Cross-Resistance to Pyrethroids and Neonicotinoids in Malaria Vectors from Vegetable Farms in the Northern Benin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Knowledge, Attitudes, and Practices (KAP) Survey
2.3. Sampling and Rearing of An. gambiae s.l.
2.4. Insecticides Susceptibility Tests
2.4.1. WHO Bioassays
2.4.2. CDC Bottle Bioassay
2.4.3. Synergists Assays
2.5. Species Identification
2.6. PCR Detection of the Kdr-L1014F, Kdr-L1014S, N1775Y, and ace-1-G119S
2.7. Data Analysis
3. Results
3.1. Knowledge, Attitudes, and Practices (KAP) Surveys
3.2. Resistance Status
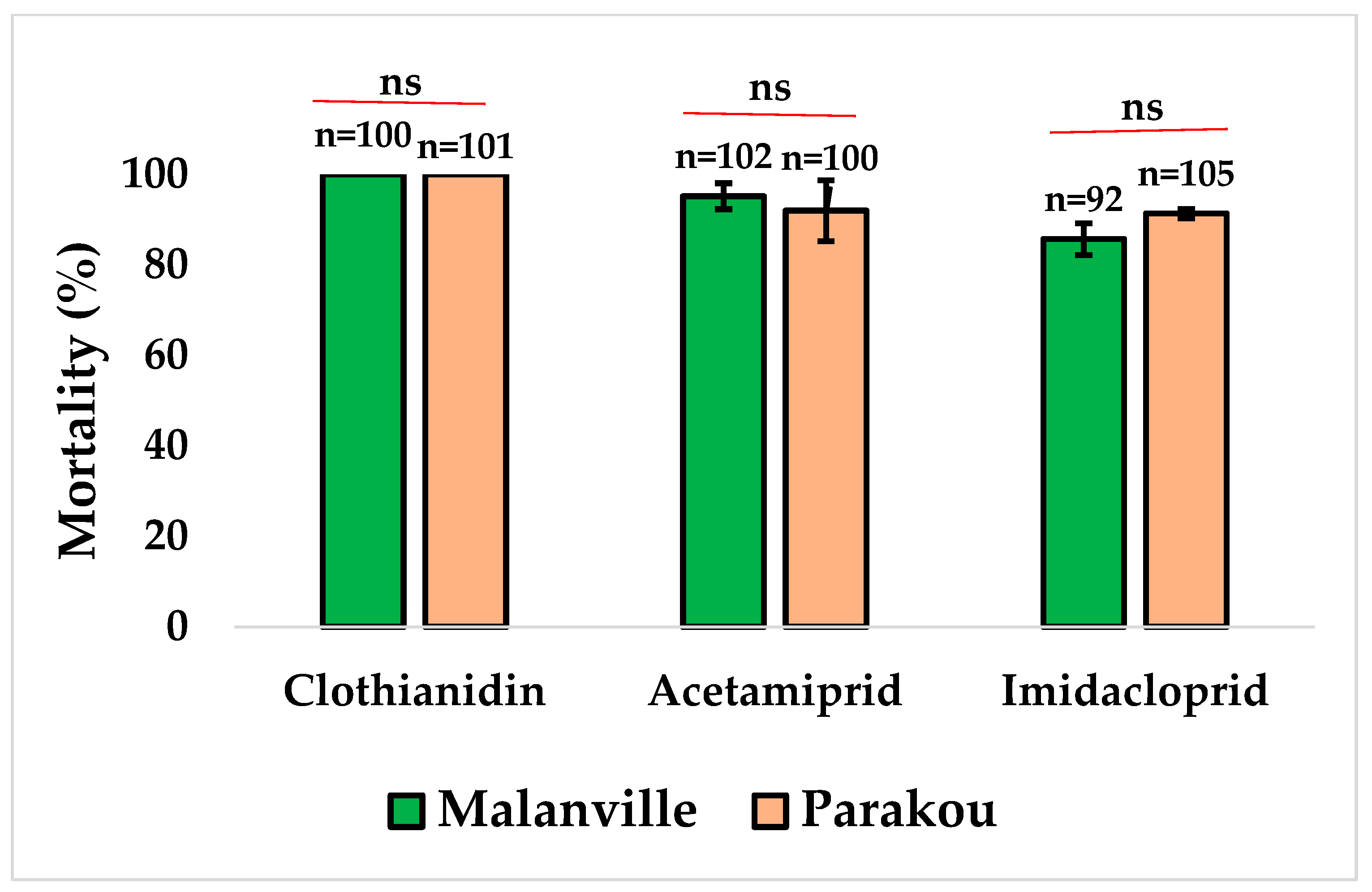
3.2.1. Susceptibility Profile of An. gambiae s.l. to Neonicotinoids
3.2.2. Susceptibility Profile of An. gambiae s.l. to Pyrethroids, Organophosphates, and DDT
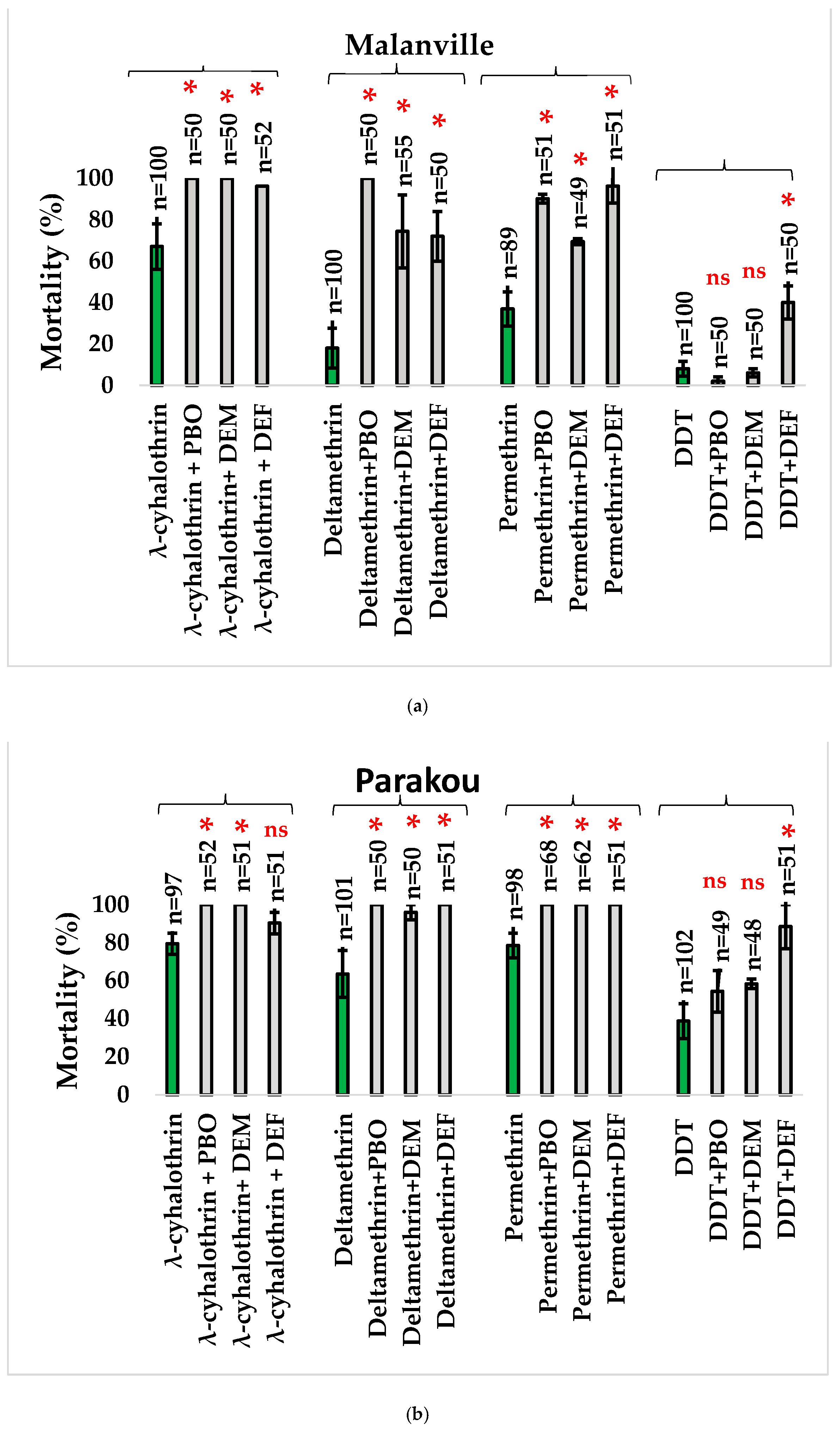
3.3. Synergists Effects
3.3.1. Synergist Tests with Imidacloprid
3.3.2. Synergist Tests with Other Classes of Insecticides
3.4. Species Identification of An. gambiae s.l.
3.5. Detection of Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleinschmidt, I.; Bradley, J.; Knox, T.B.; Mnzava, A.P.; Kafy, H.T.; Mbogo, C.; Ismail, B.A.; Bigoga, J.D.; Adechoubou, A.; Raghavendra, K.; et al. Implications of Insecticide Resistance for Malaria Vector Control with Long-Lasting Insecticidal Nets: A WHO-Coordinated, Prospective, International, Observational Cohort Study. Lancet Infect. Dis. 2018, 18, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The Effect of Malaria Control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H.; Magill, A.; Kolaczinski, J.; Fornadel, C.; Gimnig, J.; Coetzee, M.; Simard, F.; Roch, D.K.; Hinzoumbe, C.K.; et al. Averting a Malaria