Severe Systemic Chromobacterium violaceum Infection: A Case Study of a German Long-Term Resident in French Guyana
Abstract
1. Background
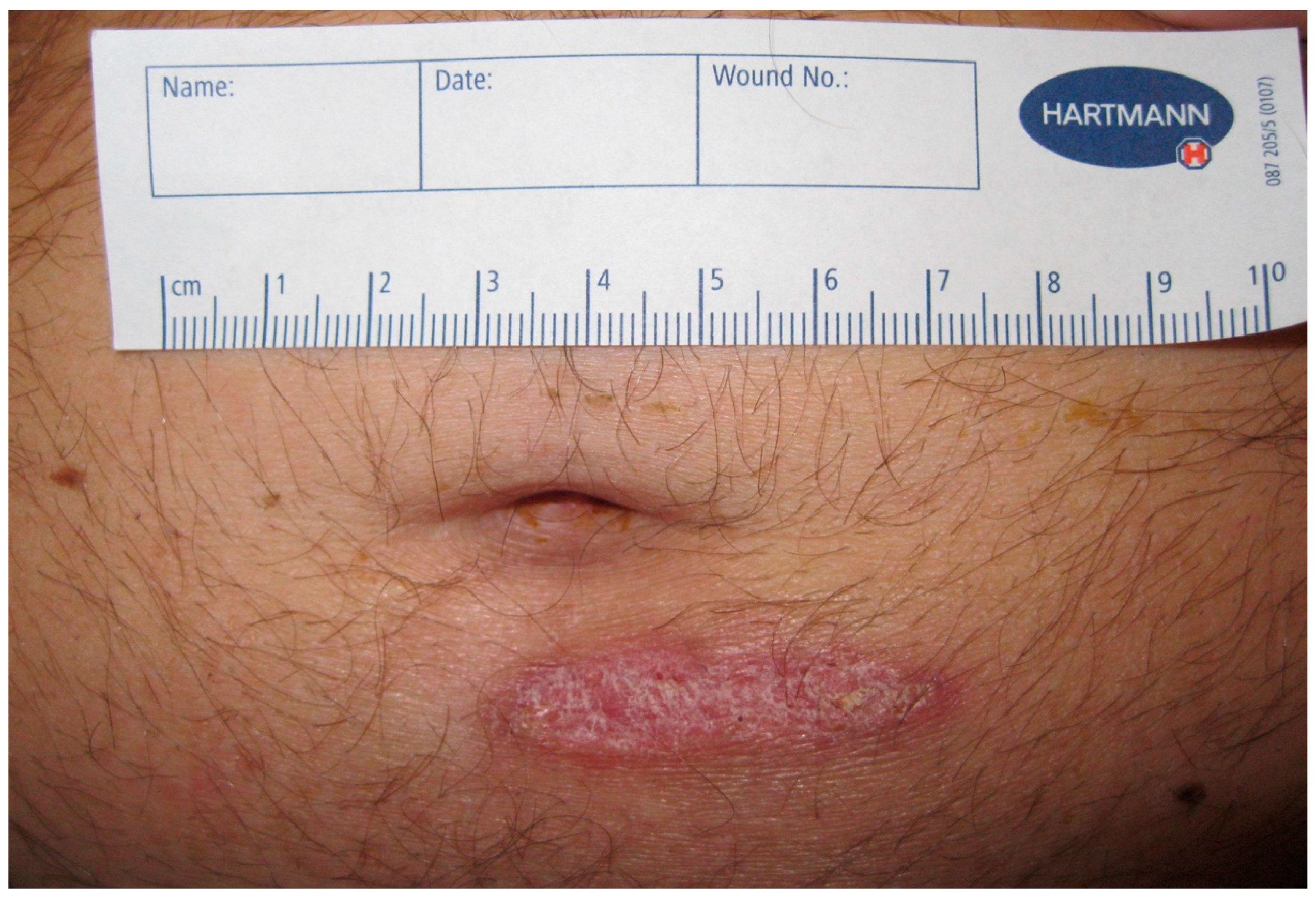
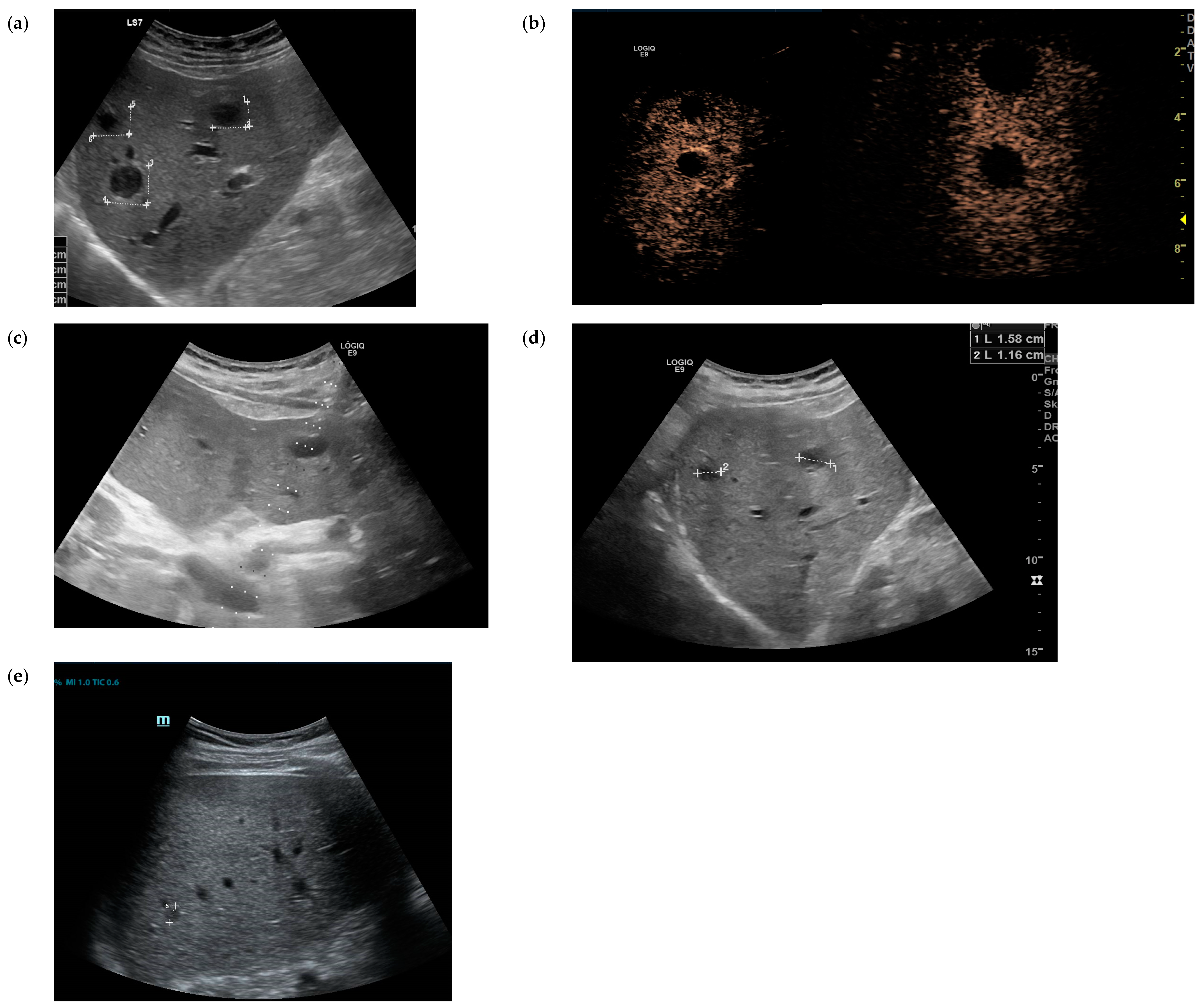
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, S.; Paudel, P.; Gautam, K.; Shrestha, S.; Thapa, S.; Gautam, R. Chromobacterium violaceum Isolated from a Wound Sepsis: A Case Study from Nepal. Case Rep. Infect. Dis. 2015, 2015, 181946. [Google Scholar] [CrossRef] [PubMed]
- Richard, K.R.; Lovvorn, J.J.; Oliver, S.E.; Ross, S.A.; Benner, K.W.; Kong, M.Y. Chromobacterium violaceum Sepsis: Rethinking Conventional Therapy to Improve Outcome. Am. J. Case Rep. 2015, 16, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Li, Y.H. Chromobacterium violaceum infection: A clinical review of an important but neglected infection. J. Chin. Med. Assoc. 2011, 74, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, Y.; Chen, Y.; Yang, H.; Luo, M.; Li, Y.; Huang, L.; Luo, H. Critical bloodstream infection caused by Chromobacterium violaceum: A case report in a 15-year-old male with sepsis-induced cardiogenic shock and purpura fulminans. Front. Med. 2024, 11, 1342706. [Google Scholar] [CrossRef]
- Fantinatti-Garboggini, F.; Almeida, R.; Portillo Vdo, A.; Barbosa, T.A.; Trevilato, P.B.; Neto, C.E.; Coelho, R.D.; Silva, D.W.; Bartoleti, L.A.; Hanna, E.S.; et al. Drug resistance in Chromobacterium violaceum. Genet. Mol. Res. 2004, 3, 134–147. [Google Scholar]
- Darmawan, G.; Kusumawardhani, R.N.Y.; Alisjahbana, B.; Fadjari, T.H. Chromobacterium violaceum: The Deadly Sepsis. Acta Med. Indones. 2018, 50, 80–81. [Google Scholar]
- Matsuura, N.; Miyoshi, M.; Doi, N.; Yagi, S.; Aradono, E.; Imamura, T.; Koga, R. Multiple Liver Abscesses with a Skin Pustule due to Chromobacterium violaceum. Intern. Med. 2017, 56, 2519–2522. [Google Scholar] [CrossRef][Green Version]
- Young, A.; Smith, S.; Horne, P.; Thomsett, B.; Hanson, J. Chromobacterium violaceum in Northern Australia: A Sheep in Wolf’s Clothing? Am. J. Trop. Med. Hyg. 2018, 99, 844–848. [Google Scholar] [CrossRef]
