Single-Center Experience of Control of Ventilator-Circuit-Transmitted Burkholderia cepacia Outbreak in an Intensive Care Unit
Abstract
:1. Background
2. Materials and Methods
2.1. Hospital Setting and Identification of B. cepacia
2.2. Active Surveillance and Discontinuation of Reusable Ventilator Circuits
2.3. Monitoring Infective State of Neighboring Beds (Spatial Effect)
2.4. Yearly Isolate Numbers of B. cepacia from Clinical Samples (Surveillance Cultures Not Included) and Nosocomial Infection Caused by B. cepacia
2.5. Statistical Analysis
3. Results
3.1. Secular Change of the Outbreak
3.2. Characteristics of Patients and Results of Active Surveillance
3.3. Intervention of Discontinuing Reusable Ventilator Circuits
3.4. Effects of Infective State of Neighboring Beds
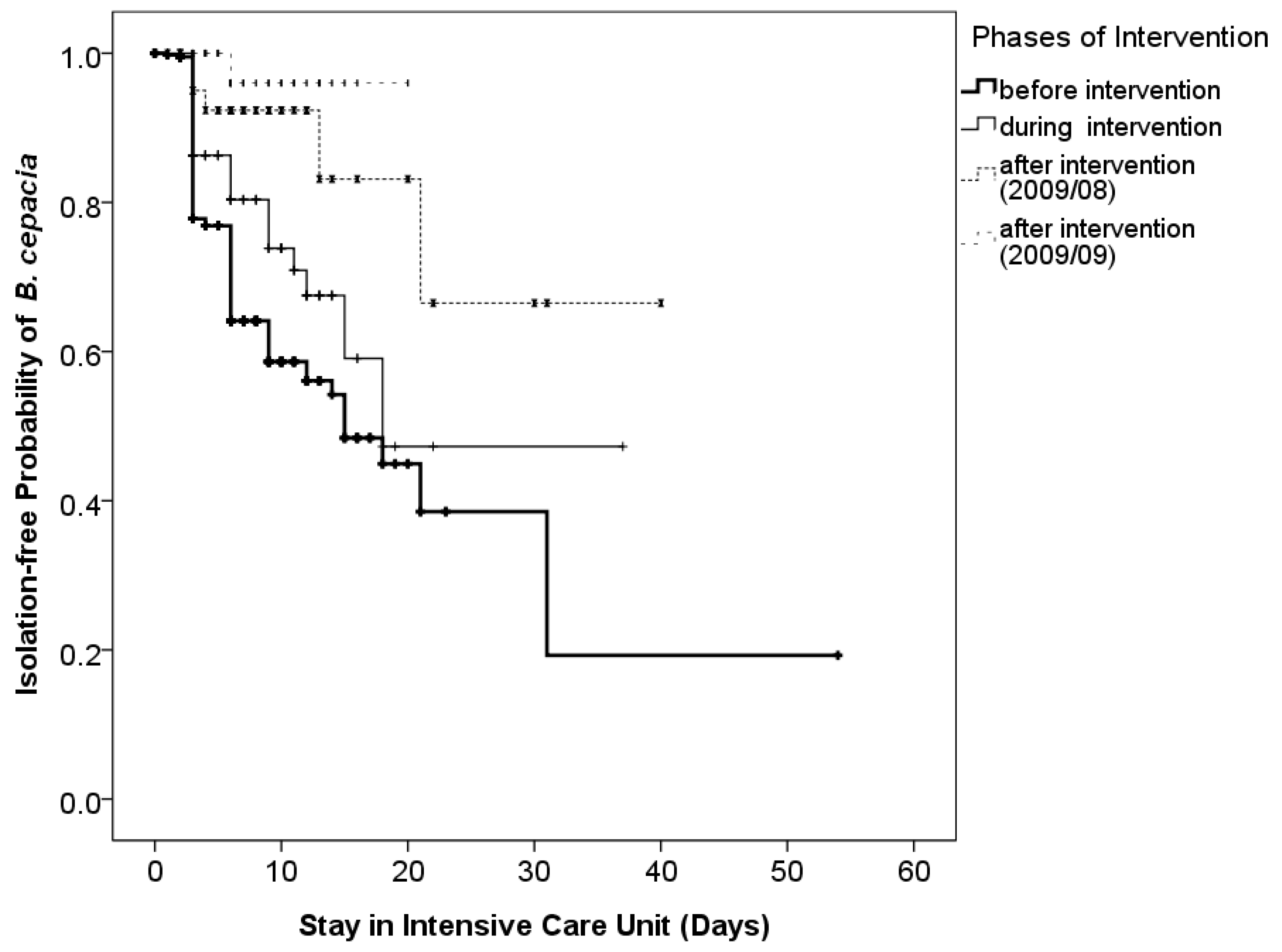
3.5. Effects of Discontinuing Reusable Ventilator Circuits
3.6. Follow-Up of Total Isolates of B. cepacia and Infection Caused by B. cepacia at FEMH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isles, A.; Maclusky, I.; Corey, M.; Gold, R.; Prober, C.; Fleming, P.; Levison, H. Pseudomonas cepacia infection in cystic fibrosis: An emerging problem. J. Pediatr. 1984, 104, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.R.; Hughes, J.E.; Vandamme, P. Burkholderia cepacia: Medical, taxonomic and ecological issues. J. Med. Microbiol. 1996, 45, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Hamill, R.J.; Houston, E.D.; Georghiou, P.R.; Wright, C.E.; Koza, M.A.; Cadle, R.M.; Goepfert, P.A.; Lewis, D.A.; Zenon, G.J.; Clarridge, J.E. An outbreak of Burkholderia (formerly Pseudomonas) cepacia respiratory tract colonization and infection associated with nebulized albuterol therapy. Ann. Intern. Med. 1995, 122, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Seelman, S.L.; Bazaco, M.C.; Wellman, A.; Hardy, C.; Fatica, M.K.; Huang, M.J.; Brown, A.M.; Garner, K.; Yang, W.C.; Norris, C.; et al. Burkholderia cepacia complex outbreak linked to a no-rinse cleansing foam product, United States—2017–2018. Epidemiol. Infect. 2022, 150, e154. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Baker, M.A.; Tucker, R.; Vaidya, V.; Holtzman, M.; Seethala, R.R.; Bentain-Melanson, M.; Lenox, J.; Smith, A.R.; Boyer, J.C.; et al. Cluster of Burkholderia cepacia Complex Infections Associated With Extracorporeal Membrane Oxygenation Water Heater Devices. Clin. Infect. Dis. 2022, 75, 1610–1617. [Google Scholar] [CrossRef]
- Bressler, A.M.; Kaye, K.S.; LiPuma, J.J.; Alexander, B.D.; Moore, C.M.; Reller, L.B.; Woods, C.W. Risk Factors for Burkholderia cepacian Complex Bacteremia Among Intensive Care Unit Patients Without Cystic Fibrosis: A Case-Control Study. Infect. Control Hosp. Epidemiol. 2007, 28, 951–958. [Google Scholar] [CrossRef]
