Natural History of Hepatosplenic Schistosomiasis (HSS) Non–Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Ethics Statement
2.4. Definitions
- Varicose gastrointestinal bleeding (VGIB)—clinically relevant digestive bleeding caused by the rupture of gastric esophageal varices in patients with portal hypertension [1].
- Ascites—abnormal accumulation of fluid inside the peritoneal cavity, identified by complementary exams (US/CT) or physical examination. Ascites can be graded from 1 to 3 according to the amount of fluid in the abdominal cavity [12].
- Spontaneous bacterial peritonitis (SBP)—bacterial infection of ascitic fluid without any intra-abdominal, surgically treatable source of infection [12].
- Hepatic encephalopathy (HE)—neuropsychiatric complication with a wide spectrum of symptoms that can affect patients with acute or chronic liver failure. HE is diagnosed and graded according to West-Haven criteria [13].
- Anemia, leucopenia and thrombocytopenia were defined by a serum hemoglobin value lower than 13 g/dL, a leucocyte count lower than 4.0 × 109/L and a platelet count lower than <150 × 109/L, respectively.
2.5. Statistical Analysis
3. Results
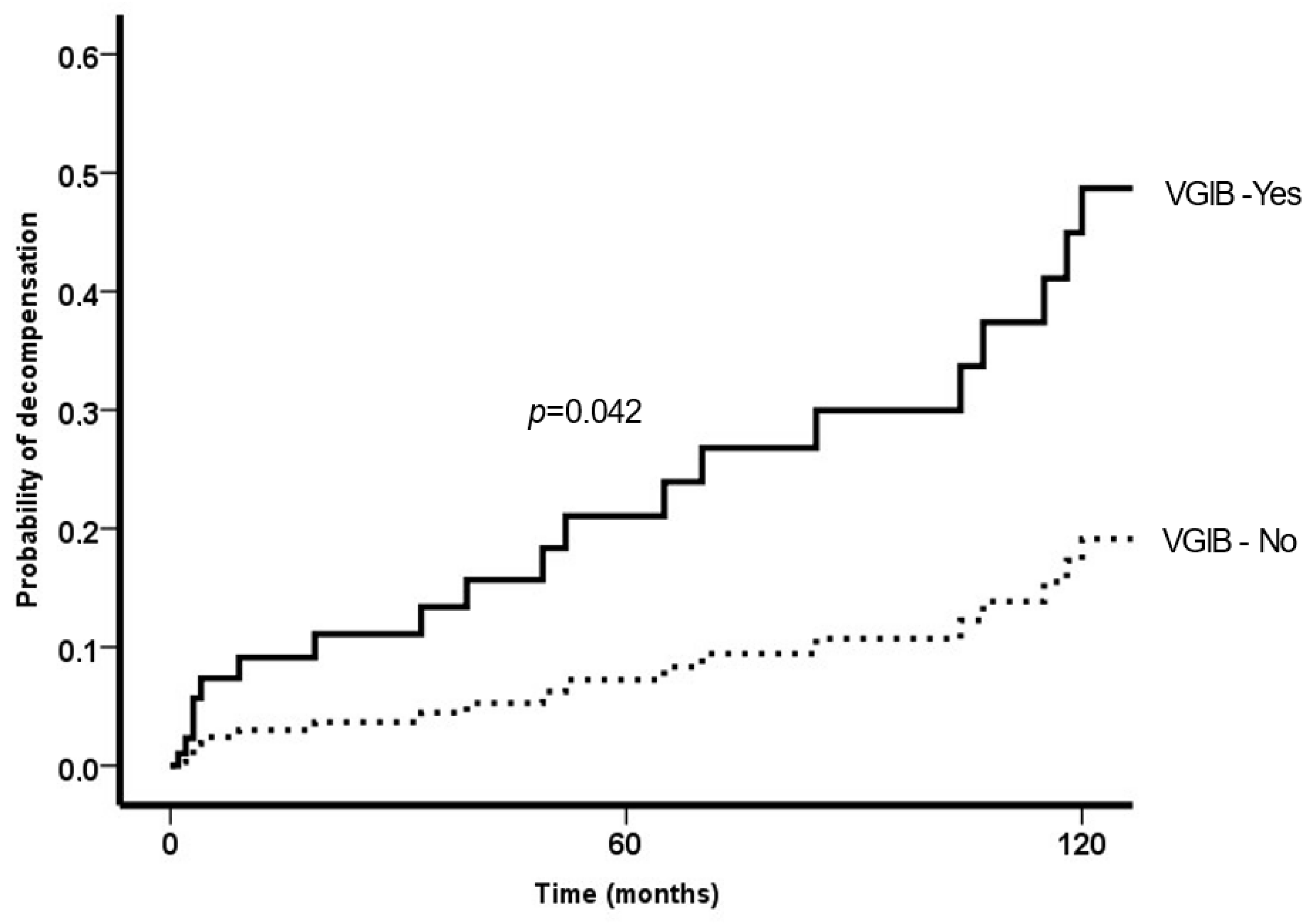
3.1. Variceal Bleeding
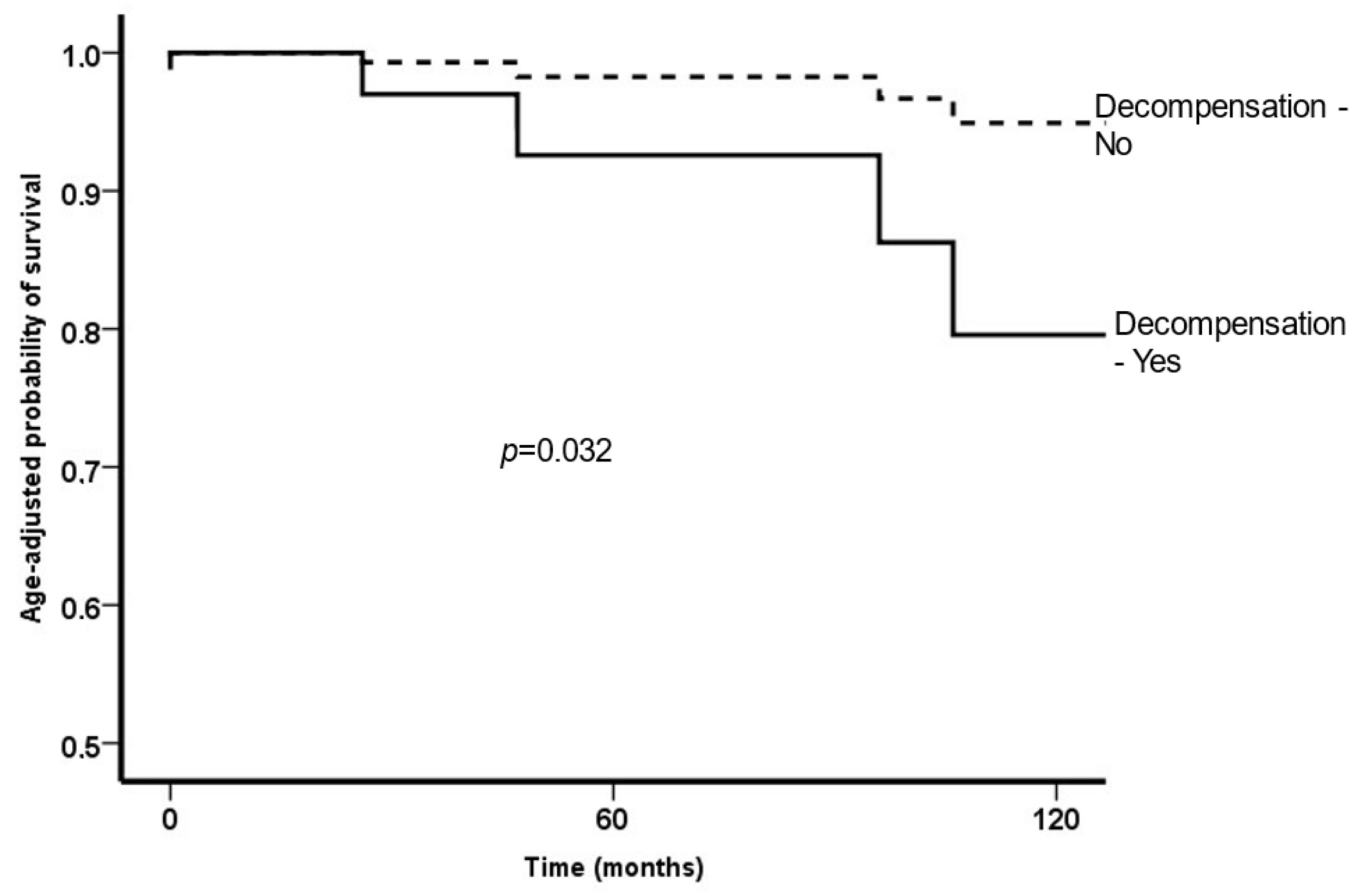
3.2. Decompensation
3.3. Survival
3.4. Other Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII. Renewing Consens Portal Hypertens. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Schouten, J.N.; Garcia-Pagan, J.C.; Valla, D.C.; Janssen, H.L. Idiopathic noncirrhotic portal hypertension. Hepatology 2011, 54, 1071–1081. [Google Scholar] [CrossRef]
- De Gottardi, A.; Rautou, P.E.; Schouten, J.; Rubbia-Brandt, L.; Leebeek, F.; Trebicka, J.; Murad, S.D.; Vilgrain, V.; Hernandez-Gea, V.; Nery, F.; et al. Porto-sinusoidal vascular disease: Proposal and description of a novel entity. Lancet Gastroenterol. Hepatol. 2019, 4, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schistosomiasis: Fact sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 22 January 2023).
