
| Rio Grande do Sul | 0.93 | 0.87 | 18.49 | 9 | 25 | < 0.001 |
| Significant Predictive Variables | ||||||
| Predictor | Region/State | Estimate | SE | t | p | Stand. Estimate |
| Annual precipitation | Southern region | −1.53 | 0.70 | −2.21 | 0.030 | −0.16 |
| Paraná | −3.77 | 1.65 | −2.29 | 0.027 | −0.37 | |
| Average maximum temperature | Rio Grande do Sul | 14.13 | 4.09 | 3.45 | 0.002 | 0.66 |
| Average minimum temperature | Southern region | 4.18 | 1.93 | 2.17 | 0.032 | 0.20 |
| Average humidity | Southern region | −9.57 | 1.80 | −5.32 | <0.001 | −0.66 |
| Natural forest | Southern region | −1.31 | 0.48 | −2.71 | 0.008 | −0.33 |
| Agricultural | Rio Grande do Sul | 1.11 | 0.50 | 2.19 | 0.038 | 0.64 |
| Urban infrastructure | Southern region | 1.12 | 0.28 | 4.00 | <0.001 | 0.36 |
| Paraná | 1.26 | 0.43 | 2.93 | 0.006 | 0.37 | |
| Rio Grande do Sul | 1.22 | 0.50 | 2.19 | 0.038 | 0.64 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, J.G.; Netto, S.A.; Francisco, E.O.; Vieira, C.P.; Variza, P.F.; Iser, B.P.M.; Lima-Camara, T.N.; Lorenz, C.; Prophiro, J.S. Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors. Trop. Med. Infect. Dis. 2023, 8, 77. https://doi.org/10.3390/tropicalmed8020077
de Oliveira JG, Netto SA, Francisco EO, Vieira CP, Variza PF, Iser BPM, Lima-Camara TN, Lorenz C, Prophiro JS. Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors. Tropical Medicine and Infectious Disease. 2023; 8(2):77. https://doi.org/10.3390/tropicalmed8020077
Chicago/Turabian Stylede Oliveira, Joice Guilherme, Sérgio Antônio Netto, Edenilson Osinski Francisco, Caroline Pereira Vieira, Paula Fassicolo Variza, Betine Pinto Moehlecke Iser, Tamara Nunes Lima-Camara, Camila Lorenz, and Josiane Somariva Prophiro. 2023. "Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors" Tropical Medicine and Infectious Disease 8, no. 2: 77. https://doi.org/10.3390/tropicalmed8020077
APA Stylede Oliveira, J. G., Netto, S. A., Francisco, E. O., Vieira, C. P., Variza, P. F., Iser, B. P. M., Lima-Camara, T. N., Lorenz, C., & Prophiro, J. S. (2023). Aedes aegypti in Southern Brazil: Spatiotemporal Distribution Dynamics and Association with Climate and Environmental Factors. Tropical Medicine and Infectious Disease, 8(2), 77. https://doi.org/10.3390/tropicalmed8020077