- Häfliger, E.; Atkinson, A.; Marschall, J. Systematic review of healthcare-associated Burkholderia cepacia complex outbreaks: Presentation, causes and outbreak control. Infect. Prev. Pract. 2020, 2, 100082. [Google Scholar] [CrossRef]
- Shaban, R.Z.; Sotomayor-Castillo, C.; Nahidi, S.; Li, C.; Macbeth, D.; Mitchell, B.G.; Russo, P.L. Global burden, point sources, and outbreak management of healthcare-associated Burkholderia cepacia infections: An integrative review. Infect. Control Hosp. Epidemiol. 2020, 41, 777–783. [Google Scholar] [CrossRef]
- Hudson, M.J.; Park, S.C.; Mathers, A.; Parikh, H.; Glowicz, J.; Dar, D.; Nabili, M.; LiPuma, J.J.; Bumford, A.; Pettengill, M.A.; et al. Outbreak of Burkholderia stabilis infections associated with contaminated nonsterile, multiuse ultrasound gel-10 States, May-September 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1517–1521. [Google Scholar] [CrossRef]
- Liao, C.-H.; Chang, H.-T.; Lai, C.-C.; Huang, Y.-T.; Hsu, M.-S.; Liu, C.-Y.; Yang, C.-J.; Hsueh, P.-R. Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn. Microbiol. Infect. Dis. 2011, 70, 260–266. [Google Scholar] [CrossRef]
- Segonds, C.; Heulin, T.; Marty, N.; Chabanon, G. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J. Clin. Microbiol. 1999, 37, 2201–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, L.-J.; Hsueh, P.-R.; Pan, H.-J.; Ho, S.-W.; Luh, K.-T. Persistent Bacteraemia Caused by a Single Clone of Burkholderia cepacia with Unusual Phenotype. J. Infect. 2001, 42, 202–205. [Google Scholar] [CrossRef]
- Wang, J.-T.; Liao, C.-H.; Fang, C.-T.; Chie, W.-C.; Lai, M.-S.; Lauderdale, T.-L.; Chang, S.-C. Incidence of and Risk Factors for Community-Associated Methicillin-Resistant Staphylococcus aureus Acquired Infection or Colonization in Intensive-Care-Unit Patients. J. Clin. Microbiol. 2010, 48, 4439–4444. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Heo, S.; Kim, S.; Jeong, Y.; Bae, I.; Jin, J.; Lee, J. Hospital outbreak of Burkholderia stabilis bacteraemia related to contaminated chlorhexidine in haematological malignancy patients with indwelling catheters. J. Hosp. Infect. 2008, 70, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Estivariz, C.F.; Bhatti, L.I.; Pati, R.; Jensen, B.; Arduino, M.J.; Jernigan, D.; LiPuma, J.J.; Srinivasan, A. An Outbreak of Burkholderia cepacia Associated With Contamination of Albuterol and Nasal Spray. Chest 2006, 130, 1346–1353. [Google Scholar] [CrossRef]
