Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Study Population
2.2. Data Collection
2.3. Nutritional Assessment
2.4. Stool Collection
2.5. Laboratory Evaluations
2.5.1. Microscopic Examination
2.5.2. Cultivation of Intestinal Parasites
2.5.3. Identification of Entamoeba histolytica, G. lamblia, and Cryptosporidium by ELISA
2.5.4. Purification and Excystation of G. lamblia Cysts
2.5.5. Drug Susceptibility Testing of G. lamblia
2.5.6. G. lamblia Detection and Genotyping
2.6. Statistical Analysis
3. Results
3.1. Prevalence of Intestinal Parasitic Infection
3.2. Association between Intestinal Parasitic Infection and Nutritional Status
3.3. Excystation of G. lamblia Cysts
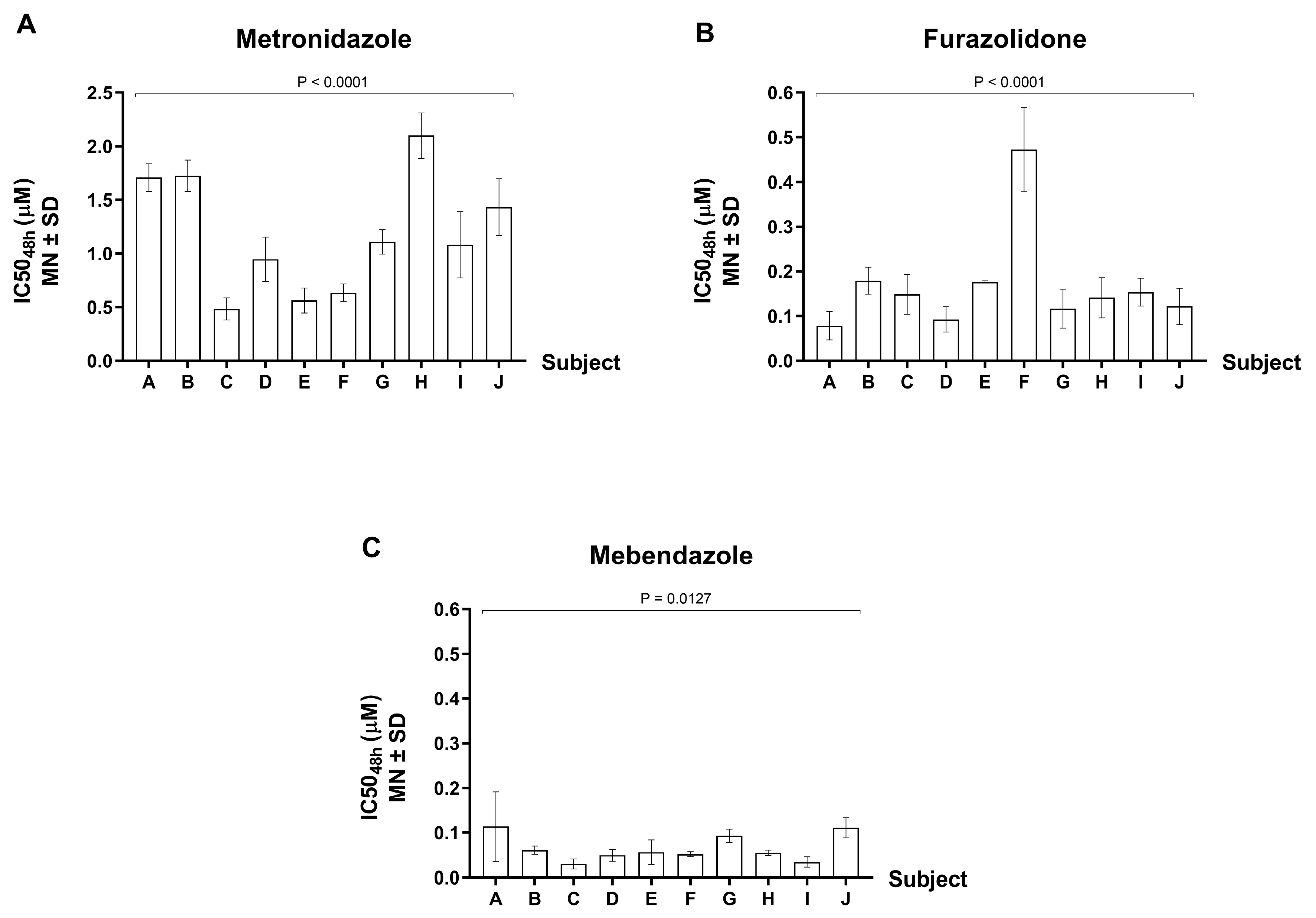
3.4. Drug Susceptibility Test of G. lamblia Isolates
3.5. G. lamblia Genotypes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- World Health Organization. Soil-Transmitted Helminth Infections. Fact Sheet, Updated January 2017. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 5 July 2023).
