What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide
Abstract
:1. Introduction
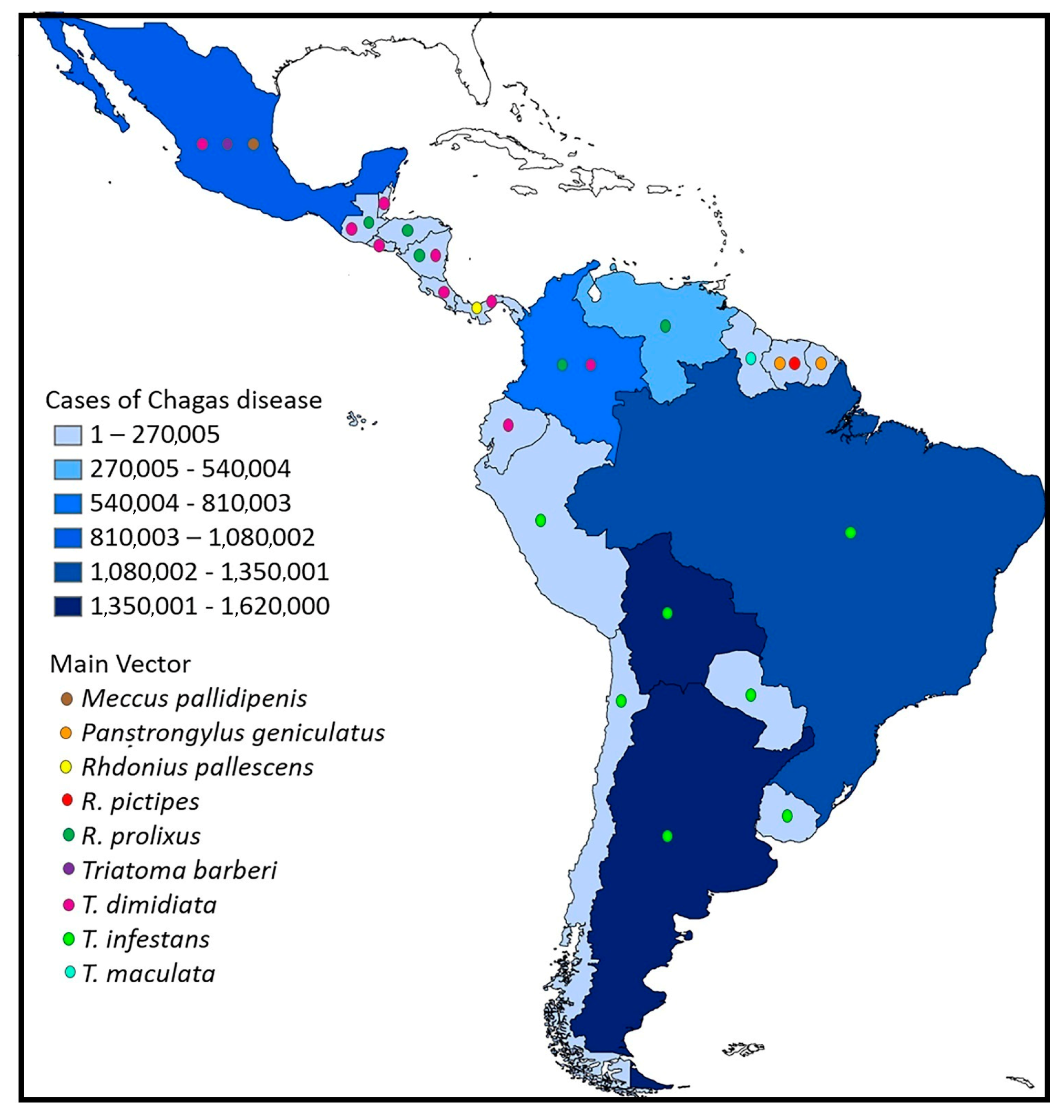
2. A Brief History of Chagas Disease and the Current Situation
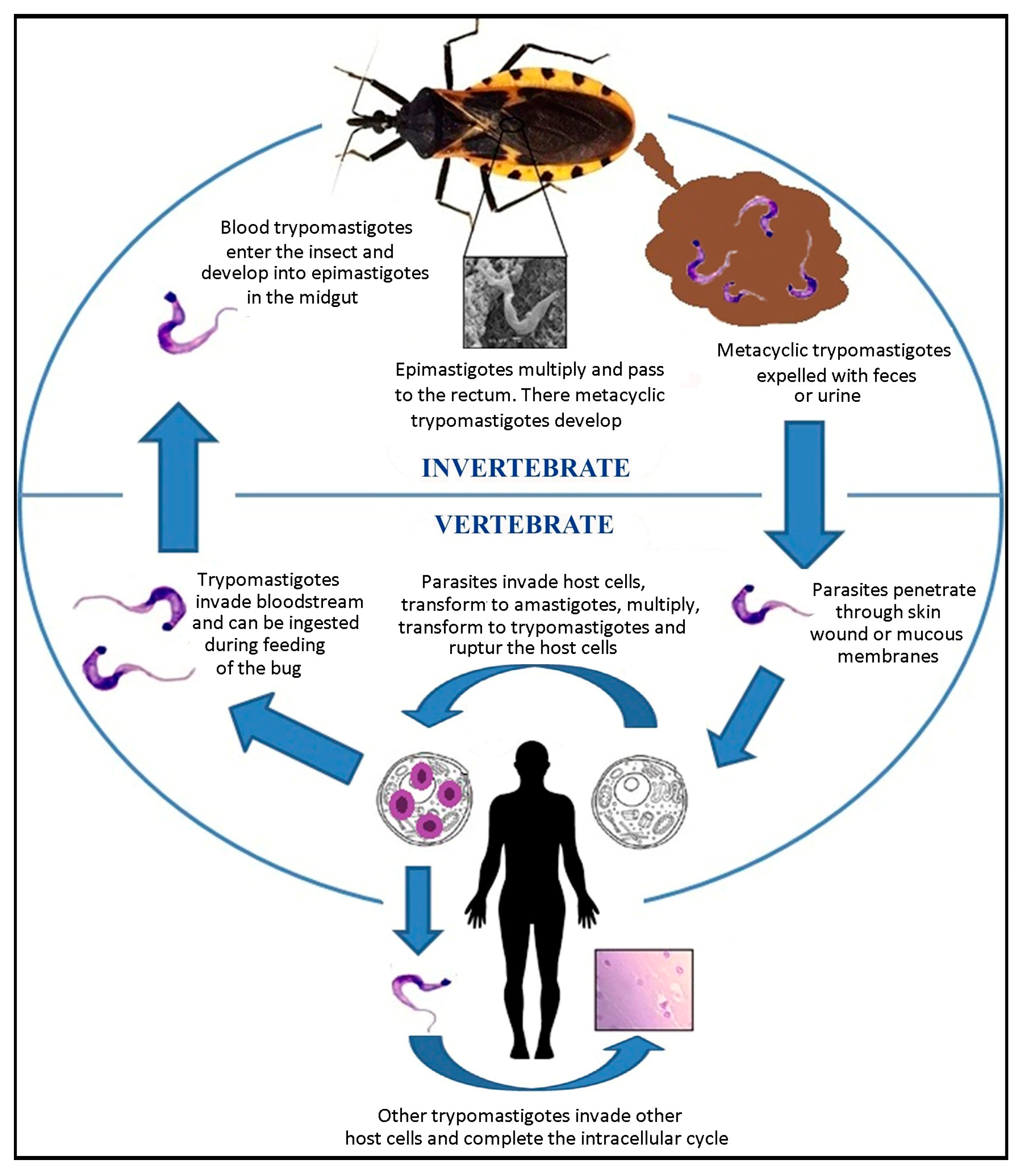
3. Life Cycle of Trypanosoma cruzi
4. Generalities of Trypanosoma cruzi
5. Origin and Genetic Variation of Trypanosoma cruzi
6. Vector Insects (Hemiptera, Reduviidae, and Triatominae)
7. Distribution and Richness of Triatomines
8. Overview of the Ecology of Triatomine Bugs
9. T. cruzi Reservoirs
10. Clinical Forms and Diagnosis of Chagas Disease
11. Immunopathogenesis of Chagas Disease
12. Final Comments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cholewiński, M.; Derda, M.; Hadás, E. Parasitic diseases in humans transmitted by vectors. Ann. Parasitol. 2015, 61, 137–157. [Google Scholar] [PubMed]
- WHO. Vector-Bornse Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases#:~:text=Vector%2Dborne%20diseases%20account%20for,infection%20transmitted%20by%20Anopheline%20mosquitoes (accessed on 11 April 2022).
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front. Public Health 2021, 5, 715759. [Google Scholar] [CrossRef] [PubMed]
- Guhl, F.; Ramírez, J.D. Poverty, migration, and Chagas disease. Curr. Trop. Med. Rep. 2021, 8, 52–58. [Google Scholar] [CrossRef]