- Lin, Y.; Majumdar, S.S.; Hennessy, J.; Baird, R.W. The Spectrum of Chromobacterium violaceum Infections from a Single Geographic Location. Am. J. Trop. Med. Hyg. 2016, 94, 710–716. [Google Scholar] [CrossRef]
- Sirinavin, S.; Techasaensiri, C.; Benjaponpitak, S.; Pornkul, R.; Vorachit, M. Invasive Chromobacterium violaceum infection in children: Case report and review. Pediatr. Infect. Dis. J. 2005, 24, 559–561. [Google Scholar] [CrossRef]
- Alisjahbana, B.; Debora, J.; Susandi, E.; Darmawan, G. Chromobacterium violaceum: A Review of an Unexpected Scourge. Int. J. Gen. Med. 2021, 14, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Kumar, V.; Bhat, N.; Rao, P. Chromobacterium violaceum infection: A rare but frequently fatal disease. J. Pediatr. Surg. 2002, 37, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Meher-Homji, Z.; Mangalore, R.P.; Johnson, P.D.R.; Chua, K.Y.L. Chromobacterium violaceum infection in chronic granulomatous disease: A case report and review of the literature. JMM Case Rep. 2017, 4, e005084. [Google Scholar] [CrossRef] [PubMed]
- Mamlok, R.J.; Mamlok, V.; Mills, G.C.; Daeschner, C.W., III; Schmalstieg, F.C.; Anderson, D.C. Glucose-6-phosphate dehydrogenase deficiency, neutrophil dysfunction and Chromobacterium violaceum sepsis. J. Pediatr. 1987, 111, 852–854. [Google Scholar] [CrossRef]
- Thwe, P.M.; Ortiz, D.A.; Wankewicz, A.L.; Hornak, J.P.; Williams-Bouyer, N.; Ren, P. The Brief Case: Recurrent Chromobacterium violaceum Bloodstream Infection in a Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Patient with a Severe Neutrophil Defect. J. Clin. Microbiol. 2020, 58, e00312-19. [Google Scholar] [CrossRef]
- Li, K.; Han, D.; Alhaskawi, A.; Liu, T.; Wang, X.; Yang, W.; Lu, H.; Fang, X. Sepsis and Hepatapostema Secondary to Chromobacterium Violaceum Infection on Lower Limb Skin: A Case Report. Infect. Drug Resist. 2024, 17, 1003–1010. [Google Scholar] [CrossRef]
