QAC Resistance Genes in ESBL-Producing E. coli Isolated from Patients with Lower Respiratory Tract Infections in the Central Slovenia Region—A 21-Year Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Bacterial DNA Extraction and PCR Screening of QAC Resistance Genes
2.3. Statistical Analysis
3. Results
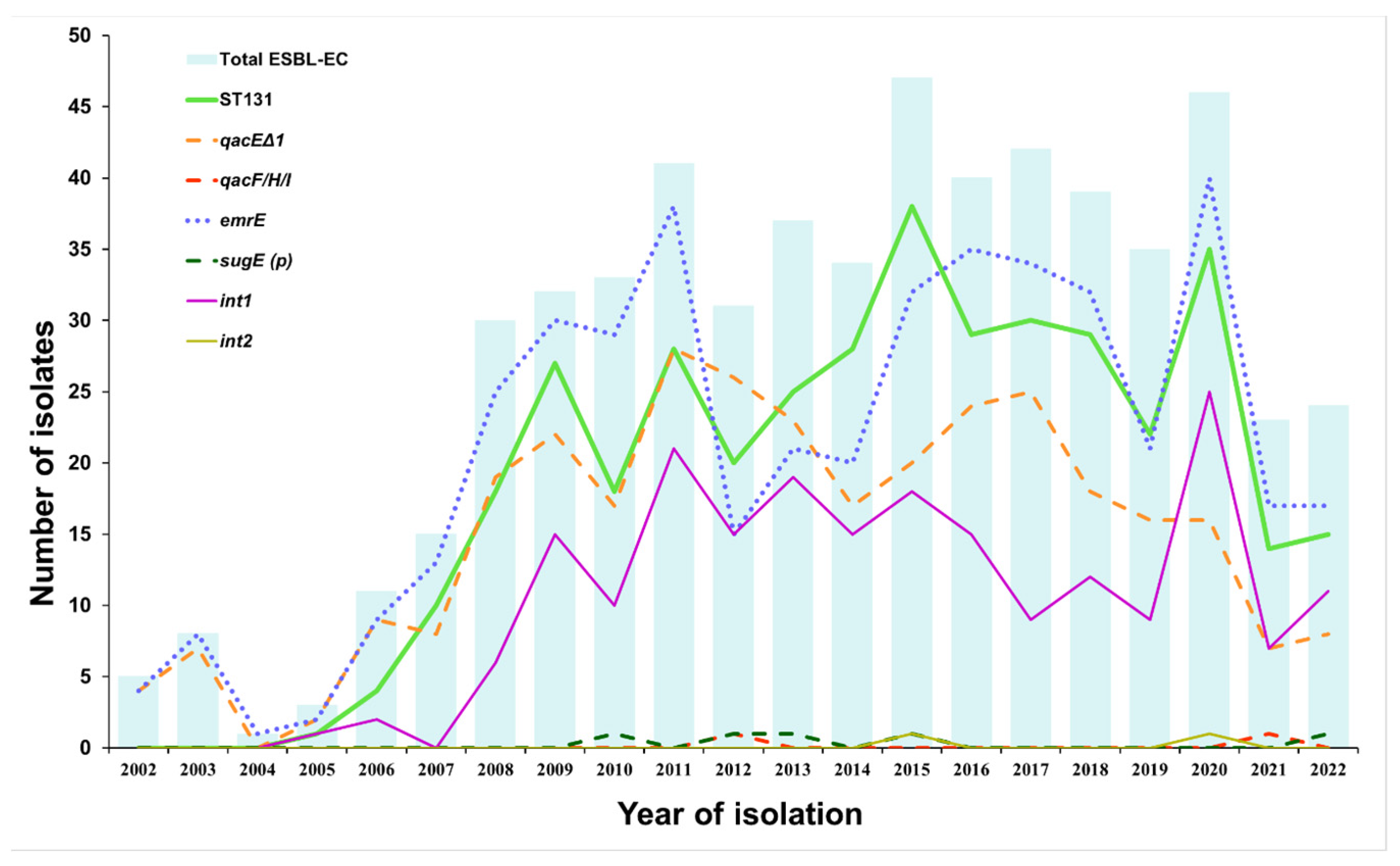
3.1. Prevalence of QAC Resistance Genes, Integrons, and ST131 Group in ESBL-EC Isolated from LRT Samples between 2002 and 2022
3.2. Correlation between QAC Resistance Genes, Integrons, Sequence Type Group ST131, and β-Lactamase Genes Detected in ESBL-EC Isolates from LRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Poolman, J.T.; Wacker, M. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J. Infect. Dis. 2016, 213, 6–13. [Google Scholar] [CrossRef]
- Rogers, B.A.; Sidjabat, H.E.; Paterson, D.L. Escherichia coli O25b-ST131: A pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 2011, 66, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Massella, E.; Giacometti, F.; Bonilauri, P.; Reid, C.J.; Djordjevic, S.P.; Merialdi, G.; Bacci, C.; Fiorentini, L.; Massi, P.; Bardasi, L.; et al. Antimicrobial Resistance Profile and ExPEC Virulence Potential in Commensal Escherichia coli of Multiple Sources. Antibiotics 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Biran, D.; Ron, E.Z. Extraintestinal Pathogenic Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog. Dis. 2007, 4, 134–163. [Google Scholar] [CrossRef]