- Messenger, L.A.; Miles, M.A.; Bern, C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert. Rev. Anti-Infect. Ther. 2015, 13, 995–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagas, C. Nova tripanosomiase humana. Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., sp., agente etiologico de nova entidade morbida do homen. Mem. Inst. Oswaldo Cruz 1909, 1, 158–218. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, B.; Solorzano-Domínguez, N.; Vizcaino-Castillo, A.; Martínez, I.; Elias-López, A.L.; Rodríguez-Martínez, J.A. Gastrointestinal infection with Mexican TcI Trypanosoma cruzi strains: Different degrees of colonization and diverse immune responses. Int. J. Biol. Sci. 2011, 7, 1357–1370. [Google Scholar] [CrossRef] [Green Version]
- De Fuentes-Vicente, J.A.; Cabrera-Bravo, M.; Enríquez-Vara, J.N.; Bucio-Torres, M.I.; Gutiérrez-Cabrera, A.E.; Vidal-López, D.G.; Martínez-Ibarra, J.A.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. Relationships between altitude, triatomine (Triatoma dimidiata) immune response and virulence of Trypanosoma cruzi, the causal agent of Chagas’ disease. Med. Vet. Entomol. 2017, 3, 63–71. [Google Scholar] [CrossRef]
- De Fuentes-Vicente, J.A.; Vidal-López, D.G.; Flores-Villegas, A.L.; Moreno-Rodríguez, A.; De Alba-Alvarado, M.C.; Salazar-Schettino, P.M.; Rodríguez-López, M.H.; Gutiérrez-Cabrera, A.E. Trypanosoma cruzi: A review of biological and methodological factors in Mexican strains. Acta Trop. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- WHO. World Chagas Disease Day 2023-Time to Integrate Chagas Disease into Primary Health Care. Available online: https://www.who.int/campaigns/world-chagas-disease-day/2023 (accessed on 11 April 2023).