- WHO. The Control of Schistosomiasis–Second Report of WHO Experts Committee; Technical Report Series 830; WHO: Geneva, Switzerland, 1993.
- Pinto-Silva, R.A.; Abrantes, W.L.; Antunes, C.M.; Lambertucci, J.R. Sonographic features of portal hypertension in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1994, 36, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Rebouças, G. Clinical aspects of hepatosplenic schistosomiasis: A contrast with cirrhosis. Yale J. Biol. Med. 1975, 48, 369–376. [Google Scholar]
- Lambertucci, J.R. Revisiting the concept of hepatosplenic schistosomiasis and its challenges using traditional and new tools. Rev. Soc. Bras. Med. Trop. 2014, 47, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.C.; Chieffi, P.P.; Carrilho, F.J. Schistosomiasis mansoni–clinical features. Gastroenterol. Hepatol. 2005, 28, 30–39. [Google Scholar] [CrossRef]
- Filgueira, N.A.; Saraiva, C.M.A.; Jucá, N.T.; Bezerra, M.F.; Lacerda, C.M. Schistosomal liver fibrosis and hepatocellular carcinoma–case series of patients submitted to liver transplantation. Braz. J. Infect. Dis. 2018, 22, 352–354. [Google Scholar] [CrossRef]
- EASL. Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Andrade, Z.A. Schistosomal hepatopathy. Mem. Inst. Oswaldo Cruz 2004, 99, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raia, S.; da Silva, L.C.; Gayotto, L.C.; Forster, S.C.; Fukushima, J.; Strauss, E. Portal hypertension in schistosomiasis: A long-term follow-up of a randomized trial comparing three types of surgery. Hepatology 1994, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- el Tourabi, H.; el Amin, A.A.; Shaheen, M.; Woda, S.A.; Homeida, M.; Harron, D.W. Propranolol reduces mortality in patients with portal hypertension secondary to schistosomiasis. Ann. Trop. Med. Parasitol. 1994, 88, 493–500. [Google Scholar] [CrossRef]
- Schouten, J.N.; Nevens, F.; Hansen, B.; Laleman, W.; van den Born, M.; Komuta, M. Idiopathic noncirrhotic portal hypertension is associated with poor survival: Results of a long-term cohort study. Aliment. Pharm. Ther. 2012, 35, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Gioia, S.; Nardelli, S.; Pasquale, C.; Pentassuglio, I.; Nicoletti, V.; Aprile, F.; Merli, M.; Riggio, O. Natural history of patients with non cirrhotic portal hypertension: Comparison with patients with compensated cirrhosis. Dig. Liver Dis. 2018, 50, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Liu, J.; Yao, J.; Zhao, J.; Wang, Y.; Ju, S.; Wang, C.; Yang, C.; Bai, Y.; Bin Xiong, B. Efficacy and safety of transjugular intrahepatic portosystemic shunt for the treatment of schistosomiasis-induced portal hypertension: A retrospective case series. Eur. J. Gastroenterol. Hepatol. 2022, 34, 1090–1097. [Google Scholar] [CrossRef]
- Farias, A.Q.; Kassab, F.; da Rocha, E.C.; Dos Santos Bomfim, V.; Vezozzo, D.C.; Bittencourt, P.L.; Carrilho, F.J. Propranolol reduces variceal pressure and wall tension in schistosomiasis presinusoidal portal hypertension. J. Gastroenterol. Hepatol. 2009, 24, 1852–1856. [Google Scholar] [CrossRef]
