Infection of Leishmania donovani in Phlebotomus orientalis Sand Flies at Different Microhabitats of a Kala-Azar Endemic Village in Eastern Sudan
Abstract
1. Introduction
2. Materials and Methods
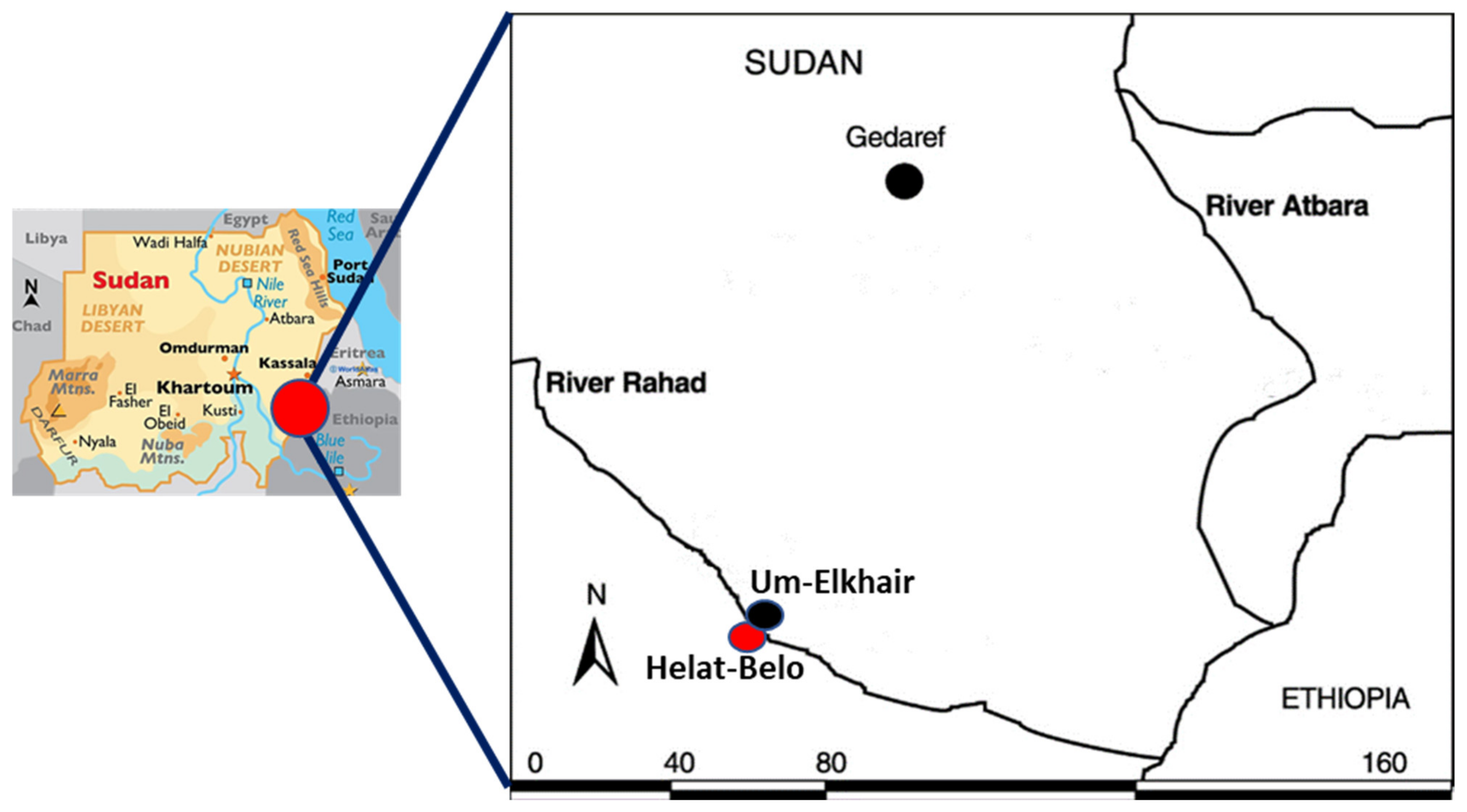
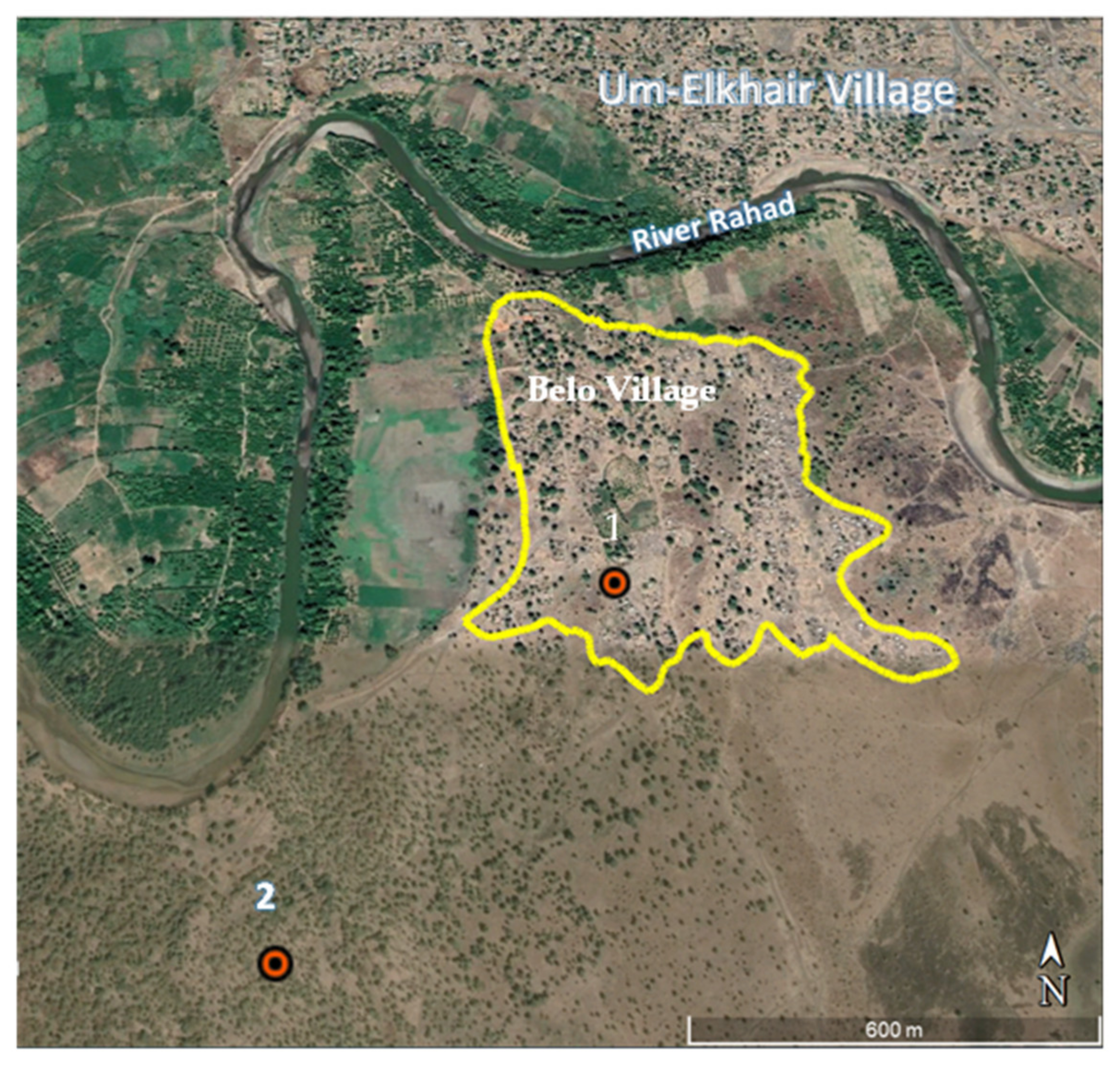
2.1. Study Area
2.2. Sand Fly Collection Sites and Protocol
2.3. Sand Fly Collection, Identification and DNA Extraction
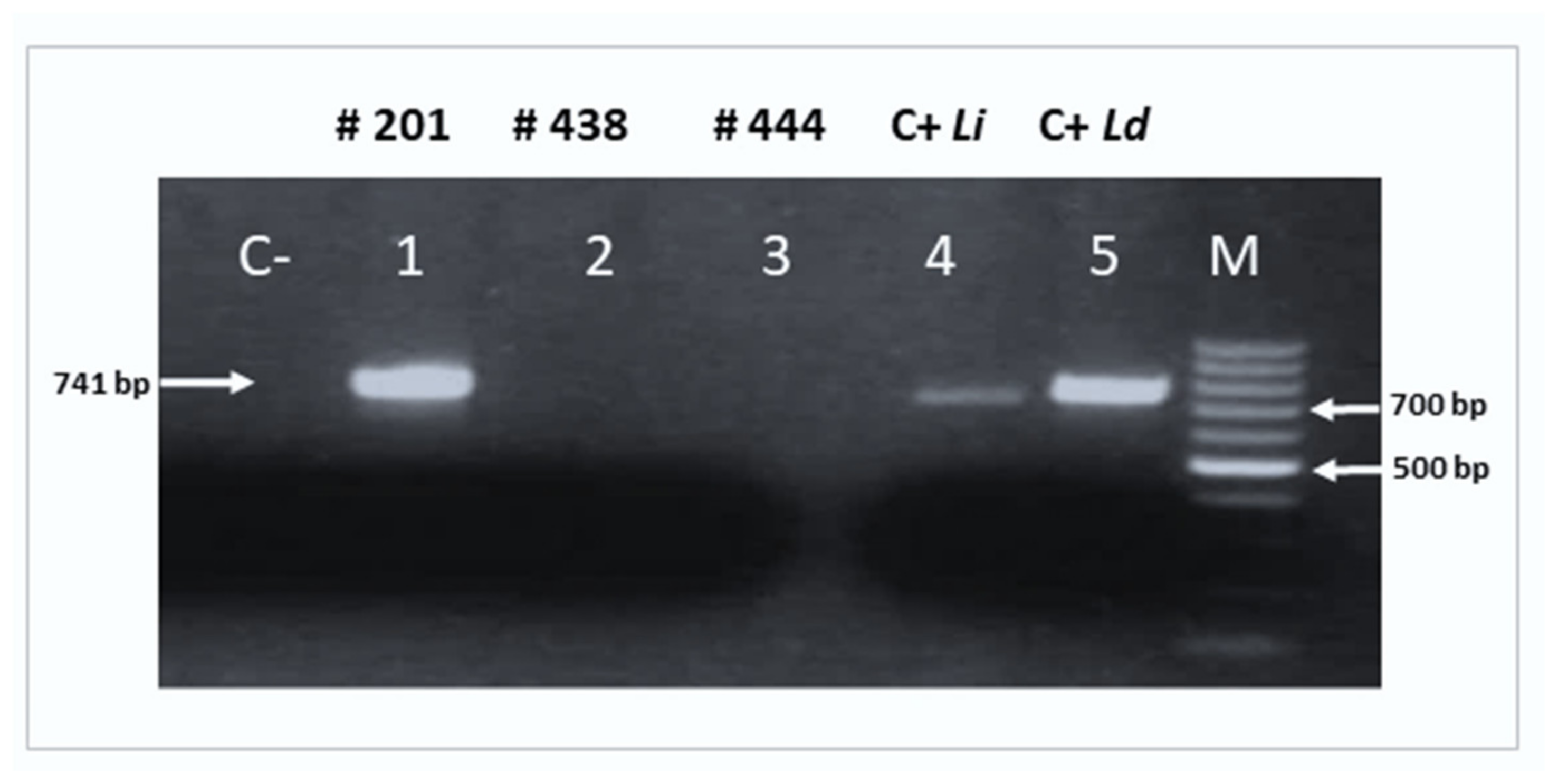
2.4. Molecular Detection of Leishmania spp.
2.5. Amplification of the cpb Gene
2.6. Characterization of Leishmania Species
2.7. Blood Meal Identification
2.8. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; den Boer, M.; Dagne, D.A. Towards the elimination of visceral leishmaniasis as a public health problem in east Africa: Reflections on an enhanced control strategy and a call for action. Lancet Glob. Health 2021, 9, e1763–e1769. [Google Scholar] [CrossRef] [PubMed]
- WHO. Operational Manual on Leishmaniasis Vector Control, Surveillance, Monitoring and Evaluation; World Health Organization: Geneva, Switzerland, 2022; Available online: https://iris.who.int/handle/10665/365615 (accessed on 21 December 2023).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, S.; Dondi, A.; Totaro, C.; Biagi, C.; Melchionda, F.; Zama, D.; Pierantoni, L.; Gennari, M.; Campagna, C.; Prete, A.; et al. Visceral leishmaniasis: Epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms 2022, 10, 1887. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leishmaniasis. WHO 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 21 December 2023).