- Hesham, M.S.; Edariah, A.B.; Norhayati, M. Intestinal parasitic infections and micronutrient deficiency: A review. Med. J. Malays. 2004, 59, 284–293. [Google Scholar]
- Cappello, M. Global health impact of soil-transmitted nematodes. Pediatr. Infect. Dis. J. 2004, 23, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, L.S.; Latham, M.C.; Ottesen, E.A. Malnutrition and parasitic helminth infections. Parasitology 2000, 121, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Lunn, P.G.; Northrop-Clewes, C.A. The impact of gastrointestinal parasites on protein-energy malnutrition in man. Proc. Nutr. Soc. 1993, 52, 101–111. [Google Scholar] [CrossRef] [PubMed]
- OpenDevelopment Thailand. SDG 6 Clean Water and Sanitation. Available online: https://thailand.opendevelopmentmekong.net/topics/sdg-6-clean-water-and-sanitation/ (accessed on 26 June 2023).
- Waikagul, J.; Krudsood, S.; Radomyos, P.; Radomyos, B.; Chalemrut, K.; Jonsuksuntigul, P.; Kojima, S.; Looareesuwan, S.; Thaineau, W. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33, 218–223. [Google Scholar]
- Warunee, N.; Choomanee, L.; Sataporn, P.; Rapeeporn, Y.; Nuttapong, W.; Sompong, S.; Thongdee, S.; Bang-On, S.; Rachada, K. Intestinal parasitic infections among school children in Thailand. Trop. Biomed. 2007, 24, 83–88. [Google Scholar]
- Yanola, J.; Nachaiwieng, W.; Duangmano, S.; Prasannarong, M.; Somboon, P.; Pornprasert, S. Current prevalence of intestinal parasitic infections and their impact on hematological and nutritional status among Karen hill tribe children in Omkoi District, Chiang Mai Province, Thailand. Acta Trop. 2018, 180, 1–6. [Google Scholar] [CrossRef]
- Sarasombath, P.T. Prevalence and Health Effects of Intestinal Parasitic Infection in School Children in Satun Province, Thailand: A Cross-Sectional Study. Siriraj Med. J. 2017, 69, 167–174. [Google Scholar]
- Punsawad, C.; Phasuk, N.; Bunratsami, S.; Thongtup, K.; Viriyavejakul, P.; Palipoch, S.; Koomhin, P.; Nongnaul, S. Prevalence of intestinal parasitic infections and associated risk factors for hookworm infections among primary schoolchildren in rural areas of Nakhon Si Thammarat, southern Thailand. BMC Public Health 2018, 18, 1118. [Google Scholar] [CrossRef]
- Mørch, K.; Hanevik, K. Giardiasis treatment: An update with a focus on refractory disease. Curr. Opin. Infect. Dis. 2020, 33, 355–364. [Google Scholar] [CrossRef]
- Heyworth, M.F. Giardia duodenalis genetic assemblages and hosts. Parasite 2016, 23, 13. [Google Scholar] [CrossRef]
- Tungtrongchitr, A.; Sookrung, N.; Indrawattana, N.; Kwangsi, S.; Ongrotchanakun, J.; Chaicumpa, W. Giardia intestinalis in Thailand: Identification of genotypes. J. Health Popul. Nutr. 2010, 28, 42–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wongjindanon, N.; Suksrichavalit, T.; Subsutti, W.; Sarachart, T.; Worapisuttiwong, U.; Norramatha, P. Current infection rate of Giardia lamblia in two provinces of Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36 (Suppl. S4), 21–25. [Google Scholar]
- Watkins, R.R.; Eckmann, L. Treatment of giardiasis: Current status and future directions. Curr. Infect. Dis. Rep. 2014, 16, 396. [Google Scholar] [CrossRef]
- Yereli, K.; Balcioglu, I.C.; Ertan, P.; Limoncu, E.; Onag, A. Albendazole as an alternative therapeutic agent for childhood giardiasis in Turkey. Clin. Microbiol. Infect. 2004, 10, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Loderstadt, U.; Frickmann, H. Antimicrobial resistance of the enteric protozoon Giardia duodenalis—A narrative review. Eur. J. Microbiol. Immunol. 2021, 11, 29–43. [Google Scholar] [CrossRef]
- Lalle, M.; Hanevik, K. Treatment-refractory giardiasis: Challenges and solutions. Infect. Drug Resist. 2018, 11, 1921–1933. [Google Scholar] [CrossRef]
- Ansell, B.R.; McConville, M.J.; Ma’ayeh, S.Y.; Dagley, M.J.; Gasser, R.B.; Svard, S.G.; Jex, A.R. Drug resistance in Giardia duodenalis. Biotechnol. Adv. 2015, 33, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Nabarro, L.E.; Lever, R.A.; Armstrong, M.; Chiodini, P.L. Increased incidence of nitroimidazole-refractory giardiasis at the Hospital for Tropical Diseases, London: 2008–2013. Clin. Microbiol. Infect. 2015, 21, 791–796. [Google Scholar] [CrossRef]
- Peters, T.E.; Kreuels, B.; Addo, M.M.; Tannich, E.; Rothe, C. Risk factors for and management of metronidazole-refractory giardiasis in international travellers: A retrospective analysis. Travel. Med. Infect. Dis. 2021, 43, 102090. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 20 July 2022).