- de Abreu, E.S.; Nardelli, M.J.; Lima, A.M.C.; Cardoso, J.B.; Osório, F.M.F.; Ferrari, T.C.A.; Faria, L.C.; Couto, C.A.; Cançado, G.G.L. Carvedilol as secondary prophylaxis for variceal bleeding in hepatosplenic schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 663–667. [Google Scholar] [CrossRef]
- Wynn, T.A.; Thompson, R.W.; Cheever, A.W.; Mentink-Kane, M.M. Immunopathogenesis of schistosomiasis. Immunol. Rev. 2004, 201, 156–167. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Burroughs, A.K.; Ripoll, C.; Maurer, R.; Planas, R.; Escorsell, A.; et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. 2009, 7, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Hua, R.; Cao, H.; Wu, Z.Y. Effects of hemoglobin concentration on hyperdynamic circulation associated with portal hypertension. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 215–218. [Google Scholar]
- Denié, C.; Vachiery, F.; Elman, A.; Soupison, T.; Gadano, A.; Moreau, R.; Lebrec, D. Systemic and splanchnic hemodynamic changes in patients with hepatic schistosomiasis. Liver 1996, 16, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R.K.; Chawla, Y.; Vasishta, R.K.; Kakkar, N.; Dilawari, J.B.; Trehan, M.S. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): Experience with 151 patients and a review of the literature. J. Gastroenterol. Hepatol. 2002, 17, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Giustina, G.; Degos, F.; Tremolada, F.; Diodati, G.; Almasio, P.; Nevens, F.; Solinas, A.; Mura, D.; Brouwer, J.T.; et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology 1997, 112, 463–472. [Google Scholar] [CrossRef]
- Siramolpiwat, S.; Seijo, S.; Miquel, R.; Berzigotti, A.; Garcia-Criado, A.; Darnell, A.; Turon, F.; Hernandez-Gea, V.; Bosch, J.; Garcia-Pagán, J.C. Idiopathic portal hypertension: Natural history and long-term outcome. Hepatology 2014, 59, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Resendes, A.P.; Souza-Santos, R.; Barbosa, C.S. Hospitalization and mortality from mansoni schistosomiasis in the state of Pernambuco, Brazil, 1992/2000. Cad Saude Publica 2005, 21, 1392–1401. [Google Scholar] [CrossRef] [Green Version]
- Martins-Melo, F.R.; Pinheiro, M.C.; Ramos, A.N., Jr.; Alencar, C.H.; Bezerra, F.S.; Heukelbach, J. Trends in schistosomiasis-related mortality in Brazil, 2000–2011. Int. J. Parasitol. 2014, 44, 1055–1062. [Google Scholar] [CrossRef]
- Krasinskas, A.M.; Eghtesad, B.; Kamath, P.S.; Demetris, A.J.; Abraham, S.C. Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transpl. 2005, 11, 627–634. [Google Scholar] [CrossRef]


| Overall | Previous Decompensation | |||
|---|---|---|---|---|
| No (n = 94) | Yes (n = 11) | p-Value | ||
| Demographic data | ||||
| Age (years) | 50 ± 13 | 50 ± 13 | 54 ± 16 | 0.40 |
| Male sex | 38 (36%) | 35% | 45% | 0.52 |
| Diabetes mellitus | 26 (25%) | 24% | 27% | >0.99 |
| Arterial hypertension | 32 (31%) | 30% | 36% | 0.73 |
| Obesity | 7 (7%) | 7% | 0% | 0.34 |
| Dyslipidemia | 8 (8%) | 9% | 0% | 0.59 |
| Previous complications and procedures | ||||
| Variceal bleeding | 48 (46%) | 41% | 73% | 0.06 |
| Surgery for portal hypertension | 12 (11%) | 12% | 9% | 0.79 |
| Clinical characteristics | ||||
| Propranolol | 86 (82%) | 81% | 90% | 0.68 |