- Makau-Barasa, L.K.; Ochol, D.; Yotebieng, K.A.; Adera, C.B.; de Souza, D.K. Moving from control to elimination of visceral leishmaniasis in East Africa. Front. Trop. Dis. 2022, 3, 1–7. [Google Scholar] [CrossRef]
- Hoogstraal, J.; Heyneman, D. Leishmaniasis in the Sudan Republic. Am. J. Trop. Med. Hyg. 1969, 18, 1091–1210. [Google Scholar] [CrossRef]
- Gebre-Michael, T.; Lane, R.P. The roles of Phlebotomus martini and P. celiae (Diptera: Phlebotominae) as vectors of visceral leishmaniasis in the Aba Roba focus, southern Ethiopia. Med. Vet. Entomol. 1996, 10, 53–62. [Google Scholar] [CrossRef]
- Elnaiem, D.A. Ecology and control of the sand fly vectors of Leishmania donovani in East Africa, with special emphasis on Phlebotomus orientalis. J. Vector Ecol. 2011, 36, S23–S31. [Google Scholar] [CrossRef]
- Gebresilassie, A.; Abbasi, I.; Kirstein, O.D.; Aklilu, E.; Yared, S.; Tekie, H.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-Michael, T. Physiological age structure and Leishmania spp. detection in Phlebotomus (Larroussius) orientalis (Parrot, 1936) (Diptera: Psychodidae) at an endemic focus of visceral leishmaniasis in Northern Ethiopia. J. Trop. Med. 2015, 2015, 710528. [Google Scholar] [CrossRef]
- Elnaiem, D.E.A.; Dakein, O.; Alawad, A.M.A.; Alsharif, B.; Khogali, A.; Jibreel, T.; Osman, O.F.; Has’san, H.; Atia, A.M.; Elhag, M.; et al. Outdoor residual insecticide spraying (ODRS), a new approach for the control of the Exophilic vectors of human visceral Leishmaniasis: Phlebotomus Orientalis in East Africa. PLoS Negl. Trop. Dis. 2020, 14, e0008774. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Heyneman, D.; Dietlein, D.R.; Browne, H.G.; Reid, T.P., Jr.; Van Peenen, P.F.; Saber, A.H.; Rohrs, L.C. Leishmaniasis in the Sudan Republic: Epidemiological findings. Bull. World Health Organ. 1963, 28, 263. [Google Scholar]
- Hoogstraal, H.; Dietlein, D.R. Leishmaniasis in the Sudan Republic. Ecological relationships of sandfly species and leishmaniasis infection. Am. J. Trop. Med. Hyg. 1963, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.A.; Ward, R.D.; Hassan, K.H.; Miles, M.A.; Frame, I.A. Infection rates of Leishmania donovani in Phlebotomus orientalis from a focus of visceral leishmaniasis in eastern Sudan. Ann. Trop. Med. Parasitol. 1998, 92, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Elraba’a, F.M.; Ward, R.D.; Maingon, R.; Elnaiem, D.A. Detection of high rates of in-village transmission of Leishmania donovani in eastern Sudan. Acta Trop. 2004, 92, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.A.; Connor, S.J.; Thomson, M.C.; Hassan, M.M.; Hassan, H.K.; Aboud, M.A. Environmental determinants of the distribution of Phlebotomus orientalis in Sudan. Ann. Trop. Med. Parasitol. 1998, 92, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C.; Elnaiem, D.A.; Ashford, R.W.; Connor, S.J. Towards a kala azar risk map for Sudan: Mapping the potential distribution of Phlebotomus orientalis using digital data of environmental variables. Trop. Med. Int. Health 1999, 4, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.A.; Hassan, H.K.; Ward, R.D. Phlebotomine sandflies in a focus of visceral leishmaniasis in a border area of eastern Sudan. Ann. Trop. Med. Parasitol. 1997, 91, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Gebresilassie, A.; Yared, S.; Aklilu, E.; Kirstein, O.D.; Moncaz, A.; Tekie, H.; Balkew, M.; Warburg, A.; Hailu, A.; Gebre-Michael, T. Host choice of Phlebotomus orientalis (Diptera: Psychodidae) in animal baited experiments: A field study in Tahtay Adiyabo district, northern Ethiopia. Parasites Vectors 2015, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Jibreel, T.; Khogali, A.; Jiménez, M.; Raiyed, A.; Dakein, O.; Alsharif, B.; Khalid, N.M.; Osman, O.F.; Nour, B.Y.; Mohamed, G.H.; et al. Host preference and human blood index of Phlebotomus orientalis, an exophilic sand fly vector of visceral leishmaniasis in eastern Sudan. Med. Vet. Entomol. 2023, 37, 782–792. [Google Scholar] [CrossRef]