- Nam, B.V.; Hà, B.T.; Thúy, Đ.T.; van Doorn, H.R.; Huy, B.V. Clinical presentation and treatment of 2 patients with infection caused by Chromobacterium violaceum in Vietnam. BMC Infect. Dis. 2024, 24, 508. [Google Scholar] [CrossRef]
- Yavuz, L.; Alhammadi, M.; Musa, R.; Hamdi, M.; Aldirawi, M. A Rare Infection of Chromobacterium violaceum in an Immunocompetent Patient: A Case Report. Cureus 2023, 15, e51148. [Google Scholar] [CrossRef]
- Barnes, P.; Gonzales, J.; Hammond, D. Chromobacterium violaceum: A rare opportunistic pathogen and clue for pediatric chronic granulomatous disease. Pediatr. Dermatol. 2023, 40, 396–397. [Google Scholar] [CrossRef]
- Gomez, S.A.; Sanz, M.B.; Rapoport, M.; Sucin, G.; Corallo, T.A.; Poklepovich, T.; Campos, J.; Ceriana, P.; de Mendieta, J.M.; Prieto, M.; et al. Novel Metallo-β-Lactamase bla(CVI-1) Isolated from a Chromobaterium violaceum Clinical Strain Resistant to Colistin. Pathogens 2023, 12, 961. [Google Scholar] [CrossRef]
- Attonito, J.; Tomasello, G.; Barrett, B.; Wauters, R.; Adams, A.; Gilbert, L. Chromobacterium violaceum in a U.S. Marine: A Case Report. Mil. Med. 2023, 189, e928–e931. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyan, G.; Bhaskaran, P.; Vijay, S.; Shashindran, N.; Jayakumar, C. Chromobacterium violaceum sepsis with extensive vesiculobullous eruptions. Int. J. Dermatol. 2022, 61, 106–108. [Google Scholar] [CrossRef]
- Mohammed, J.M.; Sajana, T.M.; Raj, R.; Rachana, B.A.; Paul, G.; Pillai, P.R. ‘The Violet Killer’-Successful Treatment of an Infant with Chromobacterium violaceum Sepsis. J. Trop. Pediatr. 2021, 67, fmab076. [Google Scholar] [CrossRef]
- Laghu, U.; Yanagawa, M.; Morimoto, K.; Dhoubhadel, B.G. Chromobacterium violaceum: A Rare Cause of Urinary Tract Infection. Case Rep. Infect. Dis. 2021, 2021, 5840899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Zhang, Y.Z.; Li, X.N. Chromobacterium violaceum infection on lower limb skin: A case report. Medicine 2021, 100, e24696. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Lee, N.; Wey, E.Q. Chromobacterium violaceum causing disseminated soft tissue and pulmonary abscesses in a traveller returning from the Azores. Access Microbiol. 2021, 3, 000251. [Google Scholar] [CrossRef]