- Pitout, J.D. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Fagerström, A.; Mölling, P.; Khan, F.A.; Sundqvist, M.; Jass, J.; Söderquist, B. Comparative distribution of extended-spectrum beta-lactamase-producing Escherichia coli from urine infections and environmental waters. PLoS ONE 2019, 14, e0224861. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef]
- Azargun, R.; Sadeghi, M.R.; Soroush Barhaghi, M.H.; Samadi Kafil, H.; Yeganeh, F.; Ahangar Oskouee, M.; Ghotaslou, R. The prevalence of plasmid-mediated quinolone resistance and ESBL-production in Enterobacteriaceae isolated from urinary tract infections. Infect. Drug Resist. 2018, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- García-Fulgueiras, V.; Bado, I.; Mota, M.I.; Robino, L.; Cordeiro, N.F.; Varela, A.; Algorta, G.; Gutkind, G.; Ayala, J.A.; Vignoli, R. Extended-spectrum β-lactamases and plasmid-mediated quinolone resistance in enterobacterial clinical isolates in the paediatric hospital of Uruguay. J. Antimicrob. Chemother. 2011, 66, 1725–1729. [Google Scholar] [CrossRef]
- Bado, I.; Gutiérrez, C.; García-Fulgueiras, V.; Cordeiro, N.F.; Araújo Pirez, L.; Seija, V.; Bazet, C.; Rieppi, G.; Vignoli, R. CTX-M-15 in combination with aac(6′)-Ib-cr is the most prevalent mechanism of resistance both in Escherichia coli and Klebsiella pneumoniae, including K. pneumoniae ST258, in an ICU in Uruguay. J. Glob. Antimicrob. Resist. 2016, 6, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Machuca, J.; Ortiz, M.; Recacha, E.; Diaz-De-Alba, P.; Docobo-Perez, F.; Rodriguez-Martinez, J.M.; Pascual, A. Impact of AAC(6′)-Ib-cr in combination with chromosomal-mediated mechanisms on clinical quinolone resistance in Escherichia coli. J. Antimicrob. Chemother. 2016, 71, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 15 April 2023).