Disaster: Will Insecticide Resistance Derail Malaria Control? Lancet 2016, 387, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Sagbohan, H.; Kpanou, C.; Sovi, A.; Osse, R.; Aboubakar, S.; Adoha, C.; Yovogan, B.; Salako, A.; Ahogni, I.B.; Fassinou, A.; et al. Pyrethroid Resistance Intensity in Anopheles gambiae s.l. from Different Agricultural Production Zones in Benin, West Africa. Vector Borne Zoonotic Dis. Larchmt. N 2022, 22, 39–47. [Google Scholar]
- Djouaka, R.; Riveron, J.M.; Yessoufou, A.; Tchigossou, G.; Akoton, R.; Irving, H.; Djegbe, I.; Moutairou, K.; Adeoti, R.; Tamò, M.; et al. Multiple Insecticide Resistance in an Infected Population of the Malaria Vector Anopheles funestus in Benin. Parasit. Vectors 2016, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Aïkpon, R.; Agossa, F.; Ossè, R.; Oussou, O.; Aïzoun, N.; Oké-Agbo, F.; Akogbéto, M. Bendiocarb Resistance in Anopheles gambiae s.l. Populations from Atacora Department in Benin, West Africa: A Threat for Malaria Vector Control. Parasit. Vectors 2013, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Tchigossou, G.; Djouaka, R.; Akoton, R.; Riveron, J.M.; Irving, H.; Atoyebi, S.; Moutairou, K.; Yessoufou, A.; Wondji, C.S. Molecular Basis of Permethrin and DDT Resistance in an Anopheles funestus Population from Benin. Parasit. Vectors 2018, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Aïzoun, N.; Adjatin, A.; Assongba, F.; Alowanou, G. Dynamics of Insecticide Resistance in Anopheles gambiae Sensu Lato Populations from Bohicon in Sudano-Guinean Area of Benin, West Africa. World J. Adv. Res. Rev. 2022, 14, 476–482. [Google Scholar] [CrossRef]