- Cubides Diaz, D.A.; Arsanios Martin, D.; Bernal Ortiz, N.; Ovalle Monroy, A.L.; Hernandez Angarita, V.; Mantilla Florez, Y.F. Chromobacterium violaceum Periareolar Infection, First Non-Lethal Case in Colombia: Case Report and Literature Review. Infect. Dis. Rep. 2021, 13, 571–581. [Google Scholar] [CrossRef]
- Lang, L.; Wang, M.; Huang, X.; Zhou, H.; Zhou, Z.; Huang, L.; Zheng, H.; Zeng, K.; Li, L. Successful treatment of a patient with recurrent infection of Chromobacterium violaceum. BMC Infect. Dis. 2021, 21, 484. [Google Scholar] [CrossRef]
- Er, C.J.; Chun, W.K.; Chiang, L.M.; Nasir, M. Pediatric osteomyelitis due to rare tropical multi-drug resistance (MDR) organisms: A treatment quandary. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e7. [Google Scholar] [CrossRef]
- Takeda, S.; Tanaka, Y.; Maeda, M.; Hayakawa, H.; Mitsuya, S.; Yamauchi, K.I. The First Fatal Case of Chromobacterium violaceum Infection in Japan. Am. J. Case Rep. 2021, 22, e932037. [Google Scholar] [CrossRef]
- Sachu, A.; Antony, S.; Mathew, P.; Sunny, S.; Koshy, J.; Kumar, V.; Mathew, R. Chromobacterium violaceum causing deadly sepsis. Iran. J. Microbiol. 2020, 12, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, R.; Sadique, T.; Sen, D.; Mozumder, P.; Rahman, T.; Chowdhury, A.; Halim, F.; Akter, N.; Ahmed, D. Agricultural Injury-Associated Chromobacterium violaceum Infection in a Bangladeshi Farmer. Am. J. Trop. Med. Hyg. 2020, 103, 1039–1042. [Google Scholar] [CrossRef]
- Moretti, E.; Baggi Menozzi, F.; Elzi, L.; Lepori, M. Chromobacterium violaceum bacteraemia: A new entity in Switzerland. Swiss Med. Wkly. 2020, 150, w20220. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, S.; Jahan, A.A.; Kamal, S.M.M.; Sarker, P. Fatal Infection Caused by Chromobacterium violaceum: A Case Report from a Tertiary Care Hospital in Bangladesh. Case Rep. Infect. Dis. 2019, 2019, 6219295. [Google Scholar] [CrossRef] [PubMed]