- Martins, I.S.; Pellegrino, F.L.P.C.; Freitas, A.D.; Santos, M.D.S.; Ferraiuoli, G.I.D.; Vasques, M.R.G.; Amorim, E.L.T.; Oliveira, S.; Nouér, S.A.; Cardoso, F.L.L.; et al. Case-Crossover Study of Burkholderia cepacia Complex Bloodstream Infection Associated with Contaminated Intravenous Bromopride. Infect. Control Hosp. Epidemiol. 2010, 31, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; D’Angelo, M.T.; Sunenshine, R.; Noble-Wang, J.; Cope, J.; Jensen, B.; Srinivasan, A. Outbreak of Burkholderia cepacia Bloodstream Infection at an Outpatient Hematology and Oncology Practice. Infect. Control Hosp. Epidemiol. 2007, 28, 1311–1313. [Google Scholar] [CrossRef]
- Pegues, D.A.; Carson, L.A.; Anderson, R.L.; Norgard, M.J.; Argent, T.A.; Jarvis, W.R.; Woernle, C.H. Outbreak of Pseudomonas cepacia Bacteremia in Oncology Patients. Clin. Infect. Dis. 1993, 16, 407–411. [Google Scholar] [CrossRef]
- Kutty, P.K.; Moody, B.; Gullion, J.S.; Zervos, M.; Ajluni, M.; Washburn, R.; Sanderson, R.; Kainer, M.A.; Powell, T.A.; Clarke, C.F.; et al. Multistate Outbreak of Burkholderia cenocepacia Colonization and Infection Associated with the Use of Intrinsically Contaminated Alcohol-Free Mouthwash. Chest 2007, 132, 1825–1831. [Google Scholar] [CrossRef]
- Martin, M.; Christiansen, B.; Caspari, G.; Hogardt, M.; von Thomsen, A.; Ott, E.; Mattner, F. Hospital-wide outbreak of Burkholderia contaminans caused by prefabricated moist washcloths. J. Hosp. Infect. 2011, 77, 267–270. [Google Scholar] [CrossRef]
- Loukil, C.; Saizou, C.; Doit, C.; Bidet, P.; Mariani-Kurkdjian, P.; Aujard, Y.; Beaufils, F.; Bingen, E. Epidemiologic Investigation of Burkholderia cepacian Acquisition in Two Pediatric Intensive Care Units. Infect. Control Hosp. Epidemiol. 2003, 24, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Fomda, B.; Velayudhan, A.; Siromany, V.A.; Bashir, G.; Nazir, S.; Ali, A.; Katoch, O.; Karoung, A.; Gunjiyal, J.; Wani, N.; et al. An outbreak of Burkholderia cepacia bloodstream infections in a tertiary-care facility in northern India detected by a healthcare-associated infection surveillance network. Infect. Control Hosp. Epidemiol. 2022, 44, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Chuang, Y.-C.; Wang, J.-T.; Sheng, W.-H. Clinical characteristics and outcomes of non-cystic fibrosis patients with Burkholderia cepacia complex bacteremia at a medical center in Taiwan. J. Microbiol. Immunol. Infect. 2021, 55, 1301–1309. [Google Scholar] [CrossRef]