- Kpanou, C.D.; Sagbohan, H.W.; Dagnon, F.; Padonou, G.G.; Ossè, R.; Salako, A.S.; Sidick, A.; Sewadé, W.; Sominahouin, A.; Condo, P.; et al. Characterization of Resistance Profile (Intensity and Mechanisms) of Anopheles gambiae in Three Communes of Northern Benin, West Africa. Malar. J. 2021, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Djouaka, R.F.; Bakare, A.A.; Bankole, H.S.; Doannio, J.M.; Coulibaly, O.N.; Kossou, H.; Tamo, M.; Basene, H.I.; Popoola, O.; Akogbeto, M.C. Does the Spillage of Petroleum Products in Anopheles Breeding Sites Have an Impact on the Pyrethroid Resistance? Malar. J. 2007, 6, 159. [Google Scholar] [CrossRef]
- Chouaibou, M.; de Souza, S.S.; Fodjo, B.K.; Zoh, M.G.; Bli, N.K.; Koudou, B.G. Evidence of Insecticide Resistance Selection in Wild Anopheles coluzzii Mosquitoes Due to Agricultural Pesticide Use. Infect. Dis. Poverty 2019, 8, 64. [Google Scholar]
- Oumbouke, W.A.; Rowland, M.; Koffi, A.A.; Alou, L.P.A.; Camara, S.; N’Guessan, R. Evaluation of an Alpha-Cypermethrin + PBO Mixture Long-Lasting Insecticidal Net VEERALIN® LN against Pyrethroid Resistant Anopheles gambiae s.s.: An Experimental Hut Trial in M’bé, Central Côte d’Ivoire. Parasit. Vectors 2019, 12, 544. [Google Scholar] [CrossRef]
- Zoungbédji, D.M.; Padonou, G.G.; Keller, A.; Konkon, A.S.S.; Adoha, C.J.; Fassinou, A.J.Y.; Sina, H.; Baba-Mouss, L.; Akogbéto, M. Evaluation of the Susceptibility of An. gambiae s.l. to Deltamethrin, Chlorfenapyr and Clothianidin in Four Agricultural Areas of Benin. Int. J. Mosq. Res. 2023, 10, 39–46. [Google Scholar]
- WHO. Vector Control Product List. 2020. Available online: https://extranet.who.int/prequal/vector-control-products/prequalified-product-list/export (accessed on 4 May 2024).