| Mean arterial pressure (mmHg) | 94 ± 15 | 94 ± 15 | 100 ± 13 | 0.36 |
| Heart rate (bpm) | 75 ± 11 | 75 ± 11 | 74 ± 8 | 0.87 |
| Laboratorial characteristics | ||||
| ALT (U/L) | 37 ± 21 | 37 ± 21 | 33 ± 14 | 0.52 |
| AST (U/L) | 42 ± 21 | 41 ± 20 | 48 ± 26 | 0.32 |
| Alkaline phosphatase (U/L) | 140 ± 86 | 142 ± 88 | 127 ± 48 | 0.62 |
| GGT (U/L) | 96 ± 83 | 94 ± 84 | 118 ± 80 | 0.37 |
| Albumin (g/L) | 39 ± 6 | 40 ± 5 | 34 ± 9 | 0.09 |
| Bilirubin (mg/dL) | 1.5 ± 1.6 | 1.5 ± 1.7 | 1.5 ± 1.9 | 0.93 |
| INR | 1.22 ± 0.23 | 1.2 ± 0.2 | 1.4 ± 0.3 | 0.20 |
| Hemoglobin (g/dL) | 12.0 ± 2.4 | 12.1 ± 2.4 | 10.7 ± 2.3 | 0.05 |
| Leucocyte count (× 109/L) | 4.5 ± 2.3 | 4.4 ± 2.2 | 5.0 ± 2.5 | 0.38 |
| Platelet count (× 109/L) | 104 ± 68 | 101 ± 66 | 134 ± 82 | 0.14 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.98 |
| Sodium (mEq/L) | 139 ± 4 | 139 ± 4 | 138 ± 2 | 0.10 |
| Endoscopic and radiological characteristics | ||||
| Esophagogastric varices (either present or treated) | 90 (86%) | 85% | 91% | 0.60 |
| Portal hypertensive gastropathy | 30 (29%) | 29% | 27% | 0.92 |
| Portal vein thrombosis | 10 (10%) | 10% | 9% | 0.96 |
| Univariate | Multivariate | |
|---|---|---|
| Age (years) | 1.03 (0.97–1.08) | |
| Male sex | 0.58 (014–1.84) | |
| Diabetes mellitus | 1.97 (0.51–7.52) | |
| Arterial hypertension | 0.34 (0.07–1.59) | |
| Previous surgery | 0.04 (0.001–183) | |
| Propranolol | 32.5 (0.09–11.332) | |
| Mean arterial pressure (mmHg) | 0.98 (0.93–1.02) | |
| Heart rate (bpm) | 0.97 (0.91–1.01) | |
| ALT (U/L) | 1.036 (1.013–1.058) | 1.03 (1.01–1.06) |
| AST (U/L) | 1.02 (0.998–1.046) | |
| Alkaline phosphatase (U/L) | 1.004 (0.999–1.009) | |
| GGT (U/L) | 1.003 (0.996–1.010) | |
| Albumin (g/L) | 1.19 (0.34–4.18) | |
| Bilirubin (mg/dL) | 0.57 (0.20–1.62) | |
| INR | 4.55 (0.12–173) | |
| Hemoglobin (g/dL) | 0.82 (0.65–1.03) | 0.76 (0.59–0.97) |
| Leucocyte count (×109/L) | 0.89 (0.65–1.03) | |
| Platelet count (×109/L) | 0.99 (0.98–1.009) | |
| Creatinine (mg/dL) | 2.37 (0.50–11.2) | |
| Esophageal varices | 31.29 (0.08–12,029) | |
| Large esophageal varices | 9.01 (1.14–71.21) | 13.2 (1.45–133) |
| Portal hypertensive gastropathy | 0.67 (0.14–3.13) | |
| Portal vein thrombosis | 0.78 (0.09–6.71) |
| Univariate | Multivariate | |
|---|---|---|
| Age (years) | 0.99 (0.96–1.04) | |
| Male sex | 1.55 (0.61–3.92) | |
| Diabetes mellitus | 1.28 (0.47–3.57) | |
| Arterial hypertension | 2.41 (0.81–7.20) | |
| Previous complications | ||
| Variceal bleeding (previous) | 0.47 (0.19–1.15) | |
| Variceal bleeding (follow-up) | 1.76 (0.59–5.26) | |
| Variceal bleeding (previous + after) | 3.43 (1.14–10.25) | 3.32 (1.01–10.0) |
| Surgery | 2.32 (0.53–10.2) | |
| Clinical and Laboratorial | ||
| Propranolol | 25.6 (0.11–6056) | |
| Mean arterial pressure (mmHg) | 0.98 (0.96–1.02) | |
| Heart rate (bpm) | 0.98(0.95–1.03) | |
| ALT (U/L) | 1.01 (0.99–1.03) | |
| AST (U/L) | 0.99 (0.98–1.02) | |
| Alkaline phosphatase (U/L) | 1.005 (1.001–1.009) | |
| GGT (U/L) | 1.00 (0.99–1.006) | |
| Albumin (g/L) | 0.86 (0.40–1.86) | |
| Bilirubin (mg/dL) | 1.34 (1.12–1.60) | 1.34 (1.12–1.60) |
| INR | 2.07 (0.32–13.2) | |
| Hemoglobin (g/dL) | 0.93 (0.78–1.10) | |