- Backman, C.; Taylor, G.; Sales, A.; Marck, P.B. An integrative review of infection prevention and control programs for multidrug-resistant organisms in acute care hospitals: A socio-ecological perspective. Am. J. Infect. Control. 2011, 39, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, D.; Maor, Y.; Keller, N.; Regev-Yochay, G.; Tal, I.; Shachar, D.; Zlotkin, A.; Smollan, G.; Rahav, G. Potential Role of Active Surveillance in the Control of a Hospital-Wide Outbreak of Carbapenem-Resistant Klebsiella pneumoniae Infection. Infect. Control Hosp. Epidemiol. 2010, 31, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.-C.; Huang, L.-M.; Lin, H.-C.; Chang, L.-Y.; Chen, M.-L.; Lu, C.-Y.; Lee, P.-I.; Chen, J.-M.; Lee, C.-Y.; Pan, H.-J.; et al. Control of an Outbreak of Pandrug-Resistant Acinetobacter baumannii Colonization and Infection in a Neonatal Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2007, 28, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, P.; Grattard, F.; Mahul, P.; Jospe, R.; Pozzetto, B.; Ros, A.; Gaudin, O.G.; Auboyer, C. Ventilator temperature sensors: An unusual source of Pseudomonas cepacia in nosocomial infection. J. Hosp. Infect. 1993, 25, 33–43. [Google Scholar] [CrossRef]
- Conly, J.M.; Klass, L.; Larson, L.; Kennedy, J.; Low, D.E.; Harding, G.K. Pseudomonas cepacia colonization and infection in intensive care units. Can. Med Assoc. J. 1986, 134, 363–366. [Google Scholar]
- Weems, J.J. Nosocomial outbreak of Pseudomonas cepacia associated with contamination of reusable electronic ventilator temperature probes. Infect. Control Hosp. Epidemiol. 1993, 14, 583–586. [Google Scholar]
- Hartstein, A.I.; Rashad, A.L.; Liebler, J.M.; Actis, L.A.; Freeman, J.; Rourke, J.W., Jr.; Stibolt, T.B.; Tolmasky, M.E.; Ellis, G.R.; Crosa, J.H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am. J. Med. 1988, 85, 624–631. [Google Scholar] [CrossRef]
- Jagtap, S.R.; Pantvaidya, S.H.; Golam, K.K.; Gogate, A.S. The sterilization of ventilatory circuit. J. Postgrad. Med. 1988, 34, 29–33. [Google Scholar] [PubMed]
- Das, I.; Fraise, A.P. How useful are microbial filters in respiratory apparatus? J. Hosp. Infect. 1997, 37, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K. Two outbreaks of Burkholderia cepacia nosocomial infection in a neonatal intensive care unit. J. Paediatr. Child Health 2007, 44, 62–66. [Google Scholar] [CrossRef]
- Hsu, M.-S.; Wu, M.-Y.; Huang, Y.-T.; Liao, C.-H. Efficacy of chlorine dioxide disinfection to non-fermentative Gram-negative bacilli and non-tuberculous mycobacteria in a hospital water system. J. Hosp. Infect. 2016, 93, 22–28. [Google Scholar] [CrossRef] [PubMed]



| Variable | Results of Serial Sputum Cultures | Total (%) | p Value | ||
|---|---|---|---|---|---|
| All Negative (%) | All Positive (%) | Converting to Positive (%) | |||