- Elamathi, N.; Vaishali Verma, V.V.; Sharma, V.P.; Sreehari, U.; Raghavendra, K. Neonicotinoids in Vector Control: In Silico Approach. Asian J. Pharm. Sci. 2014, 4, 25–29. [Google Scholar]
- Krupke, C.H.; Long, E.Y. Intersections between Neonicotinoid Seed Treatments and Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 8–13. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide Resistance in Malaria Vectors: An Update at a Global Scale. In Towards Malaria Elimination—A Leap Forward; IntechOpen: London, UK, 2018; Volume 2, pp. 149–175. [Google Scholar]
- Ahouangninou, C.C.A. Durabilité de la Production Maraîchère au Sud-Bénin: Un Essai de l’Approche Ecosystémique. PhD Thesis, Université d’Abomey, Calavi, Bénin, 2013. [Google Scholar]
- Houndji, M.A.B.; Toko, I.I.; Guedegba, L.; Agbohessi, P.T.; Yacouto, E.; Schiffers, B.; Scippo, M.-L.; Kestemont, P. Toxicité Aigüe et Subaigüe de l’Insecticide Acer 35 EC et de la Lambda-cyhalothrine Chez les Œufs Fécondés et les Géniteurs du Poisson-chat africain (Clarias gariepinus). Ann. L’Univ. Parakou Sér. Sci. Nat. Agron. 2019, 9, 79–90. [Google Scholar] [CrossRef]
- Zoumenou, B.; Aina, M.; Toko, I.; Igout, A.; Douny, C.; Brose, F.; Schiffers, B.; Gouda, I.; Sika, K.; Kestemont, P.; et al. Occurrence of Acetamiprid Residues in Water Reservoirs in the Cotton Basin of Northern Benin. Bull. Environ. Contam. Toxicol. 2019, 102, 7–12. [Google Scholar] [CrossRef]
- Zakari, S. Vulnérabilités des Parcours Naturels Aux Changements Climatiques Dans Le Bassin de La Sota à L’exutoire de Couberi (Bénin). Ph.D. Thesis, Université d’Abomey, Calavi, Bénin, 2015. [Google Scholar]
- Yadouleton, A.; Yvette, B.; Falilath, S.; Gildas, H.; Carine, T.; Praise, A.; Lamine, B.-M. Development of Rice Farming: A Cause of the Emergence of Multiple Insecticide Resistance in Populations of Anopheles gambiae s.l. and Its Impact on Human Health in Malanville, Bénin. Malawi Med. J. 2023, 35, 170–176. [Google Scholar]
- Gnanguenon, V.; Agossa, F.R.; Badirou, K.; Govoetchan, R.; Anagonou, R.; Oke-Agbo, F.; Azondekon, R.; AgbanrinYoussouf, R.; Attolou, R.; Tokponnon, F.T.; et al. Malaria Vectors Resistance to Insecticides in Benin: Current Trends and Mechanisms Involved. Parasit. Vectors 2015, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- INSAE. Effectifs de La Population Des Villages et Quartiers de Ville Du Benin (RGPH–4., 2013). Institut National de La Statistique et de l’Analyse Economique. Available online: https://www.google.com/search? (accessed on 26 April 2024).
- Silver, J.B. Mosquito Ecology: Field Sampling Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 978-1-4020-6666-5. [Google Scholar]
- WHO. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests. 2022. Available online: https://iris.who.int/bitstream/handle/10665/352316/9789240043831-eng.pdf?sequence=1 (accessed on 4 May 2024).
