Propofol Induces the Expression of Nrf2 and HO-1 in Echinococcus granulosus via the JNK and p38 Pathway In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug Assays
2.2. Parasite Sample Collection and Culture
2.3. Evaluation of Drug Treatment on PSCs of E. granulosus
2.4. ROS Levels Was Detected by Fluorescence Microscopy
2.5. Examinations about HO-1 Activity Assay
2.6. Western Blotting
2.7. Statistical Analyses
3. Results
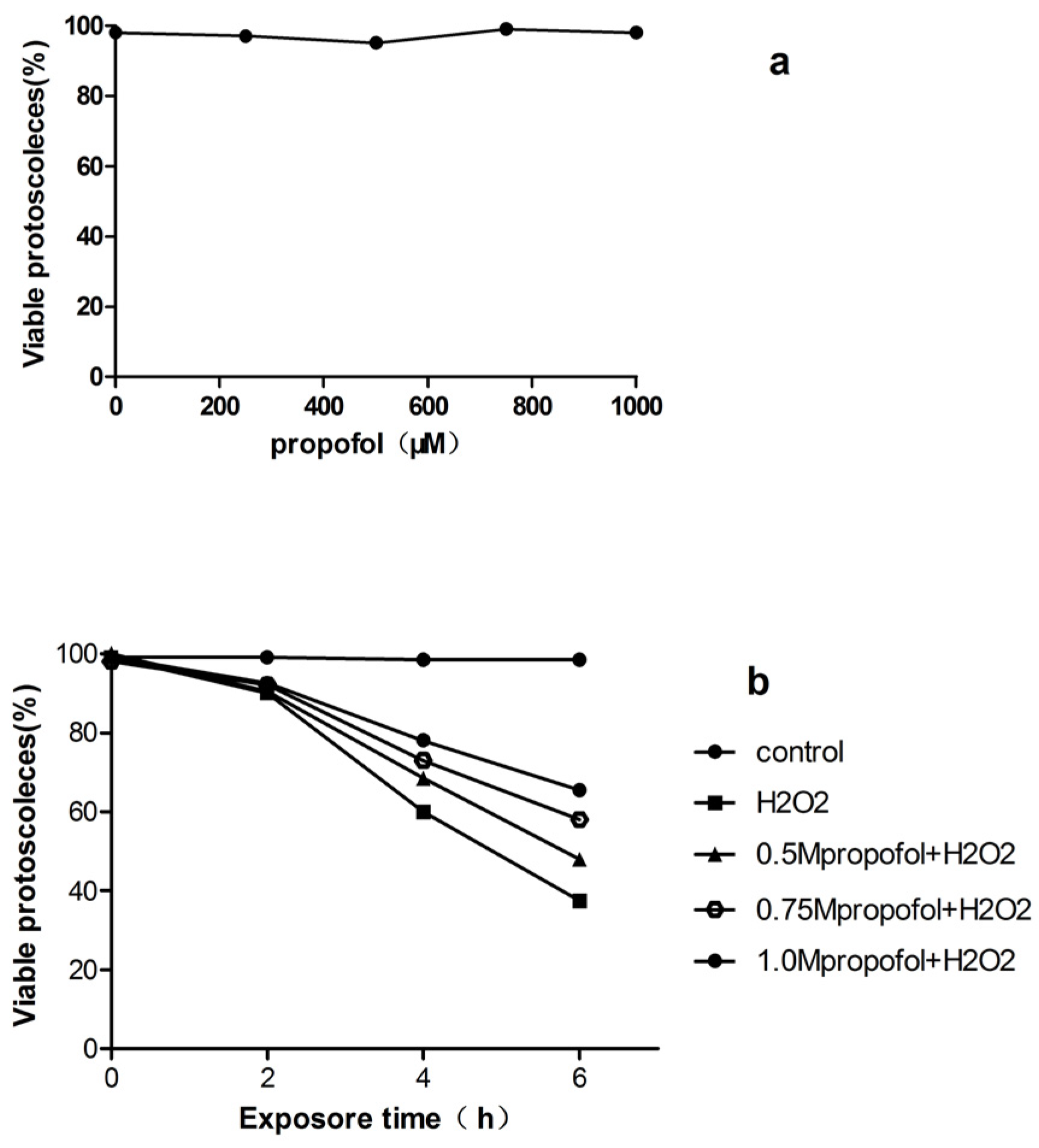
3.1. In Vitro Effects of H2O2 and Propofol on E. granulosus PSCs Viability
3.2. Effects of Propofol on H2O2-Induced BCL2 Expression in E. granulosus PSCs
3.3. Effects of H2O2, Propofol and MAPK Inhibitors on ROS in E. granulosus PSCs In Vitro
3.4. MAPK Signaling Pathways Participate in Propofol-induced Nrf2 and HO-1 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woolsey, I.D.; Miller, A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharafi, S.M.; Sefiddashti, R.R.; Sanei, B.; Yousefi, M.; Darani, H.Y. Scolicidal agents for protoscolices of Echinococcus granulosus hydatid cyst: Review of literature. J. Res. Med. Sci. 2017, 22, 92. [Google Scholar]
- Fakharzadeh, J.K.; Rafiei, A.; Rahdar, M.; Bahreini, A. Evaluation of the Protoscolicidal Effectiveness of Hypertonic Saline, Silver Nitrate, Ethanol, Using Sponge Pad Method and Injecting into Fertile Hydatid Cysts. Iran J. Parasitol. 2022, 17, 223–230. [Google Scholar]
- Mahmoudvand, H.; Saedi, D.E.; Sharififar, F.; Ezatpour, B.; Jahanbakhsh, S.; Fasihi, H.M. Protoscolecidal Effect of Berberis vulgaris Root Extract and Its Main Compound, Berberine in Cystic Echinococcosis. Iran J. Parasitol. 2014, 9, 503–510. [Google Scholar]
- Norouzi, R.; Ataei, A.; Hejazy, M.; Noreddin, A.; El, Z.M. Scolicidal Effects of Nanoparticles Against Hydatid Cyst Protoscolices in vitro. Int. J. Nanomed. 2020, 15, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Moghadaszadeh, M.; Khayyati, M.; Spotin, A.; Norouzi, R.; Pagheh, A.S.; Oliveira, S.M.; Pereira, M.D.L.; Ahmadpour, E. Scolicidal and Apoptotic Activities of 5-hydroxy-1, 4-naphthoquinone as a Potent Agent against Echinococcus granulosus Protoscoleces. Pharmaceuticals 2021, 14, 623. [Google Scholar] [CrossRef]
- Cancela, M.; Paes, J.A.; Moura, H.; Barr, J.R.; Zaha, A.; Ferreira, H.B. Unraveling oxidative stress response in the cestode parasite Echinococcus granulosus. Sci. Rep. 2019, 9, 15876. [Google Scholar] [CrossRef] [Green Version]
- Callahan, H.L.; Crouch, R.K.; James, E.R. Helminth anti-oxidant enzymes: A protective mechanism against host oxidants? Parasitol. Today 1988, 4, 218–225. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Pi, J.; Woods, C.G.; Andersen, M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010, 244, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Imamura, T.; Hiasa, Y. Roles of protein kinase R in cancer: Potential as a therapeutic target. Cancer Sci. 2018, 109, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Ellis, E.M.; Neal, G.E.; Harrison, D.J.; Manson, M.M. Cellular response to cancer chemopreventive agents: Contribution of the antioxidant responsive element to the adaptive response to oxidative and chemical stress. Biochem. Soc. Symp. 1999, 64, 141–168. [Google Scholar] [PubMed]