- Lurchachaiwong, W.; Serichantalergs, O.; Lertsethtakarn, P.; Ruamsap, N.; Srijan, A.; Oransathid, W.; Khemnu, N.; Vesely, B.A.; Demons, S.T.; Waters, N.C.; et al. Enteric etiological surveillance in acute diarrhea stool of United States Military Personnel on deployment in Thailand, 2013–2017. Gut Pathog. 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. DPDx—Laboratory Identification of Parasites of Public Health Concern. Available online: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/microexam.html (accessed on 29 June 2023).
- Saksirisampant, W.; Nuchprayoon, S.; Pradniwat, P.; Lamchuan, D. Boeck and Drbohlav Locke egg serum medium fordetection of Blastocystis hominisBlastocystis homin. Chulalongkorn Med. J. 2010, 54, 528–536. [Google Scholar]
- Davids, B.J.; Gillin, F.D. Methods for Giardia Culture, Cryopreservation, Encystation, and Excystation In Vitro. In Giardia: A Model Organism, 1st ed.; Lujan, H.D., Svärd, S., Eds.; Springer: Vienna, Austria, 2011. [Google Scholar] [CrossRef]
- Hahn, J.; Seeber, F.; Kolodziej, H.; Ignatius, R.; Laue, M.; Aebischer, T.; Klotz, C. High Sensitivity of Giardia duodenalis to Tetrahydrolipstatin (Orlistat) In Vitro. PLoS ONE 2013, 8, e71597. [Google Scholar] [CrossRef] [PubMed]
- Popruk, S.; Thima, K.; Udonsom, R.; Chiabchalard, R.; Mahittikorn, A.; Palukul, K.; Thepouypom, A. Activity of Plant Essential Oils against Giardia duodenalis. Southeast Asian J. Trop. Med. Public Health 2017, 48, 756–761. [Google Scholar]
- Liu, J.; Gratz, J.; Amour, C.; Kibiki, G.; Becker, S.; Janaki, L.; Verweij, J.J.; Taniuchi, M.; Sobuz, S.U.; Haque, R.; et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 2013, 51, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gratz, J.; Maro, A.; Kumburu, H.; Kibiki, G.; Taniuchi, M.; Howlader, A.M.; Sobuz, S.U.; Haque, R.; Talukder, K.A.; et al. Simultaneous detection of six diarrhea-causing bacterial pathogens with an in-house PCR-luminex assay. J. Clin. Microbiol. 2012, 50, 98–103. [Google Scholar] [CrossRef]
- Llewellyn, S.; Inpankaew, T.; Nery, S.V.; Gray, D.J.; Verweij, J.J.; Clements, A.C.A.; Gomes, S.J.; Traub, R.; McCarthy, J.S. Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial. PLoS Neglected Trop. Dis. 2016, 10, e0004380. [Google Scholar] [CrossRef]
- Almeida, A.; Pozio, E.; Cacciò, S.M. Genotyping of Giardia duodenalis cysts by new real-time PCR assays for detection of mixed infections in human samples. Appl. Environ. Microbiol. 2010, 76, 1895–1901. [Google Scholar] [CrossRef]
- Assavapongpaiboon, B.; Bunkasem, U.; Sanprasert, V.; Nuchprayoon, S. A Cross-Sectional Study on Intestinal Parasitic Infections in Children in Suburban Public Primary Schools, Saraburi, the Central Region of Thailand. Am. J. Trop. Med. Hyg. 2018, 98, 763–767. [Google Scholar] [CrossRef]
- Belizario, V.Y., Jr.; Totañes, F.I.; de Leon, W.U.; Lumampao, Y.F.; Ciro, R.N. Soil-transmitted helminth and other intestinal parasitic infections among school children in indigenous people communities in Davao del Norte, Philippines. Acta Trop. 2011, 120 (Suppl. S1), S12–S18. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Pullan, R.L.; Breitling, L.P.; Geiger, S.M.; Cundill, B.; Correa-Oliveira, R.; Brooker, S.; Bethony, J.M. Genetic and Household Determinants of Predisposition to Human Hookworm Infection in a Brazilian Community. J. Infect. Dis. 2010, 202, 954–961. [Google Scholar] [CrossRef]
- Pullan, R.L.; Kabatereine, N.B.; Quinnell, R.J.; Brooker, S. Spatial and Genetic Epidemiology of Hookworm in a Rural Community in Uganda. PLoS Neglected Trop. Dis. 2010, 4, e713. [Google Scholar] [CrossRef]
- World Health Organization. Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 29 December 2022).