- Quate, L.W. Phlebotomus sandflies of the Paloich Area in the Sudan (Diptera, Psychodidae). J. Med. Entomol. 1964, 1, 213–268. [Google Scholar] [CrossRef]
- Lemma, W.; Tekie, H.; Abassi, I.; Balkew, M.; Gebre-Michael, T.; Warburg, A.; Hailu, A. Nocturnal activities and host preferences of Phlebotomus orientalis in extra-domestic habitats of Kafta-Humera lowlands, Kala-azar endemic, Northwest Ethiopia. Parasites Vectors 2014, 7, 594. [Google Scholar] [CrossRef][Green Version]
- Yared, S.; Gebresilassie, A.; Abbasi, I.; Aklilu, E.; Kirstein, O.D.; Balkew, M.; Brown, A.S.; Clouse, R.M.; Warburg, A.; Hailu, A.; et al. A molecular analysis of sand fly blood meals in a visceral leishmaniasis endemic region of northwestern Ethiopia reveals a complex host-vector system. Heliyon 2019, 5, e02132. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, O.D.; Skrip, L.; Abassi, I.; Iungman, T.; Horwitz, B.Z.; Gebresilassie, A.; Spitzova, T.; Waitz, Y.; Gebre-Michael, T.; Volf, P.; et al. A fine scale eco-epidemiological study on endemic visceral leishmaniasis in north Ethiopian villages. Acta Trop. 2018, 183, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.A.; Hassan, M.M.; Maingon, R.; Nureldin, G.H.; Mekawi, A.M.; Miles, M.; Ward, R.D. The Egyptian mongoose, Herpestes ichneumon, is a possible reservoir host of visceral leishmaniasis in eastern Sudan. Parasitology 2001, 122, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.E.A.; Schorscher, J.; Bendall, A.; Obsomer, V.; Osman, M.E.; Mekkawi, A.M.; Connor, S.J.; Ashford, R.W.; Thomson, M.C. Risk mapping of visceral leishmaniasis: The role of local variation in rainfall and altitude on the presence and incidence of kala-azar in eastern Sudan. Am. J. Trop. Med. Hyg. 2003, 68, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Abonnenc, E. Les Phlébotomes de la région éthiopienne (Diptera: Psychodidae). Mémoire ORSTOM 1972, 55, 1–289. [Google Scholar]
- González, E.; Jiménez, M.; Hernández, S.; Martín, M.I.; Molina, R. Phlebotomine sand fly survey in the focus of leishmaniasis in Madrid, Spain (2012–2014): Seasonal dynamics, Leishmania infantum infection rates and blood meal preferences. Parasites Vectors 2017, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, L.; Prina, E.; Lang, T. Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J. Clin. Microbiol. 2002, 40, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Hide, M.; Bañuls, A. Species-specific PCR assay for L. infantum/L. donovani discrimination. Acta Trop. 2006, 100, 241–245. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Iriso, A.; Marco, E.; Alegret, A.; Fúster, F.; Molina, R. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniasis in Madrid, Spain. Parasitol. Res. 2013, 112, 2453–2459. [Google Scholar] [CrossRef]
- González, E.; Molina, R.; Iriso, A.; Ruiz, S.; Aldea, I.; Tello, A.; Fernández, D.; Jiménez, M. Opportunistic feeding behaviour and Leishmania infantum detection in Phlebotomus perniciosus females collected in the human leishmaniasis focus of Madrid, Spain (2012–2018). PLoS Negl. Trop. Dis. 2021, 15, e0009240. [Google Scholar] [CrossRef]
- El Tai, N.O.; Osman, O.F.; El Far, M.; Presber, W.; Schönian, G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 575–579. [Google Scholar] [CrossRef]