- Tchouakui, M.; Assatse, T.; Mugenzi, L.M.J.; Menze, B.D.; Nguiffo-Nguete, D.; Tchapga, W.; Kayondo, J.; Watsenga, F.; Manzambi, E.Z.; Osae, M.; et al. Comparative Study of the Effect of Solvents on the Efficacy of Neonicotinoid Insecticides against Malaria Vector Populations across Africa. Infect. Dis. Poverty 2022, 11, 35. [Google Scholar] [CrossRef]
- Fouet, C.; Ashu, A.F.; Ambadiang, M.M.; Tchapga, W.; Wondji, C.S.; Kamdem, C. Clothianidin-Resistant Anopheles gambiae Adult Mosquitoes From Yaoundé, Cameroon, Display Reduced Susceptibility to SumiShield® 50WG, a Neonicotinoid Formulation for Indoor Residual Spraying. BMC Infect. Dis. 2024, 24, 133. [Google Scholar] [CrossRef]
- Livak, K.J. Organization and Mapping of a Sequence on the Drosophila melanogaster X and Y Chromosomes that is Transcribed During Spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Brogdon, W.; Collins, F. Identification of Single Specimens of the Anopheles gambiae Complex by the Polymerase Chain Reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion Polymorphisms of SINE200 Retrotransposons within Speciation Islands of Anopheles gambiae Molecular Forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Donnelly, M.J.; Williamson, M.S.; Ranson, H.; Ball, A.; Vontas, J.; Field, L.M. Detection of Knockdown Resistance (Kdr) Mutations in Anopheles gambiae: A Comparison of Two New High-Throughput Assays with Existing Methods. Malar. J. 2007, 6, 111. [Google Scholar] [CrossRef]
- Jones, C.M.; Liyanapathirana, M.; Agossa, F.R.; Weetman, D.; Ranson, H.; Donnelly, M.J.; Wilding, C.S. Footprints of Positive Selection Associated with a Mutation (N1575Y) in the Voltage-Gated Sodium Channel of Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2012, 109, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of High-Throughput Real-Time PCR Assays for the Identification of Insensitive Acetylcholinesterase (Ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Yadouleton, A.; Martin, T.; Padonou, G.; Chandre, F.; Asidi, A.; Djogbenou, L.; Dabiré, R.; Aïkpon, R.; Boko, M.; Glitho, I.; et al. Cotton Pest Management Practices and the Selection of Pyrethroid Resistance in Anopheles gambiae Population in Northern Benin. Parasit. Vectors 2011, 4, 60. [Google Scholar] [CrossRef]
- Kpanou, C.D.; Sagbohan, H.W.; Sovi, A.; Osse, R.; Padonou, G.G.; Salako, A.; Tokponnon, F.; Fassinou, A.J.; Yovogan, B.; Nwangwu, U.C. Assessing Insecticide Susceptibility and Resistance Intensity of Anopheles gambiae s.l. Populations from Some Districts of Benin Republic, West Africa. J. Med. Entomol. 2022, 59, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Medjigbodo, A.A.; Djogbénou, L.S.; Djihinto, O.Y.; Akoton, R.B.; Abbey, E.; Kakossou, R.M.; Sonounameto, E.G.; Salavi, E.B.J.; Djossou, L.; Badolo, A. Putative Pleiotropic Effects of the Knockdown Resistance (L1014F) Allele on the Life-History Traits of Anopheles gambiae. Malar. J. 2021, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Djegbe, I.; Dramane, G.; Zeukeng, F.; Akogbeto, M.; Djouaka, R. Relationship between Kdr L1014F Genotypes and Phenotypic-Resistance to Pyrethroids and DDT Insecticides in Anopheles gambiae Ss. Int. J. Innov. Appl. Stud. 2017, 20, 983–993. [Google Scholar]