- Chen, B.; Han, J.; Dai, H.; Jia, P. Biocide-tolerance and antibiotic-resistance in community environments and risk of direct transfers to humans: Unintended consequences of community-wide surface disinfecting during COVID-19? Environ. Pollut. 2021, 283, 117074. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar] [PubMed]
- Vincent, J.L. Nosocomial infections in adult intensive-care units. Lancet 2003, 361, 2068–2077. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Biocidal Resistance in Clinically Relevant Microbial Species: A Major Public Health Risk. Pathogens 2021, 10, 598. [Google Scholar] [CrossRef]
- Zou, L.; Meng, J.; McDermott, P.F.; Wang, F.; Yang, Q.; Cao, G.; Hoffmann, M.; Zhao, S. Presence of disinfectant resistance genes in Escherichia coli isolated from retail meats in the USA. J. Antimicrob. Chemother. 2014, 69, 2644–2649. [Google Scholar] [CrossRef]
- Maertens, H.; Demeyere, K.; De Reu, K.; Dewulf, J.; Vanhauteghem, D.; Van Coillie, E.; Meyer, E. Effect of subinhibitory exposure to quaternary ammonium compounds on the ciprofloxacin susceptibility of Escherichia coli strains in animal husbandry. BMC Microbiol. 2020, 20, 155. [Google Scholar] [CrossRef]
- Wand, M.E. Bacterial Resistance to Hospital Disinfection. In Modeling the Transmission and Prevention of Infectious Disease; Hurst, C.J., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–54. [Google Scholar]
- Mc Carlie, S.; Belter, B.; Van Der Walt, B.; Bragg, R. Molecular Tools for the Study of Resistance to Disinfectants. In The Global Antimicrobial Resistance Epidemic; Guillermo, T.-I., Ed.; IntechOpen: Rijeka, Croatia, 2022; p. 55. [Google Scholar]
- Roedel, A.; Vincze, S.; Projahn, M.; Roesler, U.; Robé, C.; Hammerl, J.A.; Noll, M.; Al Dahouk, S.; Dieckmann, R. Genetic but No Phenotypic Associations between Biocide Tolerance and Antibiotic Resistance in Escherichia coli from German Broiler Fattening Farms. Microorganisms 2021, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Versions 4.0 to 9.0. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 10 March 2023).
- EUCAST. Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0. pp. 1–43. Available online: http://www.eucast.org/resistance_mechanisms/ (accessed on 10 March 2023).
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef]
- Hansen, L.H.; Sørensen, S.J.; Jørgensen, H.S.; Jensen, L.B. The prevalence of the OqxAB multidrug efflux pump amongst olaquindox-resistant Escherichia coli in pigs. Microb. Drug Resist. 2005, 11, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Tian, Y.; Zhang, L.; Jiang, C.; Dong, D.; Li, Z.; Mao, E.; Peng, Y. Prevalence and quinolone resistance of fecal carriage of extended-spectrum β-lactamase-producing Escherichia coli in 6 communities and 2 physical examination center populations in Shanghai, China. Diagn. Microbiol. Infect. Dis. 2016, 86, 428–433. [Google Scholar] [CrossRef]