- Olalekan, A.; Itua, F.; Mutiu, B.; Egwuatu, T.; Akinloye, O.; Iwalokun, B. Case Report on Pleural Empyema Thoracis and Urinary Tract Infection Caused by Chromobacterium violaceum from Lagos, Nigeria. Case Rep. Med. 2019, 2019, 5321484. [Google Scholar] [CrossRef]
- Jędruszczak, A.; Węgrzyn-Bąk, M.; Budzyńska-Nosal, R.; Maciejewski, M.; Marczewski, K. Sepsis caused by Chromobacterium violaceum—probably the first case in Europe, or Macbeth read anew. Ann. Agric. Environ. Med. 2019, 26, 508–510. [Google Scholar] [CrossRef]
- Bansie, R.; Harkisoen, S.; Lachman, V.; Lai, A.F.E.; Ramdhani, N.; van Laar, J.A.M. A rare infection in the tropics that is not uncommon in cases of chronic granulomatous disease. Access Microbiol. 2019, 1, e000039. [Google Scholar] [CrossRef]
- Dzupova, O.; Benes, J. Serious imported infections: A focus on Chromobacterium violaceum. Bratisl. Lek. Listy 2019, 120, 730–733. [Google Scholar] [CrossRef]
- Victorica, B.; Baer, H.; Ayoub, E.M. Successful treatment of systemic Chromobacterium violaceum infection. JAMA 1974, 230, 578–580. [Google Scholar] [CrossRef]
- Madi, D.R.; Vidyalakshmi, K.; Ramapuram, J.; Shetty, A.K. Successful Treatment of Chromobacterium violaceum Sepsis in a South Indian Adult. Am. J. Trop. Med. Hyg. 2015, 93, 1066–1067. [Google Scholar] [CrossRef]
- Khadanga, S.; Karuna, T.; Dugar, D.; Satapathy, S.P. Chromobacterium violaceum-induced sepsis and multiorgan dysfunction, resembling melioidosis in an elderly diabetic patient: A case report with review of literature. J. Lab. Physicians 2017, 9, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.D.; Acharya, S.P.; Bhandari, R.; Yadav, U.N.; Saru, D.B.; Sharma, M. Bacteremia and Urinary Tract Infection Caused by Chromobacterium violaceum: Case Reports from a Tertiary Care Hospital in Kathmandu, Nepal. Case Rep. Med. 2017, 2017, 7929671. [Google Scholar] [CrossRef]