- Cossa-Moiane, I.; Roucher, C.; Mac Quene, T.; Campos-Ponce, M.; de Deus, N.; Polman, K.; Doak, C. Association between Intestinal Parasite Infections and Proxies for Body Composition: A Scoping Review. Nutrients 2022, 14, 2229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Watanabe, C.; Ohtsuka, R. Impacts of dietary intake and helminth infection on diversity in growth among schoolchildren in rural south China: A four-year longitudinal study. Am. J. Hum. Biol. 2007, 19, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, L.M.; Incani, R.N.; Franco, C.R.; Ugarte, A.; Cadenas, Y.; Sierra Ruiz, C.I.; Hermans, P.W.M.; Hoek, D.; Campos Ponce, M.; de Waard, J.H.; et al. High Malnutrition Rate in Venezuelan Yanomami Compared to Warao Amerindians and Creoles: Significant Associations with Intestinal Parasites and Anemia. PLoS ONE 2013, 8, e77581. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Botelho, A.; Brooker, S.; Geiger, S.M.; Fleming, F.; Souza Lopes, A.C.; Diemert, D.J.; Corrêa-Oliveira, R.; Bethony, J.M. Age patterns in undernutrition and helminth infection in a rural area of Brazil: Associations with ascariasis and hookworm. Trop. Med. Int. Health 2008, 13, 458–467. [Google Scholar] [CrossRef]
- Jinatham, V.; Maxamhud, S.; Popluechai, S.; Tsaousis, A.D.; Gentekaki, E. Blastocystis One Health Approach in a Rural Community of Northern Thailand: Prevalence, Subtypes and Novel Transmission Routes. Front. Microbiol. 2021, 12, 746340. [Google Scholar] [CrossRef]
- Oyofo, B.A.; Subekti, D.; Tjaniadi, P.; Machpud, N.; Komalarini, S.; Setiawan, B.; Simanjuntak, C.; Punjabi, N.; Corwin, A.L.; Wasfy, M.; et al. Enteropathogens associated with acute diarrhea in community and hospital patients in Jakarta, Indonesia. FEMS Immunol. Med. Microbiol. 2002, 34, 139–146. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Shiff, C.K.; Tamang, L.; Munsaka, F.; Beitin, A.M.; Moss, W.J. The association of Blastocystis hominis and Endolimax nana with diarrheal stools in Zambian school-age children. Parasitol. Res. 2005, 98, 38–43. [Google Scholar] [CrossRef]
- Shah, M.; Tan, C.B.; Rajan, D.; Ahmed, S.; Subramani, K.; Rizvon, K.; Mustacchia, P. Blastocystis hominis and Endolimax nana Co-Infection Resulting in Chronic Diarrhea in an Immunocompetent Male. Case Rep. Gastroenterol. 2012, 6, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Sousa, M.I.; Azeredo, Z.; Leite, E.; Figueiredo de Sousa, J.C.; Cabral, M. Isolation, excystation and axenization of Giardia lamblia isolates: In vitro susceptibility to metronidazole and albendazole. J. Antimicrob. Chemother. 2003, 51, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Hautus, M.A.; Kortbeek, L.M.; Vetter, J.C.; Laarman, J.J. In vitro excystation and subsequent axenic growth of Giardia lamblia. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 858–861. [Google Scholar] [CrossRef]