- Mazel, D.; Dychinco, B.; Webb, V.A.; Davies, J. Antibiotic resistance in the ECOR collection: Integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 2000, 44, 1568–1574. [Google Scholar] [CrossRef]
- Goldstein, C.; Lee, M.D.; Sanchez, S.; Hudson, C.; Phillips, B.; Register, B.; Grady, M.; Liebert, C.; Summers, A.O.; White, D.G.; et al. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob. Agents Chemother. 2001, 45, 723–726. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Disinfectant resistance in bacteria: Mechanisms, spread, and resolution strategies. Environ. Res. 2021, 195, 110897. [Google Scholar] [CrossRef]
- Zhang, A.; He, X.; Meng, Y.; Guo, L.; Long, M.; Yu, H.; Li, B.; Fan, L.; Liu, S.; Wang, H.; et al. Antibiotic and Disinfectant Resistance of Escherichia coli Isolated from Retail Meats in Sichuan, China. Microb. Drug Resist. 2016, 22, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Guo, D.; Shi, C.; Zhang, C.; Peng, X.; Yang, H.; Xia, X. Disinfectant Resistance Profiles and Biofilm Formation Capacity of Escherichia coli Isolated from Retail Chicken. Microb. Drug Resist. 2019, 25, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bengtsson-Palme, J.; Rensing, C.; Kristiansson, E.; Larsson, D.G. BacMet: Antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 2014, 42, D737–D743. [Google Scholar] [CrossRef]
- Sahin, S.; Mogulkoc, M.N.; Kürekci, C. Disinfectant and heavy metal resistance profiles in extended spectrum β-lactamase (ESBL) producing Escherichia coli isolates from chicken meat samples. Int. J. Food Microbiol. 2022, 377, 109831. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Sandle, T. A review on biocide reduced susceptibility due to plasmid-borne antiseptic-resistant genes-special notes on pharmaceutical environmental isolates. J. Appl. Microbiol. 2019, 126, 1011–1022. [Google Scholar] [CrossRef]
- Habibollah-Pourzereshki, N.; Peymani, A.; Keshavarz-Saleh, F. The Emergence of Quaternary Ammonium Compounds Resistance in Escherichia coli Isolated from Hospitals of Qazvin, Iran. Infect. Disord. Drug Targets 2020, 20, 455–460. [Google Scholar] [CrossRef]
- Hadadi, F.; Ghaznavirad, E.; Almasi-Hashiani, A.; Abtahi, H. Detection of qacEΔ1, qacG, qacE, qacF resistance genes in Escherichia coli producing broad-spectrum beta-lactamases to benzalkonium chloride. J. Babol Univ. Med. Sci. 2019, 21, 286–292. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Blok, H.E.M.; Donders, A.R.T.; Paauw, A.; Fluit, A.C.; Verhoef, J. Multidrug Resistance among Enterobacteriaceae Is Strongly Associated with the Presence of Integrons and Is Independent of Species or Isolate Origin. J. Infect. Dis. 2003, 187, 251–259. [Google Scholar] [CrossRef]
- Martinez-Freijo, P.; Fluit, A.C.; Schmitz, F.J.; Grek, V.S.; Verhoef, J.; Jones, M.E. Class I integrons in Gram-negative isolates from different European hospitals and association with decreased susceptibility to multiple antibiotic compounds. J. Antimicrob. Chemother. 1998, 42, 689–696. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, F.; Wang, F.; Wu, K.; Wang, Q.; Chen, Q.; Yu, S.; Rui, Y. Class 1 integrons in urinary isolates of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Southern China during the past five years. Microb. Drug Resist. 2013, 19, 289–294. [Google Scholar] [CrossRef]