- Djègbè, I.; Toponon, F.; Gbankoto, A.; Tchigossou, G.; Djossou-Hessou, D.; Dossou, C.; Yessoufou, A.; Akogbéto, M.; Djogbénou, L.; Djouaka, R. Typologie Des Gîtes Larvaires et Résistance Des Vecteurs Du Paludisme a La Deltaméthrine Dans Les Milieux Urbain et Rural Du Département de l’Atlantique Au Sud Du Bénin: Données Préliminaires. Eur. Sci. J. 2019, 15, 1857–7881. [Google Scholar] [CrossRef]
- Djènontin, A.; Bio-Bangana, S.; Moiroux, N.; Henry, M.-C.; Bousari, O.; Chabi, J.; Ossè, R.; Koudénoukpo, S.; Corbel, V.; Akogbéto, M.; et al. Culicidae Diversity, Malaria Transmission and Insecticide Resistance Alleles in Malaria Vectors in Ouidah-Kpomasse-Tori District from Benin (West Africa): A Pre-Intervention Study. Parasit. Vectors 2010, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Djogbenou, L.; Benoit, A.; Laurette, D.; Michel, M. Indirect Evidence That Agricultural Pesticides Select for Insecticide Resistance in the Malaria Vector Anopheles Gambiae. J. Vector Ecol. 2016, 41, 34–40. [Google Scholar]
- Akogbeto, M.C.; Djouaka, R.; Noukpo, H. Use of agricultural insecticides in Benin. Bull. Soc. Pathol. Exot. 2005, 98, 400–405. [Google Scholar] [PubMed]
- Talom, A.D.; Essoung, M.A.; Gbankoto, A.; Tchigossou, G.; Akoton, R.; Sahabi, B.B.A.; Atoyebi, S.M.; Fotso Kuate, A.; Verspoor, R.L.; Tamò, M.; et al. A Preliminary Analysis on the Effect of Copper on Anopheles coluzzii Insecticide Resistance in Vegetable Farms in Benin. Sci. Rep. 2020, 10, 6392. [Google Scholar] [CrossRef]
- Bradford, B.Z.; Huseth, A.S.; Groves, R.L. Widespread Detections of Neonicotinoid Contaminants in Central Wisconsin Groundwater. PLoS ONE 2018, 13, 17. [Google Scholar] [CrossRef]
- Assatse, T.; Tchouakui, M.; Mugenzi, L.; Menze, B.; Nguiffo-Nguete, D.; Tchapga, W.; Kekeunou, S.; Wondji, C.S. Anopheles funestus Populations across Africa Are Broadly Susceptible to Neonicotinoids but with Signals of Possible Cross-Resistance from the GSTe2 Gene. Trop. Med. Infect. Dis. 2023, 8, 244. [Google Scholar] [CrossRef]
- Ngufor, C.; Fongnikin, A.; Rowland, M.; N’Guessan, R. Indoor Residual Spraying with a Mixture of Clothianidin (a Neonicotinoid Insecticide) and Deltamethrin Provides Improved Control and Long Residual Activity against Pyrethroid Resistant Anopheles gambiae s.l. in Southern Benin. PLoS ONE 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Anderson, T.D. High Levels of Resistance in the Common Bed Bug, Cimex lectularius (Hemiptera: Cimicidae), to Neonicotinoid Insecticides. J. Med. Entomol. 2016, 53, 727–731. [Google Scholar] [CrossRef]
- Cocou, A.; Djihinto, A.; Azonkpin, S.; Affokpon, A.; Chougourou, D. Effet Du Délintage et de Six Produits de Traitement de Semences En Culture Cotonnière Au Bénin. Afr. Sci. 2022, 21, 167–179. [Google Scholar]
- Padonou, G.; Kpanou, C.; Osse, R.; Salako, A.; Yovogan, B.; Sagbohan, H.; Konkon, A.; Zoungbédji, D.; Akinro, B.; SINA, H.; et al. Evaluation of the Efficacy of 2.15% Imidacloprid (ROCO GEL) Gel against Periplaneta Americana under Laboratory and Field Conditions in Benin. Acta Entomol. Zool. 2023, 4, 95–102. [Google Scholar] [CrossRef]