- Lim, I.W.; Stride, P.J.; Horvath, R.L.; Hamilton-Craig, C.R.; Chau, P.P. Chromobacterium violaceum endocarditis and hepatic abscesses treated successfully with meropenem and ciprofloxacin. Med. J. Aust. 2009, 190, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, H.; Murase, T.; Suzuki, M.; Shibayama, K.; Kokumai, Y.; Watanabe, N.; Maki, M.; Otsuka, F. Chromobacterium violaceum nosocomial pneumonia in two Japanese patients at an intensive care unit. J. Infect. Chemother. 2014, 20, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.C.; Lane, J.E.; Stephens, J.L. Successful treatment of an infant with Chromobacterium violaceum sepsis. Clin. Infect. Dis. 2001, 32, E107–E110. [Google Scholar] [CrossRef]
| Antibiotic | Minimal Inhibitory Concentration |
|---|---|
| piperacillin | 32 µg/mL |
| ceftazidim | ≥256 µg/mL |
| meropenem | 1 µg/mL * |
| ciprofloxacin | 0.016 µg/mL * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klenk, C.; Schnieders, M.; Heinemann, M.; Wiegard, C.; Büttner, H.; Ramharter, M.; Jordan, S.; Mackroth, M.S. Severe Systemic Chromobacterium violaceum Infection: A Case Study of a German Long-Term Resident in French Guyana. Trop. Med. Infect. Dis. 2024, 9, 242. https://doi.org/10.3390/tropicalmed9100242
Klenk C, Schnieders M, Heinemann M, Wiegard C, Büttner H, Ramharter M, Jordan S, Mackroth MS. Severe Systemic Chromobacterium violaceum Infection: A Case Study of a German Long-Term Resident in French Guyana. Tropical Medicine and Infectious Disease. 2024; 9(10):242. https://doi.org/10.3390/tropicalmed9100242
Chicago/Turabian StyleKlenk, Caroline, Miriam Schnieders, Melina Heinemann, Christiane Wiegard, Henning Büttner, Michael Ramharter, Sabine Jordan, and Maria Sophia Mackroth. 2024. "Severe Systemic Chromobacterium violaceum Infection: A Case Study of a German Long-Term Resident in French Guyana" Tropical Medicine and Infectious Disease 9, no. 10: 242. https://doi.org/10.3390/tropicalmed9100242
APA StyleKlenk, C., Schnieders, M., Heinemann, M., Wiegard, C., Büttner, H., Ramharter, M., Jordan, S., & Mackroth, M. S. (2024). Severe Systemic Chromobacterium violaceum Infection: A Case Study of a German Long-Term Resident in French Guyana. Tropical Medicine and Infectious Disease, 9(10), 242. https://doi.org/10.3390/tropicalmed9100242