- Noireau, F.; Diosque, P.; Jansen, A.M. Trypanosoma cruzi: Adaptation to its vectors and its hosts. Vet. Res. 2009, 40, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef]
- Antinori, S.; Corbellino, M. Chagas disease in Europe: A long way to go. Eur. J. Intern. Med. 2018, 4, e29–e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avaria, A.; Ventura-Garcia, L.; Sanmartino, M.; Van der Laat, C. Population movements, borders, and Chagas disease. Mem. Inst. Oswaldo Cruz 2022, 8, e210151. [Google Scholar] [CrossRef] [PubMed]
- De Fuentes-Vicente, J.A.; Gutiérrez-Cabrera, A.E.; Flores-Villegas, A.L.; Lowenberger, C.; Benelli, G.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A. What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop. 2018, 183, 23–31. [Google Scholar] [CrossRef]
- Sales-Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and clinical treatment of Chagas Disease: A review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Huertas, P.; Cardona-Castro, N. Advances in the treatment of Chagas disease: Promising new drugs, plants and targets. Biomed. Pharmacother. 2021, 142, 112020. [Google Scholar] [CrossRef]
- Adasme, M.F.; Bolz, S.N.; Adelmann, L.; Salentin, S.; Haupt, V.J.; Moreno-Rodríguez, A.; Nogueda-Torres, B.; Castillo-Campos, V.; Yepez-Mulia, L.; De Fuentes-Vicente, J.A.; et al. Repositioned drugs for Chagas disease unveiled via structure-based drug repositioning. Int. J. Mol. Sci. 2020, 20, 8809. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, S.M.; Avelis, C.M.; Asti, L.; Hertenstein, D.L.; Ndeffo-Mbah, M.; Galvani, A.; Lee, B.Y. The economic value of identifying and treating Chagas disease patients earlier and the impact on Trypanosoma cruzi transmission. PLoS Negl. Trop. Dis. 2018, 12, e0006809. [Google Scholar] [CrossRef]
- Ramsey, J.M.; Elizondo-Cano, M.; Sanchez-González, G.; Peña-Nieves, A.; Figueroa-Lara, A. Opportunity cost for early treatment of Chagas disease in Mexico. PLoS Negl. Trop. Dis. 2014, 17, e2776. [Google Scholar] [CrossRef] [PubMed]
- Stillwaggon, E.; Perez-Zetune, V.; Bialek, S.R.; Montgomery, S.P. Congenital Chagas disease in the United States: Cost savings through maternal screening. Am. J. Trop. Med. Hyg. 2018, 8, 1733–1742. [Google Scholar] [CrossRef] [Green Version]
- Onyekwelu, C. Life cycle of Trypanosoma cruzi in the invertebrate and the vertebrate hosts. In Biology of Trypanosoma cruzi, 1st ed.; De Souza, W., Ed.; IntechOpen: London, UK, 2019; Volume 1, pp. 1–19. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, C.S.; Ávila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasit. Vectors 2018, 6, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onofre, T.S.; Rodrigues, J.P.F.; Shio, M.T.; Macedo, S.; Juliano, M.A.; Yoshida, N. Interaction of Trypanosoma cruzi Gp82 with host cell LAMP2 induces protein kinase C activation and promotes invasion. Front. Cell. Infect. Microbiol. 2021, 12, 627888. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.M.; Balouz, V.; Centeno, C.; Cori, C.R.; Kashiwagi, G.A.; Gil, S.A.; Macchiaverna, N.P.; Cardinal, M.V.; Guaimas, F.; Lobo, M.; et al. Trypanosoma cruzi surface mucins are involved in the attachment to the Triatoma infestans rectal ampoule. PLoS Negl. Trop. Dis. 2019, 13, e0007418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworak, E.S.; Araújo, S.M.; Gomes, M.L.; Massago, M.; Ferreira, É.; Toledo, M.J. Sympatry influence in the interaction of Trypanosoma cruzi with triatomine. Rev. Soc. Bras. Med. Trop. 2017, 50, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroga, T.B.D.; Pereira, N.S.; da Silva, D.D.; Andrade, C.M.; de Araújo Júnior, R.F.; Brito, C.R.D.N.; Galvão, L.M.D.C.; da Câmara, A.C.J.; Nascimento, M.S.L.; Guedes, P.M.M. Virulence of Trypanosoma cruzi strains is related to the differential expression of innate immune receptors in the heart. Front. Cell. Infect. Microbiol. 2021, 15, 696719. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions—A comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Kemmerling, U.; Bosco, C.; Galanti, N. Infection and invasion mechanisms of Trypanosoma cruzi in the congenital transmission of Chagas’ disease: A proposal. Biol. Res. 2010, 43, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Noya, B.A.; Díaz-Bello, Z.; Colmenares, C.; Ruiz-Guevara, R.; Mauriello, L.; Muñoz-Calderón, A.; Noya, O. Update on oral Chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem. Inst. Oswaldo Cruz 2015, 110, 377–386. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A. Emerging and reemerging forms of Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz 2022, 16, e210033. [Google Scholar] [CrossRef]