| Leucocyte count (×109/L) | 0.98 (0.83–1.17) | |
| Platelet count (×109/L) | 0.997 (0.99–1.005) | |
| Creatinine (mg/dL) | 2.26 (0.57–8.91) | |
| Sodium (mEq/L) | 0.92 (0.83–1.03) | |
| Endoscopy and Radiology | ||
| Esophageal varices (any) | 25.9 (0.13–5131) | |
| Large esophageal varices | 2.89 (0.85–9.88) | |
| Portal hypertensive gastropathy | 2.32 (0.96–5.79) | |
| Portal vein thrombosis | 1.22 (0.36–4.16) |
| Univariate | Multivariate | |
|---|---|---|
| Age (years) | 1.08 (1.01–1.16) | 1.08 (1.01–1.15) |
| Male sex | 0.32 (0.08–1.30) | |
| Diabetes mellitus | 1.41 (0.28–7.14) | |
| Arterial hypertension | 0.58 (0.11–2.78) | |
| Previous complications | ||
| Variceal bleeding | ||
| At inclusion or before | 1.86 (0.44–7.78) | |
| After inclusion | 1.83 (0.37–9.10) | |
| Before or after inclusion | 4.18 (0.51–34.1) | |
| Surgery | 0.31 (0.03–34.2) | |
| Decompensations | 5.89 (1.19–29.2) | 6.00 (1.15–31.3) |
| Clinical and Laboratorial | ||
| Propranolol | 23.77 (0.001–622,492) | |
| Mean arterial pressure (mmHg) | 0.97 (0.92–1.02) | |
| Heart rate (bpm) | 0.98 (0.93–1.04) | |
| ALT (U/L) | 0.9 (0.96–1.03) | |
| AST (U/L) | 1.01 (0.98–1.04) | |
| Alkaline phosphatase (U/L) | 1.002 (0.994–1.009) | |
| GGT (U/L) | 1.004 (0.996–1.012) | |
| Albumin (g/L) | 0.81 (0.25–2.60) | |
| Bilirubin (mg/dL) | 1.15 (0.89–1.47) | |
| INR | 1.89 (0.11–32.67) | |
| Hemoglobin (g/dL) | 0.94 (0.72–1.25) | |
| Leucocyte count (×109/L) | 1.03 (0.72–1.25) | |
| Platelet count (×109/L) | 0.99 (0.98–1.01) | |
| Creatinine (mg/dL) | 6.4 (0.45–91.3) | |
| Sodium (mEq/L) | 0.79 (0.66–0.96) | |
| Endoscopy and Radiology | ||
| Esophageal varices | 25.18 (0.003–210,931) | |
| Large esophageal varices | 2.86 (0.35–23.3) | |
| Portal hypertensive gastropathy | 1.25 (0.77–2.02) | |
| Portal vein thrombosis | 1.19 (0.38–9.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga, Z.S.T.; Fernandes, F.F.; Guimarães, L.; Piedade, J.; Pereira, G.H.S. Natural History of Hepatosplenic Schistosomiasis (HSS) Non–Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis. Trop. Med. Infect. Dis. 2023, 8, 145. https://doi.org/10.3390/tropicalmed8030145
Veiga ZST, Fernandes FF, Guimarães L, Piedade J, Pereira GHS. Natural History of Hepatosplenic Schistosomiasis (HSS) Non–Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis. Tropical Medicine and Infectious Disease. 2023; 8(3):145. https://doi.org/10.3390/tropicalmed8030145
Chicago/Turabian StyleVeiga, Zulane S. T., Flávia F. Fernandes, Lívia Guimarães, Juliana Piedade, and Gustavo Henrique S. Pereira. 2023. "Natural History of Hepatosplenic Schistosomiasis (HSS) Non–Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis" Tropical Medicine and Infectious Disease 8, no. 3: 145. https://doi.org/10.3390/tropicalmed8030145
APA StyleVeiga, Z. S. T., Fernandes, F. F., Guimarães, L., Piedade, J., & Pereira, G. H. S. (2023). Natural History of Hepatosplenic Schistosomiasis (HSS) Non–Cirrhotic Portal Hypertension (NCPH): Influence of Gastrointestinal Bleeding and Decompensation in Prognosis. Tropical Medicine and Infectious Disease, 8(3), 145. https://doi.org/10.3390/tropicalmed8030145