- Oxborough, R.; Seyoum, A.; Yihdego, Y.; Dabiré, R.; Gnanguenon, V.; Wat’senga, F.; Agossa, F.; Yohannes, G.; Coleman, S.; Samdi, L.; et al. Susceptibility Testing of Anopheles Malaria Vectors with the Neonicotinoid Insecticide Clothianidin; Results from 16 African Countries, in Preparation for Indoor Residual Spraying with New Insecticide Formulations. Malar. J. 2019, 18, 264. [Google Scholar] [CrossRef]
- Djègbè, I.; Boussari, O.; Sidick, A.; Martin, T.; Ranson, H.; Chandre, F.; Akogbéto, M.; Corbel, V. Dynamics of Insecticide Resistance in Malaria Vectors in Benin: First Evidence of the Presence of L1014S Kdr Mutation in Anopheles gambiae from West Africa. Malar. J. 2011, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; N’guessan, R.; Brengues, C.; Chandre, F.; Djogbenou, L.; Martin, T.; Akogbeto, M.; Hougard, J.-M.; Rowland, M. Multiple Insecticide Resistance Mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007, 101, 207–216. [Google Scholar] [CrossRef]
- Apetogbo, Y.; Ahadji-Dabla, K.M.; Soma, D.D.; Amoudji, A.D.; Koffi, E.; Akagankou, K.I.; Bamogo, R.; Ngaffo, K.L.; Maiga, S.; Atcha-Oubou, R.T.; et al. Insecticide Resistance Intensity and Efficacy of Synergists with Pyrethroids in Anopheles gambiae (Diptera: Culicidae) from Southern Togo. Malar. J. 2022, 21, 353. [Google Scholar] [CrossRef]
- Gross, A.D.; Bloomquist, J.R. Characterizing Permethrin and Etofenprox Resistance in Two Common Laboratory Strains of Anopheles gambiae (Diptera: Culicidae). Insects 2018, 9, 146. [Google Scholar] [CrossRef]
- Joseph, R.N.; Mwema, T.; Eiseb, S.J.; Maliti, D.V.; Tambo, M.; Iitula, I.; Eloff, L.; Lukubwe, O.; Smith-Gueye, C.; Vajda, É.A.; et al. Insecticide Susceptibility Status of Anopheles gambiae Mosquitoes and the Effect of Pre-Exposure to a Piperonyl Butoxide (PBO) Synergist on Resistance to Deltamethrin in Northern Namibia. Malar. J. 2024, 23, 77. [Google Scholar] [CrossRef]
- Agumba, S.; Gimnig, J.E.; Ogonda, L.; Ombok, M.; Kosgei, J.; Munga, S.; Guyah, B.; Omondi, S.; Ochomo, E. Diagnostic Dose Determination and Efficacy of Chlorfenapyr and Clothianidin Insecticides against Anopheles Malaria Vector Populations of Western Kenya. Malar. J. 2019, 18, 243. [Google Scholar] [CrossRef]
- Ashu, F.A.; Fouet, C.; Ambadiang, M.M.; Penlap-Beng, V.; Kamdem, C. Adult Mosquitoes of the Sibling Species Anopheles gambiae and Anopheles coluzzii Exhibit Contrasting Patterns of Susceptibility to Four Neonicotinoid Insecticides along an Urban-to-Rural Gradient in Yaoundé, Cameroon. Malar. J. 2024, 23, 65. [Google Scholar] [CrossRef]




| Primers | Sequences |
|---|---|
| kdr-Forward | 5′-CATTTTTCTTGGCCACTGTAGTGAT-3′ |
| kdr-Reverse | 5′-CGATCTTGGTCCATGTTAATTTGCA-3′ |
| probe WT | 5′-CTTACGACTAAATTTC-3′ |
| probes kdr-W | 5′-ACGACAAAATTTC-3′ |
| primer G119-R1 | 5′-CGGTGGTCGTACACGTCCAGGGT-3′ |
| G119S-F1 | 5′-GCGGGCAGGGCGGCGGGGGCGGGGCCCTGTGGATCTTCGGCGGCG-3′ |
| primer G119R-F1 | 5′-GCGGGCCTGTGGATCTTCGGCGGCA-3′ |
| Parameters | Characteristics | Malanville n (%) | Parakou n (%) |
|---|---|---|---|
| Age | less than 20 | 0 (0.00) | 5 (11.11) |
| 20 to 30 | 11 (27.50) | 24 (53.33) | |
| 31 to 40 | 14 (35.00) | 9 (20.00) | |
| 41 to 50 | 5 (12.50) | 3 (6.67) | |
| 51 to 60 | 6 (15.00) | 3 (6.67) | |
| more than 60 | 4 (10.00) | 1 (2.22) | |
| Sex | Male | 40 (100.00) | 45 (100.00) |
| Female | 0 (0.00) | 0 (0.00) | |