- Deus, D.; Krischek, C.; Pfeifer, Y.; Sharifi, A.R.; Fiegen, U.; Reich, F.; Klein, G.; Kehrenberg, C. Comparative analysis of the susceptibility to biocides and heavy metals of extended-spectrum β-lactamase-producing Escherichia coli isolates of human and avian origin, Germany. Diagn. Microbiol. Infect. Dis. 2017, 88, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genom. 2015, 16, 964. [Google Scholar] [CrossRef] [PubMed]

| Target | Primer (Sequence 5′→3′) | Amplification Conditions | PCR Product Size (bp) | Reference |
|---|---|---|---|---|
| QAC resistance genes | ||||
| qacEΔ1 | qacEΔ1 F | 95 °C—5 min | 175 | [21] |
| (AATCCATCCCTGTCGGTGTT) | 94 °C—30 s | |||
| 56 °C—30 s 30× | ||||
| qacEΔ1 R | 72 °C—30 s | |||
| (CGCAGCGACTTCCACGATGGGGAT) | 72 °C—7 min | |||
| qacE | qacE F | 95 °C—5 min | 258 | |
| (AAGTAATCGCAACATCCG) | 94 °C—30 s | |||
| 50 °C—30 s 30× | ||||
| qacE R | 72 °C—30 s | |||
| (CTACTACACCACTAACTATGAG) | 72 °C—7 min | |||
| qacF/H/I | qacF/H/I F | 95 °C—5 min | 229 | |
| (GTCGTCGCAACTTCCGCACTG) | 94 °C—30 s | |||
| 60 °C—30 s 30× | ||||
| qacF/H/I R | 72 °C—30 s | |||
| (TGCCAACGAACGCCCACA) | 72 °C—7 min | |||
| qacG | qacG F ZOU | 95 °C—5 min | 122 | |
| (TCGCCTACGCAGTTTGGT) | 94 °C—30 s | |||
| 56 °C—30 s 30× | ||||
| qacG R | 72 °C—30 s | |||
| (AACGCCGCTGATAATGAA) | 72 °C—7 min | |||
| emrE | emrE F | 95 °C—5 min | 195 | |
| (TATTTATCTTGGTGGTGCAATAC) | 94 °C—30 s | |||
| 55 °C—30 s 30× | ||||
| emrE R | 72 °C—30 s | |||
| (ACAATACCGACTCCTGACCAG) | 72 °C—7 min | |||
| mdfA | mdfA F | 95 °C—5 min | 284 | |
| (GCATTGATTGGGTTCCTAC) | 94 °C—30 s | |||
| 55 °C—30 s 30× | ||||
| mdfA R | 72 °C—30 s | |||
| (CGCGGTGATCTTGATACA) | 72 °C—7 min | |||
| sugE (c) | sugE(c) F | 95 °C—5 min | 226 | |
| (CTGCTGGAAGTGGTATGGG) | 94 °C—30 s | |||
| 56 °C—30 s 30× | ||||
| sugE(c) R | 72 °C—30 s | |||
| (GCATCGGGTTAGCGGACT) | 72 °C—7 min | |||
| sugE (p) | sugE(p) F | 95 °C—5 min | 190 | |
| (GTCTTACGCCAAGCATTATCACTA) | 94 °C—30 s | |||
| 57 °C—30 s 30× | ||||
| sugE(p) R | 72 °C—30 s | |||
| (CAAGGCTCAGCAAACGTGC) | 72 °C—7 min | |||
| ydgE | ydgE F | 95 °C—5 min | 149 | |
| (GGCAATCGTGCTGGAAAT) | 94 °C—30 s | |||
| 55 °C—30 s 30× | ||||
| ydgE R | 72 °C—30 s | |||
| (CGACAGACAAGTCGATCCCT) | 72 °C—7 min | |||
| ydgF | ydgF F | 95 °C—5 min | 330 | |
| (TAGGTCTGGCTATTGCTACGG) | 94 °C—30 s | |||
| 55 °C—30 s 30× | ||||
| ydgF R | 72 °C—30 s | |||
| (GGTTCACCTCCAGTTCAGGT) | 72 °C—7 min | |||
| oqxA | oqxA F | 671 | [31] | |
| (GATCAGTCAGTGGGATAGTTT) | 95 °C—5 min | |||
| oqxA r | 95 °C—30 s | |||
| (TACTCGGCGTTAACTGATTA) | 55 °C—30 s 30× | |||
| oqxB | oqxBx F | 72 °C—1.5 min | 544 | [32] |
| (CCACCCTTAACTGATCCCTAA) | 72 °C—10 min | |||
| oqxBx r | ||||
| (CGCCAGCTCATCCTTCAC) | ||||