- Wang, Z.; Ka, S.O.; Lee, Y.; Park, B.H.; Bae, E.J. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur. J. Pharmacol. 2017, 799, 201–210. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, B.; Huang, H.; Qu, S.; Yang, S.; Zeng, Z. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz. J. Med. Biol. Res. 2018, 51, e7439. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Nishio, Y.; Chen, J.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhao, L.; et al. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016, 479, 663–669. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e141509. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, X.; Zhang, K.; Mao, X.; Ding, X.; Zeng, Q.; Bai, S.; Xuan, Y.; Peng, H. Vanadate oxidative and apoptotic effects are mediated by the MAPK-Nrf2 pathway in layer oviduct magnum epithelial cells. Metallomics 2017, 9, 1562–1575. [Google Scholar] [CrossRef]
- Wang, B.; Shravah, J.; Luo, H.; Raedschelders, K.; Chen, D.D.; Ansley, D.M. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem. Biophys. Res. Commun. 2009, 389, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.H.; Zhou, X.; Yang, X.Y.; Zhou, Z.; Lu, D.-H.; Tang, Y.; Ling, Z.-M.; Zhou, L.-H.; Feng, X. Propofol Protects Against H2O2-Induced Oxidative Injury in Differentiated PC12 Cells via Inhibition of Ca2+-Dependent NADPH Oxidase. Cell Mol. Neurobiol. 2016, 36, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chi, M.; Sun, X.; Wang, G.; Li, M.; Liu, L.; Li, X. Propofol-induced protection of SH-SY5Y cells against hydrogen peroxide is associated with the HO-1 via the ERK pathway. Int. J. Med. Sci. 2013, 10, 599–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Guan, D.; Ma, R.; Yang, R.; Xing, G.; Shi, H.; Tang, G.; Li, J.; Lv, H.; Jiang, Y. Effects of trigonelline inhibition of the Nrf2 transcription factor in vitro on Echinococcus granulosus. Acta Biochim. Biophys. Sin. 2017, 49, 696–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Li, S.; Zhang, J.; Liang, W.; Mu, X.; Jiang, Y. In vitro effects of SB202190 on Echinococcus granulosus. Korean J. Parasitol. 2013, 51, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Zhang, X.; Qi, J.; Yu, W.; Gu, Y. p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma cells against TMZ. Med. Oncol. 2015, 32, 69. [Google Scholar] [PubMed]
- Vari, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.W.; Yin, X.; Yuan, L.; Huan, S.Y.; Zhang, X.B. A cell membrane-anchored fluorescent probe for monitoring carbon monoxide release from living cells. Chem. Sci. 2019, 10, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, F.; Peng, W.; Zhang, J.; Dong, W.; Yuan, D.; Wang, Z.; Zheng, Y. Preincubation with a low-dose hydrogen peroxide enhances anti-oxidative stress ability of BMSCs. J. Orthop. Surg. Res. 2020, 15, 392. [Google Scholar]