- Meloni, B.P.; Thompson, R.C. Comparative studies on the axenic in vitro cultivation of Giardia of human and canine origin: Evidence for intraspecific variation. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 637–640. [Google Scholar] [CrossRef]
- Charoenlarp, P.; Bunnag, D. Treatment of parasitic infections in Thailand. Southeast Asian J. Trop. Med. Public Health 1986, 17, 620–626. [Google Scholar] [PubMed]
- Krakovka, S.; Ribacke, U.; Miyamoto, Y.; Eckmann, L.; Svard, S. Characterization of Metronidazole-Resistant Giardia intestinalis Lines by Comparative Transcriptomics and Proteomics. Front. Microbiol. 2022, 13, 834008. [Google Scholar] [CrossRef]
- Carter, E.R.; Nabarro, L.E.; Hedley, L.; Chiodini, P.L. Nitroimidazole-refractory giardiasis: A growing problem requiring rational solutions. Clin. Microbiol. Infect. 2018, 24, 37–42. [Google Scholar] [CrossRef]
- Arguello-Garcia, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar] [CrossRef]
- Requena-Mendez, A.; Goni, P.; Rubio, E.; Pou, D.; Fumado, V.; Lobez, S.; Aldasoro, E.; Cabezos, J.; Valls, M.E.; Trevino, B.; et al. The Use of Quinacrine in Nitroimidazole-resistant Giardia duodenalis: An Old Drug for an Emerging Problem. J. Infect. Dis. 2017, 215, 946–953. [Google Scholar] [CrossRef]
- Canete, R.; Noda, A.L.; Rodriguez, M.; Brito, K.; Herrera, E.; Kofoed, P.E.; Ursing, J. 5-Nitroimidazole refractory giardiasis is common in Matanzas, Cuba and effectively treated by secnidazole plus high-dose mebendazole or quinacrine: A prospective observational cohort study. Clin. Microbiol. Infect. 2020, 26, 1092.e1–1092.e6. [Google Scholar] [CrossRef]
- Galeh, T.M.; Kazemi, A.; Mahami-Oskouei, M.; Baradaran, B.; Spotin, A.; Sarafraz, S.; Karamat, M. Introducing nitazoxanide as a promising alternative treatment for symptomatic to metronidazole-resistant giardiasis in clinical isolates. Asian Pac. J. Trop. Med. 2016, 9, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Saghaug, C.S.; Klotz, C.; Kallio, J.P.; Brattbakk, H.R.; Stokowy, T.; Aebischer, T.; Kursula, I.; Langeland, N.; Hanevik, K. Genetic variation in metronidazole metabolism and oxidative stress pathways in clinical Giardia lamblia assemblage A and B isolates. Infect. Drug Resist. 2019, 12, 1221–1235. [Google Scholar] [CrossRef]
- Muller, J.; Hemphill, A.; Muller, N. Physiological aspects of nitro drug resistance in Giardia lamblia. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.E.; Elwin, K.; Phot, N.; Seng, C.; Mao, S.; Suy, K.; Kumar, V.; Nader, J.; Bousfield, R.; Perera, S.; et al. Molecular Characterization of Cryptosporidium Species and Giardia duodenalis from Symptomatic Cambodian Children. PLoS Negl. Trop. Dis. 2016, 10, e0004822. [Google Scholar] [CrossRef] [PubMed]
- Boontanom, P.; Mungthin, M.; Tan-Ariya, P.; Naaglor, T.; Leelayoova, S. Epidemiology of giardiasis and genotypic characterization of Giardia duodenalis in preschool children of a rural community, central Thailand. Trop. Biomed. 2011, 28, 32–39. [Google Scholar]