- Beiyu, L.; Yanan, L.; Shawn, A.; Motyka, E.; Agbo, P.; Englund, T. Fellowship of the rings: The replication of kinetoplast DNA. Trends Parasitol. 2005, 21, 363–369. [Google Scholar] [CrossRef]
- Docampo, R.; Moreno, S.N.J. Biochemistry of Trypanosoma cruzi. In American Trypanosomiasis Chagas Disease, 1st ed.; Telleria, J., Tybairenc, M., Eds.; Elsevier: London, UK, 2010; Volume 1, pp. 365–392. [Google Scholar] [CrossRef]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Gironès, N.; Fresno, M. Trypanosoma cruzi genome: Organization, multi-gene families, transcription, and biological implications. Genes 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.M.; da Costa, K.M.; Chaves, V.S.; Freire-de-Lima, C.G.; Morrot, A.; Mendonça-Previato, L.; Previato, J.O.; Freire-de-Lima, L. Theft and reception of host cell’s sialic acid: Dynamics of Trypanosoma cruzi trans-sialidases and mucin-like molecules on Chagas’ disease immunomodulation. Front. Immunol. 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire-de-Lima, L.; Fonseca, L.M.; Oeltmann, T.; Mendonça-Previato, L.; Previato, J.O. The trans-sialidase, the major Trypanosoma cruzi virulence factor: Three decades of studies. Glycobiology 2015, 25, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Toloza, G.; Sosoniuk-Roche, E.; Valck, C.; Aguilar-Guzmán, L.; Ferreira, V.P.; Ferreira, A. Trypanosoma cruzi calreticulin: Immune evasion, infectivity, and tumorigenesis. Trends Parasitol. 2020, 36, 368–381. [Google Scholar] [CrossRef] [Green Version]
- Pech-Canul, Á.C.; Monteón, V.; Solís-Oviedo, R.L. A brief view of the surface membrane proteins from Trypanosoma cruzi. J. Parasitol. Res. 2017, 2017, 3751403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreros-Cabello, A.; Callejas-Hernández, F.; Fresno, M.; Gironès, N. Comparative proteomic analysis of trypomastigotes from Trypanosoma cruzi strains with different pathogenicity. Infec. Genet. Evol. 2019, 76, 104041. [Google Scholar] [CrossRef]
- Grisard, E.C. Salivaria or stercoraria? The Trypanosoma rangeli dilemma. Kinetoplastid Biol. Dis. 2002, 1, 5. [Google Scholar] [CrossRef]
- Suárez-Quevedo, Y.; Barbosa-Vinasco, H.J.; Gutiérrez-Garnizo, S.A.; Olaya-Morales, J.L.; Zabala-González, D.; Carranza-Martínez, J.C.; Guhl-Nannetti, F.; Cantillo-Barraza, O.; Vallejo, G.A. Factores tripanolíticos innatos contra Trypanosoma rangeli y T. cruzi en la hemolinfa de triatominos: Un estudio comparativo en ocho especies de vectores de la enfermedad de Chagas. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2020, 44, 88–104. [Google Scholar] [CrossRef]
- Díaz, S.; Villavicencio, B.; Correia, N.; Costa, J.; Haag, K.L. Triatomine bugs, their microbiota and Trypanosoma cruzi: Asymmetric responses of bacteria to an infected blood meal. Parasit. Vectors 2016, 9, 636. [Google Scholar] [CrossRef] [Green Version]
- Teotônio, I.M.S.N.; Dias, N.; Hagström-Bex, L.; Nitz, N.; Francisco, A.F.; Hecht, M. Intestinal microbiota—A modulator of the Trypanosoma cruzi-vector-host triad. Microb. Pathog. 2019, 137, 103711. [Google Scholar] [CrossRef]
- Genes, C.; Baquero, E.; Echeverri, F.; Maya, J.; Triana, O. Mitochondrial dysfunction in Trypanosoma cruzi: The role of Serratia marcescens prodigiosin in the alternative treatment of Chagas disease. Parasit. Vectors 2011, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, I.; Fieck, A.; Read, A.; Hillesland, H.; Klein, N.; Kang, A.; Durvasula, R. Paratransgenic control of vector borne diseases. Int. J. Biol. Sci. 2011, 7, 1334–1344. [Google Scholar] [CrossRef]
- Lopez-Ordoñez, T.; Flores-López, C.A.; Montejo-Lopez, R.; Cruz-Hernandez, A.; Conners, E.E. Cultivable bacterial diversity in the gut of the Chagas disease vector Triatoma dimidiata: Identification of possible bacterial candidates for a paratransgenesis approach. Front. Ecol. Evol. 2018, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Silva, E.; Morais, L.H.; Clarke, G.; Savino, W.; Peixoto, C. Targeting the gut microbiota in Chagas disease: What do we know so far? Front. Microbiol. 2020, 11, 585857. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Noyes, H.A.; Schofield, C.J.; Gibson, W. The molecular evolution of Trypanosomatidae. Adv. Parasitol. 2001, 48, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Teixeira, M.M.; Stevens, J.R. The evolution of Trypanosoma cruzi: The ’bat seeding’ hypothesis. Trends Parasitol. 2012, 28, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of Chagas disease. Parasit. Vectors 2014, 10, 317. [Google Scholar] [CrossRef]