| Duration of Stay (days) | |||||
| 1–10 | 386 (78.8) | 37 (7.6) | 66 (13.5) | 489 (100) | <0.01 |
| 11–20 | 76 (47.2) | 15 (9.3) | 70 (43.5) | 161 (100) | |
| >20 | 11 (28.2) | 2 (5.1) | 26 (66.7) | 39 (100) | |
| Date of Admission | |||||
| September 2008–May 2009 | 298 (60.3) | 49 (10.2) | 132 (27.5) | 479 (100) | <0.01 |
| June 2009–July 2009 * | 78 (73.6) | 4 (3.8) | 24 (22.6) | 106 (100) | |
| August 2009–September 2009 † | 97 (93.3) | 1 (1.0) | 6 (5.8) | 104 (100) | |
| Chamber of Stay | |||||
| Anterior Room | 253 (72.7) | 23 (6.6) | 72 (20.7) | 348 (100) | 0.07 |
| Posterior Room | 220 (64.5) | 31 (9.1) | 90 (26.4) | 341 (100) | |
| Total | 473 (68.7) | 54 (7.8) | 162 (23.5) | 689 (100) | |
| Variable | Intervention Stages | Total (%) | p Value | ||
|---|---|---|---|---|---|
| Pre-Intervention (%) | Partial Intervention (%) | Complete Intervention (%) | |||
| Duration of Stay (days) | |||||
| 1–10 | 342 (70.0) | 71 (14.5) | 76 (15.5) | 489 (100) | 0.84 |
| 11–20 | 111 (68.9) | 27 (16.8) | 23 (14.3) | 161 (100) | |
| >20 | 26 (66.7) | 8 (20.5) | 5 (12.8) | 39 (100) | |
| Chamber of Stay | |||||
| Anterior Room | 246 (70.7) | 53 (15.2) | 49 (14.1) | 348 (100) | 0.73 |
| Posterior Room | 233 (68.3) | 53 (15.5) | 55 (16.1) | 341 (100) | |
| Total | 479 (69.5) | 106 (15.4) | 104 (15.1) | 689 (100) | |
| Duration of Stay (Days) | B. cepacia (+) | B. cepacia (−) | Odds Ratio | p Value | ||
|---|---|---|---|---|---|---|
| Neighboring Bed (+) | Neighboring Bed (−) | Neighboring Bed (+) | Neighboring Bed (−) | |||
| 1–10 | 88 | 15 | 258 | 128 | 2.90 | <0.01 |
| 11–20 | 68 | 17 | 46 | 30 | 2.59 | <0.01 |
| 21–30 | 19 | 1 | 4 | 3 | 12.39 | 0.04 |
| Total | 175 | 33 | 308 | 161 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.-J.; Wang, J.-T.; Chang, H.-T.; Chang, S.-C.; Liao, C.-H. Single-Center Experience of Control of Ventilator-Circuit-Transmitted Burkholderia cepacia Outbreak in an Intensive Care Unit. Trop. Med. Infect. Dis. 2023, 8, 335. https://doi.org/10.3390/tropicalmed8070335
Shen B-J, Wang J-T, Chang H-T, Chang S-C, Liao C-H. Single-Center Experience of Control of Ventilator-Circuit-Transmitted Burkholderia cepacia Outbreak in an Intensive Care Unit. Tropical Medicine and Infectious Disease. 2023; 8(7):335. https://doi.org/10.3390/tropicalmed8070335
Chicago/Turabian StyleShen, Bing-Jie, Jann-Tay Wang, Hou-Tai Chang, Shan-Chwen Chang, and Chun-Hsing Liao. 2023. "Single-Center Experience of Control of Ventilator-Circuit-Transmitted Burkholderia cepacia Outbreak in an Intensive Care Unit" Tropical Medicine and Infectious Disease 8, no. 7: 335. https://doi.org/10.3390/tropicalmed8070335