- Chong, S.J.; Low, I.C.; Pervaiz, S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 2014, 19 Pt A, 39–48. [Google Scholar]
- Yoon, J.Y.; Baek, C.W.; Kim, E.J.; Park, B.-S.; Yu, S.-B.; Kim, E.-N. Propofol protects against oxidative-stress-induced COS-7 cell apoptosis by inducing autophagy. J. Dent. Anesth. Pain Med. 2017, 17, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, J.; Yang, J.; Zhao, B.; Shang, P. HO-1: A new potential therapeutic target to combat osteoporosis. Eur. J. Pharmacol. 2021, 906, 174219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wei, Y.; Qiu, S.; Xu, Y.; Zhang, T.; Zhang, S. Propofol Decreases Endoplasmic Reticulum Stress-Mediated Apoptosis in Retinal Pigment Epithelial Cells. PLoS ONE 2016, 11, e157590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Li, Y.; Xu, Z.; Li, W.; Fu, Z.; Wang, Y.; King, A.; Wei, H. Propofol Affects Neurodegeneration and Neurogenesis by Regulation of Autophagy via Effects on Intracellular Calcium Homeostasis. Anesthesiology 2017, 127, 490–501. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Yen, C.-J.; Chang, C.-C.; Huang, K.-T.; Chen, K.-H.; Zhang, R.-Y.; Lee, P.-Y.; Miaw, S.-C.; Huang, J.-W.; Chiang, C.-K.; et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim. Biophys. Acta 2015, 1850, 2506–2517. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Kim, Y.S.; Park, S.J.; Kim, B.I. Induction of Nrf2-mediated phase II detoxifying/antioxidant enzymes in vitro by chitosan-caffeic acid against hydrogen peroxide-induced hepatotoxicity through JNK/ERK pathway. Mol. Cell Biochem. 2017, 424, 79–86. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009, 4, e6588. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Ma, B.; Jiang, Y.; Lv, H. Propofol Induces the Expression of Nrf2 and HO-1 in Echinococcus granulosus via the JNK and p38 Pathway In Vitro. Trop. Med. Infect. Dis. 2023, 8, 306. https://doi.org/10.3390/tropicalmed8060306
Luo G, Ma B, Jiang Y, Lv H. Propofol Induces the Expression of Nrf2 and HO-1 in Echinococcus granulosus via the JNK and p38 Pathway In Vitro. Tropical Medicine and Infectious Disease. 2023; 8(6):306. https://doi.org/10.3390/tropicalmed8060306
Chicago/Turabian StyleLuo, Guangyi, Bin Ma, Yufeng Jiang, and Hailong Lv. 2023. "Propofol Induces the Expression of Nrf2 and HO-1 in Echinococcus granulosus via the JNK and p38 Pathway In Vitro" Tropical Medicine and Infectious Disease 8, no. 6: 306. https://doi.org/10.3390/tropicalmed8060306
APA StyleLuo, G., Ma, B., Jiang, Y., & Lv, H. (2023). Propofol Induces the Expression of Nrf2 and HO-1 in Echinococcus granulosus via the JNK and p38 Pathway In Vitro. Tropical Medicine and Infectious Disease, 8(6), 306. https://doi.org/10.3390/tropicalmed8060306