- Tomasini, N.; Diosque, P. Evolution of Trypanosoma cruzi: Clarifying hybridisations, mitochondrial introgressions and phylogenetic relationships between major lineages. Mem. Inst. Oswaldo Cruz 2015, 110, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.L. An evolutionary view of Trypanosoma cruzi telomeres. Front. Cell. Infect. Microbiol. 2020, 10, 439. [Google Scholar] [CrossRef]
- Schofield, C. Trypanosoma cruzi-the vector-parasite paradox. Mem. Inst. Oswaldo Cruz 2000, 95, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Souto, R.; Fernandes, O.; Macedo, A.; Campbell, D.; Zingales, B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996, 83, 141–152. [Google Scholar] [CrossRef]
- Brisse, S.; Barnabé, C.; Tibayrenc, M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int. J. Parasitol. 2000, 30, 35–44. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Campbell, D.A.; Tibayrenc, M.; Macedo, A.M.; Teixeira, M.M.; Schijman, A.G.; Llewellyn, M.S.; Lages-Silva, E.; Machado, C.R.; et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. 2012, 12, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef]
- Otálora-Luna, F.; Pérez-Sánchez, A.J.; Sandoval, C.; Aldana, E. Evolution of hematophagous habit in Triatominae (Heteroptera: Reduviidae). Rev. Chil. de His. Nat. 2015, 88, 4. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Albiter, H.M.; Ferreira, T.N.; Costa, S.G.; Rivas, G.B.; Gumiel, M.; Cavalcante, D.R.; Genta, F.A. Everybody loves sugar: First report of plant feeding in triatomines. Parasit. Vectors 2016, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo-Porras, N.; Umaña-Diaz, C.; de Oliveira Barbosa Bitencourt, R.; Lowenberger, C. The role of bacterial symbionts in triatomines: An evolutionary perspective. Microorganisms 2020, 8, 1438. [Google Scholar] [CrossRef]
- De la Cruz Pantoja, M.M. Influencia de Endosimbiontes Sobre el Desarrollo de Triatoma barberi y la Colonización de Trypanosoma cruzi. Master’s Thesis, Universidad Autónoma del Estado de Hidalgo, Hidalgo, Mexico, 2013. Available online: http://dgsa.uaeh.edu.mx:8080/bibliotecadigital/bitstream/handle/231104/1874/TESIS%20FINAL.pdf?sequence=1&isAllowed= (accessed on 23 April 2022).
- Téllez–García, A.A.; Bello-Bedoy, R.; Enríquez-Vara, J.N.; Córdoba–Aguilar, A.; Gutiérrez-Cabrera, A.E. Genital morphology and copulatory behavior in triatomine bugs (Reduviidae: Triatominae). Arthropod. Struct. Dev. 2019, 49, 103–118. [Google Scholar] [CrossRef]
- Jurberg, J.; Galvão, C. Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health. Denisia 2006, 50, 1095–1116. [Google Scholar]
- Santillán-Guayasamín, S.; Villacís, A.G.; Grijalva, M.J.; Dujardin, J.P. Triatominae: Does the shape change of non-viable eggs compromise species recognition? Parasit. Vectors 2018, 11, 543. [Google Scholar] [CrossRef]
- Galindez-Girón, I.; Carcavallo, R.U.; Jurberg, J.; Lent, H.; Barth, O.M. Estudo morfológico de Triatoma guazu Lent & Wygodzinsky, 1979 (Hemiptera, Reduviidae, Triatominae). Mem. Instit. Oswaldo Cruz 1997, 92, 539–543. [Google Scholar] [CrossRef] [Green Version]
- Fellet, M.R.; Lorenzo, M.G.; Elliot, S.L.; Carrasco, D.; Guarneri, A.A. Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS ONE 2014, 9, e105255. [Google Scholar] [CrossRef]
- Galvão, C.; Justi, S.A. An overview on the ecology of Triatominae (Hemiptera: Reduviidae). Acta Trop. 2015, 151, 116–125. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Balsalobre, A.; Medone, P.; Cano, M.E.; Gurgel Gonçalves, R.; Feliciangeli, D.; Rabinovich, J.E. DataTri, a database of American triatomine species occurrence. Sci. Data 2018, 5, 180071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargues, M.D.; Schofield, C.J.; Dujardin, J.P. Classification and phylogeny of the Triatominae. In American Trypanosomiasis, 1st ed.; Telleria, J., Tibayrenc, M., Eds.; Elsevier: London, UK, 2010; Volume 1, pp. 117–147. [Google Scholar] [CrossRef]
- Justi, S.A.; Dale, C. Designation of the neotype of Triatoma dimidiata (Latreille, 1811) (Hemiptera, Reduviidae, Triatominae), with full integrated redescription including mitogenome and nuclear ITS-2 sequences. ZooKeys 2021, 1076, 9. [Google Scholar] [CrossRef]
- De Mello, D.V.; Nhapulo, E.F.; Cesaretto, L.P.; Alevi, J.J.; Cristal, D.C.; Montanari, G.; Galvão, C.; Alevi, K.C.C. Dichotomous keys based on cytogenetic data for triatomines reported in Brazilian regions with outbreaks of orally transmitted Chagas disease (Pernambuco and Rio Grande Do Norte). Trop. Med. Infect. Dis. 2023, 8, 196. [Google Scholar] [CrossRef]