| Main activity | Market gardening | 15 (37.50) | 43 (95.56) |
| Agriculture | 25 (62.50) | 2 (4.44) | |
| Education | No formal schooling | 36 (90.00) | 13 (28.89) |
| Primary | 0 (0.00) | 11 (24.44) | |
| Secondary | 4 (10.00) | 16 (35.56) | |
| University | 0 (0.00) | 5 (11.11) | |
| Year from which these pesticides are used | 0 to 10 | 21 (52.50) | 35 (77.78) |
| 11 to 20 | 8 (20.00) | 6 (13.33) | |
| More than 20 | 11 (27.50) | 4 (4.44) | |
| Recognition of the effectiveness of pesticides | State of health of the plant | 31 (77.50) | 40 (88.89) |
| Low number of pests | 0 (0.00) | 3 (6.67) | |
| Both | 9 (22.50) | 2 (4.44) | |
| Number of persons surveyed by site | 40 | 45 | |
| Localities | Mutations | Alive | Dead | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | RR | RS | SS | f(R) | N | RR | RS | SS | f(R) | p Value (Z = Value of the Normality) | ||
| Malanville | kdr West | 60 | 22 | 19 | 19 | 0.53 | 60 | 12 | 31 | 17 | 0.46 | 0.26 (Z = 1.107) |
| kdr East | 60 | 0 | 7 | 53 | 0.06 | 60 | 0 | 0 | 60 | 0 | 0.33 (Z = −0.857) | |
| ace-1 | 60 | 0 | 1 | 59 | 0.01 | 60 | 2 | 4 | 54 | 0.07 | 0.04 (Z = −2.087) | |
| N1575Y | 60 | 0 | 4 | 56 | 0.03 | 60 | 0 | 7 | 53 | 0.06 | 0.07 (Z = −1.771) | |
| Parakou | kdr West | 60 | 33 | 9 | 18 | 0.63 | 60 | 27 | 2 | 31 | 0.47 | 0.08 (Z = 1.732) |
| kdr East | 60 | 0 | 22 | 38 | 0.18 | 60 | 0 | 15 | 45 | 0.12 | 0.93 (Z = −0.080) | |
| ace-1 | 60 | 0 | 0 | 60 | 0 | 60 | 1 | 0 | 59 | 0.02 | 0.27 (Z = −1.095) | |
| N1575Y | 60 | 0 | 0 | 60 | 0 | 60 | 0 | 0 | 60 | 0 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koto Yérima Gounou Boukari, M.; Djègbè, I.; Tepa-Yotto, G.T.; Hessou-Djossou, D.; Tchigossou, G.; Tossou, E.; Lontsi-Demano, M.; Adanzounon, D.; Gbankoto, A.; Djogbénou, L.; et al. Cross-Resistance to Pyrethroids and Neonicotinoids in Malaria Vectors from Vegetable Farms in the Northern Benin. Trop. Med. Infect. Dis. 2024, 9, 305. https://doi.org/10.3390/tropicalmed9120305
Koto Yérima Gounou Boukari M, Djègbè I, Tepa-Yotto GT, Hessou-Djossou D, Tchigossou G, Tossou E, Lontsi-Demano M, Adanzounon D, Gbankoto A, Djogbénou L, et al. Cross-Resistance to Pyrethroids and Neonicotinoids in Malaria Vectors from Vegetable Farms in the Northern Benin. Tropical Medicine and Infectious Disease. 2024; 9(12):305. https://doi.org/10.3390/tropicalmed9120305
Chicago/Turabian StyleKoto Yérima Gounou Boukari, Massioudou, Innocent Djègbè, Ghislain T. Tepa-Yotto, Donald Hessou-Djossou, Genevieve Tchigossou, Eric Tossou, Michel Lontsi-Demano, Danahé Adanzounon, Adam Gbankoto, Luc Djogbénou, and et al. 2024. "Cross-Resistance to Pyrethroids and Neonicotinoids in Malaria Vectors from Vegetable Farms in the Northern Benin" Tropical Medicine and Infectious Disease 9, no. 12: 305. https://doi.org/10.3390/tropicalmed9120305
APA StyleKoto Yérima Gounou Boukari, M., Djègbè, I., Tepa-Yotto, G. T., Hessou-Djossou, D., Tchigossou, G., Tossou, E., Lontsi-Demano, M., Adanzounon, D., Gbankoto, A., Djogbénou, L., & Djouaka, R. (2024). Cross-Resistance to Pyrethroids and Neonicotinoids in Malaria Vectors from Vegetable Farms in the Northern Benin. Tropical Medicine and Infectious Disease, 9(12), 305. https://doi.org/10.3390/tropicalmed9120305