- Saksirisampant, W.; Boontanom, P.; Mungthin, M.; Tan-Ariya, P.; Lamchuan, D.; Siripattanapipong, S.; Leelayoova, S. Prevalence of giardiasis and genotypic characterization of Giardia duodenalis in hilltribe children, Northern Thailand. Trop. Biomed. 2012, 29, 331–338. [Google Scholar]
- Fantinatti, M.; Bello, A.R.; Fernandes, O.; Da-Cruz, A.M. Identification of Giardia lamblia Assemblage E in Humans Points to a New Anthropozoonotic Cycle. J. Infect. Dis. 2016, 214, 1256–1259. [Google Scholar] [CrossRef]
| Characteristic | Total Participant N = 661 | Parasitic Infection N = 445 (67.32%) | Non-Parasitic Infection N = 216 (32.68%) | Chi2 Value, p-Value |
|---|---|---|---|---|
| Age; mean (SD); year | 8.29 (2.14) | 8.36 (2.11) | 8.12 (2.22) | 0.174 |
| Sex | ||||
| 304 (45.99) | 198 (44.49) | 106 (49.07) | 1.228, 0.268 |
| 357 (54.01) | 247 (55.51) | 110 (50.93) | |
| Height; mean (SD); cm | 126.54 (13.01) | 126.29 (12.84) | 127.07 (13.35) | 0.469 |
| Weight; mean (SD); kg | 26.72 (9.56) | 26.35 (9.34) | 27.47 (9.97) | 0.160 |
| Stool characteristics | ||||
| 216 (32.68) | 137 (30.79) | 79 (36.57) | 2.214, 0.137 |
| 392 (59.30) | 273 (61.35) | 119 (59.05) | 2.358, 0.125 |
| 49 (7.41) | 32 (7.19) | 17 (7.87) | 0.098, 0.755 |
| 4 (0.61) | 3 (0.67) | 1 (0.46) | 0.108, 1.00 |
| 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Symptoms & signs | ||||
| 64 (9.68) | 49 (11.01) | 15 (6.94) | 2.750, 0.097 |
| 10 (1.51) | 8 (1.80) | 2 (0.93) | 0.742, 0.511 |
| 30 (4.54) | 22 (4.94) | 8 (3.70) | 0.516, 0.472 |
| 27 (4.08) | 22 (4.94) | 5 (2.31) | 2.565, 0.109 |
| 36 (5.45) | 27 (6.07) | 9 (4.17) | 1.020, 0.312 |
| 0 (0) | 0 (0) | 0 (0) | 1.00 |
| 9 (1.36) | 8 (1.79) | 1 (0.46) | 1.929, 0.367 |
| Study’s site | ||||
| 226 (34.19) | 152 (67.26) | 74 (32.74) | 10.70, 0.005 |
| 110 (16.64) | 88 (80.0) | 22 (20.0) | |
| 325 (49.17) | 205 (63.08) | 120 (36.92) |
| Organisms | Prevalence (%) (95% Confidence Interval) | ||
|---|---|---|---|
| Total | Preschool Age * | School Age * | |
| Overall infection | |||
| 0.30 (0.04–1.09) | 1.10 (0.03–5.97) | 0.18 (0.004–0.97) |
| 65.05 (61.28–68.69) | 56.04 (45.25–66.44) | 66.49 (62.45–70.36) |
| 1.97 (1.05–3.34) | 3.30 (0.69–9.33) | 1.75 (0.84–3.20) |
| Protozoa infection | |||
| Non-pathogenic | |||
| 49.32 (45.44–53.22) | 36.26 (26.44–47.01) | 51.40 (47.21–55.58) |
| 26.93 (23.58–30.48) | 24.18 (15.81–34.28) | 27.37 (23.75–31.23) |
| 9.53 (7.40–12.03) | 3.30 (0.69–9.33) | 10.53 (8.13–13.34) |
| 5.45 (3.84–7.46) | 3.30 (0.69–9.33) | 5.79 (4.02–8.03) |
| 3.03 (1.86–4.63) | 1.10 (0.03–5.97) | 3.33 (2.02–5.16) |
| 1.97 (1.05–3.34) | 1.10 (0.03–5.97) | 2.11 (1.09–3.65) |
| Pathogenic | |||
| 0.76 (0.25–1.76) | 0 (0–3.97) | 0.88 (0.29–2.04) |
| 17.40 (14.58–20.51) | 28.57 (19.59–39.0) | 15.61 (12.73–18.86) |
| 0 (0–0.56) | 0 (0–3.97) | 0 (0–0.65) |
| Helminth infection | |||
| 0.91 (0.33–1.97) | 2.20 (0.27–7.71) | 0.70 (0.19–1.79) |
| 0.45 (0.09–1.32) | 0 (0–3.97) | 0.53 (0.11–1.53) |
| 0.61 (0.17–1.54) | 2.20 (0.27–7.71) | 0.35 (0.04–1.26) |