- Bargues, M.D.; Schofield, C.; Dujardin, J.P. Classification and systematics of the Triatominae. In American Trypanosomiasis Chagas Disease, 2nd ed.; Telleria, J., Tybairenc, M., Eds.; Elsevier: London, UK, 2017; Volume 1, pp. 113–143. [Google Scholar] [CrossRef]
- Hernández, C.; Aristeu da Rosa, J.; Vallejo, G.A.; Guhl, F.; Ramírez, J.D. Taxonomy, evolution, and biogeography of the Rhodniini tribe (Hemiptera: Reduviidae). Diversity 2020, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Gaunt, M.; Miles, M. The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem. Inst. Oswaldo Cruz 2000, 95, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waleckx, E.; Gourbière, S.; Dumonteil, E. Intrusive versus domiciliated triatomines and the challenge of adapting vector control practices against Chagas disease. Mem. Inst. Oswaldo Cruz 2015, 110, 324–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grijalva, M.J.; Villacís, A.G.; Moncayo, A.L.; Ocaña-Mayorga, S.; Yumiseva, C.A.; Baus, E.G. Distribution of triatomine species in domestic and peridomestic environments in central coastal Ecuador. PLoS Negl. Trop. Dis. 2017, 11, e0005970. [Google Scholar] [CrossRef] [Green Version]
- Bargues, M.D.; Klisiowicz, D.R.; Gonzalez-Candelas, F.; Ramsey, J.M.; Monroy, C.; Ponce, C.; Mas-Coma, S. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl. Trop. Dis. 2008, 2, e233. [Google Scholar] [CrossRef] [Green Version]
- Flores-Ferrer, A.; Marcou, O.; Waleckx, E.; Dumonteil, E.; Gourbière, S. Evolutionary ecology of Chagas disease; what do we know and what do we need? Evol. Appl. 2018, 11, 470–487. [Google Scholar] [CrossRef] [Green Version]
- Justi, S.A.; Galvão, C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2017, 33, 42–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, A.; Smith, K.R.; Campbell-Lendrum, D.; Chadee, D.D.; Honda, Y.; Liu, Q.; Haines, A. Climate change and health: On the latest IPCC report. Lancet 2014, 383, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, G.J.; Medone, P.; Ceccarelli, S.; Rabinovich, J.; Schilman, P.E. Geographical distribution, climatic variability and thermo-tolerance of Chagas disease vectors. Ecography 2015, 38, 851–860. [Google Scholar] [CrossRef]
- Noireau, F.; Carbajal-De-La-Fuente, A.L.; Lopes, C.M.; Diotaiuti, L. Some considerations about the ecology of Triatominae. An. Acad. Bras. Cien. 2005, 77, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medone, P.; Ceccarelli, S.; Parham, P.E.; Figuera, A.; Rabinovich, J.E. The impact of climate change on the geographical distribution of two vectors of Chagas disease: Implications for the force of infection. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, R.; Bacigalupo, A.; Peña-Gómez, F.; Bustamante, R.O.; Cattan, P.E.; Gorla, D.E.; Botto-Mahan, C. Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile: Mepraia spinolai and Mepraia gajardoi. Parasit. Vectors 2019, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, G.; Nava-Bolaños, A.; De Fuentes-Vicente, J.A.; Téllez-Rendón, J.L.; Huerta, H.; Martínez-Hernández, F.; Rocha-Ortega, M.; Gutiérrez-Cabrera, A.E.; Ibarra-Cerdeña, C.N.; Córdoba-Aguilar, A. A reduction in ecological niche for Trypanosoma cruzi-infected triatomine bugs. Parasit. Vectors 2018, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Guerenstein, P.G.; Lazzari, C.R. Host-seeking: How triatomines acquire and make use of information to find blood. Acta Trop. 2009, 110, 148–158. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Reisenman, C.E.; Guerenstein, P.; Lazzari, C.R.; Lorenzo, M.G. An inside look at the sensory biology of triatomines. J. Insect Physiol. 2017, 97, 3–19. [Google Scholar] [CrossRef] [PubMed]
- May-Concha, I.J.; Talavera, M.J.E.; Dujardin, J.P.; Waleckx, E. Does Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida: Trypanosomatidae) modify the antennal phenotype of Triatoma dimidiata (Latreille, 1811) (Hemiptera: Triatominae)? Parasit. Vectors 2022, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.; Roque, A.L.R. Ecological aspects of Trypanosoma cruzi: Wild hosts and reservoirs. In American Trypanosomiasis Chagas Disease, 2nd ed.; Telleria, J., Tybairenc, M., Eds.; Elsevier: London, UK, 2017; Volume 1, pp. 243–264. [Google Scholar] [CrossRef]
- Avalos-Borges, E.E.; Rios, L.E.; Jiménez-Coello, M.; Ortega-Pacheco, A.; Garg, N.J. Animal models of Trypanosoma cruzi congenital transmission. Pathogens 2022, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.D.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Jansen, A.M.; Leon, L.; Machado, G.M.; da Silva, M.H.; Souza-Leão, S.M.; Deane, M.P. Trypanosoma cruzi in the opossum Didelphis marsupialis: Parasitological and serological follow-up of the acute infection. Exp. Parasitol. 1991, 73, 249–259. [Google Scholar] [CrossRef]