| Integrons | ||||
| int1 | IntI1-F | 95 °C—5 min | 483 | [33] |
| (GGTCAAGGATCTGGATTTCG) | 94 °C—30 s | |||
| 62 °C—30 s 30× | ||||
| IntI1-R | 72 °C—1 min | |||
| (ACATGCGTGTAAATCATCGTC) | 72 °C—7 min | |||
| int2 | IntI2-F | 95 °C—5 min | 789 | |
| (CACGGATATGCGACAAAAAGGT) | 94 °C—30 s | |||
| 62 °C—30 s 30× | ||||
| IntI2-R | 72 °C—1 min | |||
| (GTAGCAAACGAGTGACGAAATG) | 72 °C—7 min | |||
| int3 | IntI3-F | 95 °C—5 min | 600 | [34] |
| (AGTGGGTGGCGAATGAGTG) | 94 °C—30 s | |||
| 60 °C—30 s 30× | ||||
| IntI3-R | 72 °C—1 min | |||
| (TGTTCTTGTATCGGCAGGTG) | 72 °C—7 min | |||
| QAC Resistance Genes | Total ESBL | ST131 | Non-ST131 | Pearson Chi-Square Value | p-Value 1 |
|---|---|---|---|---|---|
| N = 577 (100%) | N = 388 (100%) | N = 189 (100%) | (df 1) | ||
| n (%) | n (%) | n (%) | |||
| MGE-encoded genes | |||||
| oqxA | 0 (0%) | 0 (0%) | 0 (0%) | ||
| oqxB | 0 (0%) | 0 (0%) | 0 (0%) | ||
| qacEΔ1 | 316 (54.6%) | 230 (59.1%) | 86 (45.3%) | 9.8 | 0.002 |
| qacE | 0 (0%) | 0 (0%) | 0 (0%) | ||
| qacF/H/I | 2 (0.3%) | 0 (0%) | 2 (1.1%) | 4.1 | 0.042 |
| qacG | 0 (0%) | 0 (0%) | 0 (0%) | ||
| sugE (p) | 5 (0.9%) | 0 (0%) | 5 (2.6%) | 10.4 | 0.001 |
| Chromosome-encoded genes | |||||
| emrE | 443 (76.9%) | 340 (87.7%) | 103 (54.7%) | 78.2 | <0.001 |
| mdfA | 577 (100%) | 388 (100%) | 189 (100%) | ||
| sugE (c) | 571 (99%) | 385 (99.2%) | 186 (98.4%) | 0.8 | 0.366 |
| ydgE | 577 (100%) | 388 (100%) | 189 (100%) | ||
| ydgF | 577 (100%) | 388 (100%) | 189 (100%) | ||
| Integrons | |||||
| int1 | 210 (36.3%) | 157 (40.4%) | 53 (27.9%) | 8.7 | 0.004 |
| int2 | 2 (0.3%) | 0 (0%) | 2 (1.1%) | 4.1 | 0.042 |
| int3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrovat, K.; Zupančič, J.Č.; Seme, K.; Avguštin, J.A. QAC Resistance Genes in ESBL-Producing E. coli Isolated from Patients with Lower Respiratory Tract Infections in the Central Slovenia Region—A 21-Year Survey. Trop. Med. Infect. Dis. 2023, 8, 273. https://doi.org/10.3390/tropicalmed8050273
Hrovat K, Zupančič JČ, Seme K, Avguštin JA. QAC Resistance Genes in ESBL-Producing E. coli Isolated from Patients with Lower Respiratory Tract Infections in the Central Slovenia Region—A 21-Year Survey. Tropical Medicine and Infectious Disease. 2023; 8(5):273. https://doi.org/10.3390/tropicalmed8050273
Chicago/Turabian StyleHrovat, Katja, Jerneja Čremožnik Zupančič, Katja Seme, and Jerneja Ambrožič Avguštin. 2023. "QAC Resistance Genes in ESBL-Producing E. coli Isolated from Patients with Lower Respiratory Tract Infections in the Central Slovenia Region—A 21-Year Survey" Tropical Medicine and Infectious Disease 8, no. 5: 273. https://doi.org/10.3390/tropicalmed8050273
APA StyleHrovat, K., Zupančič, J. Č., Seme, K., & Avguštin, J. A. (2023). QAC Resistance Genes in ESBL-Producing E. coli Isolated from Patients with Lower Respiratory Tract Infections in the Central Slovenia Region—A 21-Year Survey. Tropical Medicine and Infectious Disease, 8(5), 273. https://doi.org/10.3390/tropicalmed8050273