| 0.15 (0.004–0.84) | 0 (0–3.97) | 0.18 (0.004–0.97) |
| 0.15 (0.004–0.84) | 0 (0–3.97) | 0.18 (0.004–0.97) |
| Organism | WFA | HFA | WFH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | OR (95% CI) | p-Value | Yes | No | OR (95% CI) | p-Value | Yes | No | OR (95% CI) | p-Value | |
| Overall infection | ||||||||||||
| 21 (9.72) | 195 (90.28) | 1 | 0.070 | 23 (10.65) | 193 (89.35) | 1 | 0.141 | 16 (7.41) | 200 (92.59) | 1 | 0.915 |
| 66 (14.83) | 379 (85.17) | 1.62 (0.96–2.72) | 66 (14.83) | 379 (85.17) | 1.46 (0.88–2.42) | 34 (7.64) | 411 (92.36) | 1.03 (0.56–1.92) | |||
| Helminth infection | ||||||||||||
| 84 (13.0) | 562 (87.0) | 1 | 0.433 | 86 (13.31) | 560 (86.69) | 1 | 0.457 | 48 (7.43) | 598 (92.57) | 1 | 0.401 |
| 3 (20.0) | 12 (80.0) | 1.67 (0.46–6.05) | 3 (20.0) | 12 (80.0) | 1.63 (0.45–5.89) | 2 (13.33) | 13 (86.67) | 1.92 (0.42–8.74) | |||
| Protozoa infection | ||||||||||||
| 21 (9.63) | 197 (90.37) | 1 | 0.062 | 23 (10.55) | 195 (89.45) | 1 | 0.125 | 16 (7.34) | 202 (92.66) | 1 | 0.878 |
| 66 (14.90) | 377 (85.10) | 1.64 (0.98–2.76) | 66 (14.90) | 377 (85.10) | 1.48 (0.90–2.46) | 34 (7.67) | 409 (92.33) | 1.05 (0.57–1.95) | |||
| Protozoa infection | ||||||||||||
| B. hominis | ||||||||||||
| 34 (10.15) | 301 (89.85) | 1 | 0.021 | 37 (11.04) | 298 (88.96) | 1 | 0.066 | 25 (7.46) | 310 (92.54) | 1 | 0.920 |
| 53 (16.26) | 273 (83.74) | 1.72 (1.08–2.72) | 52 (15.95) | 274 (84.05) | 1.53 (0.97–2.40) | 25 (7.67) | 301 (92.33) | 1.03 (0.58–1.83) | |||
| E. coli | ||||||||||||
| 81 (13.55) | 517 (86.45) | 1 | 0.372 | 83 (13.88) | 515 (86.12) | 1 | 0.339 | 46 (7.69) | 552 (92.31) | 1 | 0.702 |
| 6 (9.52) | 57 (90.48) | 0.67 (0.28–1.61) | 6 (9.52) | 57 (90.48) | 0.65 (0.27–1.56) | 4 (6.35) | 59 (93.65) | 0.81 (0.28–2.34) | |||
| E. nana | ||||||||||||
| 57 (11.80) | 428 (88.2) | 1 | 0.090 | 57 (11.80) | 426 (88.20) | 1 | 0.040 | 37 (7.66) | 446 (92.34) | 1 | 0.878 |
| 30 (16.85) | 148 (83.15) | 1.51 (0.94–2.45) | 32 (17.98) | 146 (82.02) | 1.64 (1.02–2.63) | 13 (7.30) | 165 (92.70) | 0.95 (0.49–1.83) | |||
| G. lamblia | ||||||||||||
| 73 (13.37) | 473 (86.63) | 1 | 0.730 | 77 (14.10) | 469 (85.90) | 1 | 0.297 | 44 (8.06) | 502 (91.94) | 1 | 0.299 |
| 14 (12.17) | 101 (87.83) | 0.90 (0.49–1.65) | 12 (10.43) | 103 (89.57) | 0.71 (0.37–1.35) | 6 (5.22) | 109 (94.78) | 0.63 (0.26–1.51) | |||
| P. hominis | ||||||||||||
| 85 (13.12) | 563 (86.88) | 1 | 0.811 | 86 (13.27) | 562 (86.73) | 1 | 0.314 | 49 (7.56) | 599 (92.44) | 1 | 0.986 |
| 2 (15.38) | 11 (84.62) | 1.20 (0.26–5.53) | 3 (23.08) | 10 (76.92) | 1.96 (0.53–7.27) | 1 (7.69) | 12 (92.31) | 1.02 (0.13–8.00) | |||
| I. buetschlii | ||||||||||||
| 79 (12.64) | 546 (87.36) | 1 | 0.104 | 80 (12.80) | 545 (87.2) | 1 | 0.042 | 47 (7.52) | 578 (92.48) | 1 | 0.858 |
| 8 (22.22) | 28 (27.78) | 1.97 (0.87–4.49) | 9 (25.0) | 27 (75.0) | 2.27 (1.03–5.00) | 3 (8.33) | 33 (91.67) | 1.12 (0.33–3.78) | |||
| Most prevalent protozoa (G. lamblia, B. Hominis, and E. nana) | ||||||||||||