- Gómez-Sánchez, E.F.; Ochoa-Díaz-López, H.; Espinoza-Medinilla, E.E.; Velázquez-Ramírez, D.D.; Santos-Hernández, N.G.; Ruiz-Castillejos, C.; Vidal-López, D.G.; Moreno-Rodríguez, A.; Flores-Villegas, A.L.; López-Argueta, E.; et al. Mini-exon gene reveals circulation of TcI Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae) in bats and small mammals in an ecological reserve in southeastern Mexico. ZooKeys 2022, 1084, 139. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; Oria-Martínez, B.; Rendón-Franco, E.; Villalobos, G.; Muñoz-García, C.I. Trypanosoma cruzi, beyond the dogma of non-infection in birds. Infect. Genet. Evol. 2022, 99, 105239. [Google Scholar] [CrossRef]
- Botto-Mahan, C.; Correa, J.P.; Araya-Donoso, R.; Farías, F.; San Juan, E.; Quiroga, N.; González-Acuña, D. Lizards as silent hosts of Trypanosoma cruzi. Emerg. Infect. Dis. 2022, 28, 1250. [Google Scholar] [CrossRef]
- Akle, V.; Agudelo-Dueñas, N.; Molina-Rodriguez, M.A.; Kartchner, L.B.; Ruth, A.M.; González, J.M.; Forero-Shelton, M. Establishment of larval zebrafish as an animal model to investigate Trypanosoma cruzi motility in vivo. J. Vis. Exp. 2017, 127, e56238. [Google Scholar] [CrossRef] [Green Version]
- Torres-Castro, M.; Cuevas-Koh, N.; Hernández-Betancourt, S.; Noh-Pech, H.; Estrella, E.; Herrera-Flores, B.; Peláez-Sánchez, R. Natural infection with Trypanosoma cruzi in bats captured in Campeche and Yucatán, México. Biomédica 2021, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zecca, I.B.; Hodo, C.L.; Slack, S.; Auckland, L.; Rodgers, S.; Killets, K.C.; Hamer, S.A. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Vet. Parasitol. 2020, 278, 109014. [Google Scholar] [CrossRef]
- Villena, F.E.; Gomez-Puerta, L.A.; Jhonston, E.J.; Del Alcazar, O.M.; Maguiña, J.L.; Albujar, C.; Laguna-Torres, A.; Recuenco, S.E.; Ballard, S.; Ampuero, J. First report of Trypanosoma cruzi infection in salivary gland of bats from the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2018, 99, 723. [Google Scholar] [CrossRef]
- Nunes, M.C.P.; Dones, W.; Morillo, C.A.; Encina, J.J.; Ribeiro, A.L. Chagas disease: An overview of clinical and epidemiological aspects. J. Am. Coll. Cardiol. 2013, 62, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Zhou, X.N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Hernández, D.A.; Franyuti-Kelly, G.A.; Díaz-López-Silva, R.; González-Chávez, A.M.; González-Hermosillo-Cornejo, D.; Vázquez-López, R. Chagas disease: Current perspectives on a forgotten disease. Rev. Médica del Hosp. Gen. de Mex. 2018, 81, 154–164. [Google Scholar] [CrossRef]
- Balouz, V.; Agüero, F.; Buscaglia, C.A. Chagas disease diagnostic applications: Present knowledge and future steps. In Advances in Parasitology; Rollinson, D., Stothard, J.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 97, pp. 1–45. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.M.; Ebell, M.H.; Tarleton, R.L. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl. Trop. Dis. 2012, 6, e1881. [Google Scholar] [CrossRef] [Green Version]
- Candia-Puma, M.A.; Machaca-Luque, L.Y.; Roque-Pumahuanca, B.M.; Galdino, A.S.; Giunchetti, R.C.; Coelho, E.A.F.; Chávez-Fumagalli, M.A. Accuracy of diagnostic tests for the detection of Chagas disease: A systematic review and meta-analysis. Diagnostics 2022, 12, 2752. [Google Scholar] [CrossRef]
- Morillo, C.A.; Echeverria, L.E. New treatment regimens for Chagas disease: Light at the end of the tunnel? Lancet Infect. Dis. 2021, 21, 1057–1058. [Google Scholar] [CrossRef]
- Andrews, N.W.; Abrams, C.K.; Slatin, S.L.; Griffiths, G. A T. cruzi-secreted protein immunologically related to the complement component C9: Evidence for membrane pore-forming activity at low pH. Cell 1990, 61, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.S.; Sabino, E.C.; Oliveira, C.D.L.; de Oliveira, L.C.; Ferreira, A.M.; Cunha-Neto, E.; Ribeiro, A.L.P. Longitudinal study of patients with chronic Chagas cardiomyopathy in Brazil (SaMi-Trop project): A cohort profile. BMJ Open 2016, 6, e011181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piacenza, L.; Peluffo, G.; Alvarez, M.N.; Martínez, A.; Radi, R. Trypanosoma cruzi antioxidant enzymes as virulence factors in Chagas disease. Antioxid. Redox Signal. 2013, 19, 723–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, G.; Valck, C.; Molina, M.C.; Ribeiro, C.H.; López, N.; Sánchez, G.; Ferreira, V.; Billeta, R.; Aguilar, L.; Maldonado, I.; et al. Trypanosoma cruzi calreticulin: A novel virulence factor that binds complement C1 on the parasite surface and promotes infectivity. Immunobiology 2011, 216, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rubin-de-Celis, S.S.; Uemura, H.; Yoshida, N.; Schenkman, S. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell. Microbiol. 2006, 8, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Cardoni, R.L.; Antunez, M.I.; Abrami, A.A. Respuesta TH1 en la infección experimental con Trypanosoma cruzi. Medicina 1999, 59, 84–90. [Google Scholar] [PubMed]