| 22 (8.94) | 224 (91.06) | 1 | 0.015 | 25 (10.16) | 221 (89.84) | 1 | 0.057 | 18 (7.32) | 228 (92.68) | 1 | 0.853 |
| 350 (84.34) | 65 (15.66) | 1.89 (1.13–3.15) | 64 (15.42) | 351 (84.58) | 1.61 (0.99–2.64) | 32 (7.71) | 383 (92.29) | 1.06 (0.58–1.93) | |||
| Helminth infection | ||||||||||||
| A. lumbricoides | ||||||||||||
| 86 (13.13) | 569 (86.87) | 1 | 0.799 | 87 (13.28) | 568 (86.72) | 1 | 0.176 | 49 (7.48) | 606 (92.52) | 1 | 0.413 |
| 1 (16.67) | 5 (83.33) | 1.32 (0.15–11.46) | 2 (33.33) | 4 (66.67) | 3.26 (0.59–18.09) | 1 (16.67) | 5 (83.33) | 2.47 (0.28–21.59) | |||
| Hookworm | ||||||||||||
| 87 (13.22) | 571 (86.78) | - | - | 89 (13.53) | 569 (86.47) | - | - | 50 (7.6) | 608 (92.4) | - | - |
| 0 (0.0) | 3 (100) | - | 0 (0.0) | 3 (100) | - | 0 (0.0) | 3 (100) | - | |||
| E. vermicularis | ||||||||||||
| 85 (12.94) | 572 (87.06) | 1 | 0.058 | 88 (13.39) | 569 (86.61) | 1 | 0.508 | 49 (7.46) | 608 (92.54) | 1 | 0.223 |
| 2 (50.0) | 2 (50.0) | 6.73 (0.94–48.41) | 1 (25.0) | 3 (75.0) | 2.16 (0.22–20.95) | 1 (25.0) | 3 (75.0) | 4.14 (0.42–40.51) | |||
| S. stercoralis | ||||||||||||
| 87 (13.18) | 573 (86.82) | - | - | 89 (13.48) | 571 (86.52) | - | - | 50 (7.58) | 610 (92.42) | - | - |
| 0 (0.0) | 1 (100) | - | 0 (0.0) | 1 (100) | - | 0 (0.0) | 1 (100) | - | |||
| T. trichiura | ||||||||||||
| 87 (13.18) | 573 (86.82) | - | - | 89 (13.48) | 571 (86.52) | - | - | 50 (7.58) | 610 (92.42) | - | - |
| 0 (0.0) | 1 (100) | - | 0 (0.0) | 1 (100) | - | 0 (0.0) | 1 (100) | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongstitwilairoong, B.; Anothaisintawee, T.; Ruamsap, N.; Lertsethtakarn, P.; Kietsiri, P.; Oransathid, W.; Oransathid, W.; Gonwong, S.; Silapong, S.; Suksawad, U.; et al. Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand. Trop. Med. Infect. Dis. 2023, 8, 394. https://doi.org/10.3390/tropicalmed8080394
Wongstitwilairoong B, Anothaisintawee T, Ruamsap N, Lertsethtakarn P, Kietsiri P, Oransathid W, Oransathid W, Gonwong S, Silapong S, Suksawad U, et al. Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand. Tropical Medicine and Infectious Disease. 2023; 8(8):394. https://doi.org/10.3390/tropicalmed8080394
Chicago/Turabian StyleWongstitwilairoong, Boonchai, Thunyarat Anothaisintawee, Nattaya Ruamsap, Paphavee Lertsethtakarn, Paksathorn Kietsiri, Wirote Oransathid, Wilawan Oransathid, Siriphan Gonwong, Sasikorn Silapong, Umaporn Suksawad, and et al. 2023. "Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand" Tropical Medicine and Infectious Disease 8, no. 8: 394. https://doi.org/10.3390/tropicalmed8080394
APA StyleWongstitwilairoong, B., Anothaisintawee, T., Ruamsap, N., Lertsethtakarn, P., Kietsiri, P., Oransathid, W., Oransathid, W., Gonwong, S., Silapong, S., Suksawad, U., Sornsakrin, S., Bodhidatta, L., Boudreaux, D. M., & Livezey, J. R. (2023). Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand. Tropical Medicine and Infectious Disease, 8(8), 394. https://doi.org/10.3390/tropicalmed8080394