- Campos, S.V.; Strabelli, T.M.V.; Neto, V.A.; Silva, C.P.; Bacal, F.; Bocchi, E.; Stolf, N.A.G. Risk factors for Chagas’ disease reactivation after heart transplantation. J. Heart Lung Transplant. 2008, 27, 597–602. [Google Scholar] [CrossRef]
- De Alba-Alvarado, M.; Salazar-Schettino, P.M.; Jiménez-Álvarez, L.; Cabrera-Bravo, M.; García-Sancho, C.; Zenteno, E.; Bucio-Torres, M.I. Th-17 cytokines are associated with severity of Trypanosoma cruzi chronic infection in pediatric patients from endemic areas of Mexico. Acta Trop. 2018, 178, 134–141. [Google Scholar] [CrossRef]
- Fretes-Zárate, R.; Medina Gutiérrez, J.; Muñoz-Rodas, D. Chagasic megacolon in Paraguay: Surgical aspects and futures perspectives. Mem. Inst. Investig. Cienc. Salud 2013, 11, 97–104. [Google Scholar]
- García-Orozco, V.H.; Villalvazo, J.E.; Solar-Aguirre, C.; Ibarra-Ocampo, C.M.; Díaz-Sandoval, C.I.; Ortiz-Gallegos, C.A.; Oregel-Camacho, D.J.; Noriega-Bucio, A. Digestive disorders in Chagas disease: Megaesophagus and Chagasic megacolon. In Chagas Disease–from Celullar and Molecular Aspects of Trypanosoma cruzi–Host Interactions to the Clinical Intervention, 1st ed.; Menna-Barreto, R., Ed.; IntechOpen: London, UK, 2022; Volume 1, pp. 1–16. [Google Scholar] [CrossRef]
- Álvarez-Hernández, D.A.; García-Rodríguez-Arana, R.; Ortiz-Hernández, A.; Álvarez-Sánchez, M.; Wu, M.; Mejia, R.; Martínez-Juárez, L.A.; Montoya, A.; Gallardo-Rincón, H.; Vázquez-López, R.; et al. A systematic review of historical and current trends in Chagas disease. Ther. Adv. Infect. Dis. 2021, 8, 20499361211033715. [Google Scholar] [CrossRef]
- Bonne, K.M.; Engman, D.M. Chagas heart disease pathogenesis: One mechanism or many? Curr. Mol. Med. 2008, 8, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Neto, E. Repensando la patogenia de la cardiopatía crónica chagásica en el fin del milenio. Medicina 1999, 59, 496–500. [Google Scholar] [PubMed]
- De Alba-Alvarado, M.C.; Torres-Gutiérrez, E.; Reynoso-Ducoing, O.A.; Zenteno-Galindo, E.; Cabrera-Bravo, M.; Guevara-Gómez, Y.; Salazar-Schettino, P.M.; Rivera-Fernández, N.; Bucio-Torres, M.I. Immunopathological mechanisms underlying cardiac damage in Chagas disease. Pathogens 2023, 12, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.C.; Neves, E.G.A.; de Souza-Silva, T.G.; Carvalho, A.C.; Pinto, C.H.R.; Galdino, A.S.; Gollob, K.J.; Dutra, W.O. Cytokine networks as targets for preventing and controlling Chagas heart disease. Pathogens 2023, 12, 171. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fuentes-Vicente, J.A.; Santos-Hernández, N.G.; Ruiz-Castillejos, C.; Espinoza-Medinilla, E.E.; Flores-Villegas, A.L.; de Alba-Alvarado, M.; Cabrera-Bravo, M.; Moreno-Rodríguez, A.; Vidal-López, D.G. What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide. Trop. Med. Infect. Dis. 2023, 8, 360. https://doi.org/10.3390/tropicalmed8070360
De Fuentes-Vicente JA, Santos-Hernández NG, Ruiz-Castillejos C, Espinoza-Medinilla EE, Flores-Villegas AL, de Alba-Alvarado M, Cabrera-Bravo M, Moreno-Rodríguez A, Vidal-López DG. What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide. Tropical Medicine and Infectious Disease. 2023; 8(7):360. https://doi.org/10.3390/tropicalmed8070360
Chicago/Turabian StyleDe Fuentes-Vicente, José A., Nancy G. Santos-Hernández, Christian Ruiz-Castillejos, Eduardo E. Espinoza-Medinilla, A. Laura Flores-Villegas, Mariana de Alba-Alvarado, Margarita Cabrera-Bravo, Adriana Moreno-Rodríguez, and Dolores G. Vidal-López. 2023. "What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide" Tropical Medicine and Infectious Disease 8, no. 7: 360. https://doi.org/10.3390/tropicalmed8070360
APA StyleDe Fuentes-Vicente, J. A., Santos-Hernández, N. G., Ruiz-Castillejos, C., Espinoza-Medinilla, E. E., Flores-Villegas, A. L., de Alba-Alvarado, M., Cabrera-Bravo, M., Moreno-Rodríguez, A., & Vidal-López, D. G. (2023). What Do You Need to Know before Studying Chagas Disease? A Beginner’s Guide. Tropical Medicine and Infectious Disease, 8(7), 360. https://doi.org/10.3390/tropicalmed8070360