- Kuhls, K.; Mauricio, I.L.; Pratlong, F.; Presber, W.; Schönian, G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005, 7, 1224–1234. [Google Scholar] [CrossRef]
- Adam, G.K.; Ali, K.M.; Abdella, Y.H.; Omar, S.M.; Ahmed, M.A.A.; Abdalla, T.M.; Ali, A.A. Trend in cumulative cases and mortality rate among visceral leishmaniasis patients in Eastern Sudan: A 14-year registry, 2002–2015. Int. J. Infect. Dis. 2016, 51, 81–84. [Google Scholar] [CrossRef]
- Schorscher, J.A.; Goris, M. Incrimination of Phlebotomus (Larroussius) orientalis as a vector of visceral leishmaniasis in western Upper Nile Province, southern Sudan. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 622–623. [Google Scholar] [CrossRef]
- Hassan, M.M.; Elamin, E.M.; Mukhtar, M.M. Isolation and identification of Leishmania donovani from Phlebotomus orientalis, in an area of eastern Sudan with endemic visceral leishmaniasis. Ann. Trop. Med. Parasitol. 2008, 102, 553–555. [Google Scholar] [CrossRef]
- Elnaiem, D.A.; Osman, O.F. Evidence for active transmission of visceral leishmaniasis within a village in eastern Sudan. Acta Trop. 1998, 71, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.; Kiilu, G.; Leeuwenburg, J. Modification of sand fly biting behavior by Leishmania leads to increased parasite transmission. Am. J. Trop. Med. Hyg. 1985, 34, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Osman, O.; El-Raba’a, F.; Schallig, H.; Elnaiem, D.A. Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan. Parasites Vectors 2009, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Rohousova, I.; Talmi-Frank, D.; Kostalova, T.; Polanska, N.; Lestinova, T.; Kassahun, A.; Yasur-Landau, D.; Maia, C.; King, R.; Votypka, J.; et al. Exposure to Leishmania spp. and sand flies in domestic animals in northwestern Ethiopia. Parasites Vectors 2015, 8, 360. [Google Scholar] [CrossRef]
- Jamjoom, M.B.; Ashford, R.W.; Bates, P.A.; Chance, M.L.; Kemp, S.J.; Watts, P.C.; Noyes, H.A. Leishmania donovani is the only cause of visceral leishmaniasis in East Africa; previous descriptions of L. infantum and “L. archibaldi” from this region are a consequence of convergent evolution in the isoenzyme data. Parasitology 2004, 129, 399–409. [Google Scholar] [CrossRef]

| Year/Habitat | Blood-Fed/Gravid Females | Unfed Females | All Samples | ||||
|---|---|---|---|---|---|---|---|
| No. Tested | No. Positive | No. Tested | No. Positive | Total No. Tested | Total No. Positive | % Infection | |
| 2016 | |||||||
| Indoor | 18 | 0 | 0 | - | 18 | 0 | 0.00 |
| Outdoor | 40 | 0 | 1 | - | 41 | 0 | 0.00 |
| Peri-domestic | 0 | - | 0 | - | 0 | - | - |
| Sylvatic | 7 | 0 | 0 | - | 7 | 0 | 0.00 |
| Total | 65 | 0 | 1 | - | 66 | 0 | 0.00 |
| 2017 | |||||||
| Indoor | 30 | 1 | 0 | - | 30 | 1 | 3.3 |
| Outdoor | 54 | 8 | 1 | - | 55 | 8 | 14.5 |
| Peri-domestic | 40 | 0 | 0 | - | 40 | 0 | 0.0 |
| Sylvatic | 22 | 3 | 1 | - | 23 | 3 | 13.0 |
| Total | 146 | 12 | 2 | - | 148 | 12 | 8.1 |
| 2018 | |||||||
| Indoor | 8 | 0 | 146 | 0 | 154 | 0 | 0.0 |
| Outdoor | 64 | 2 | 279 | 1 | 343 | 3 | 0.9 |
| Peri-domestic | 30 | 0 | 131 | 0 | 161 | 0 | 0.00 |
| Sylvatic | 11 | 0 | 195 | 0 | 206 | 0 | 0.00 |
| Total | 113 | 3 | 751 | 0 | 864 | 3 | 0.35 |
| All 3 Years Samples | |||||||
| Indoor | 56 | 1 | 146 | 0 | 202 | 1 | 0.5 |
| Outdoor | 158 | 10 | 281 | 1 | 439 | 11 | 2.5 |
| Peri-domestic | 70 | 0 | 131 | 0 | 201 | 0 | 0.0 |
| Sylvatic | 40 | 3 | 196 | 0 | 236 | 3 | 1.7 |
| Total | 324 | 15 | 754 | 0 | 1078 | 15 | 1.4 |
| Pairwise Comparisons | p Value * |
|---|---|
| Indoor vs. Outdoor | 0.1159 |
| Indoor vs. Peri-domestic | 1.0000 |
| Indoor vs. Sylvatic | 0.6278 |
| Outdoor vs. Peri-domestic | 0.0207 |
| Outdoor vs. Sylvatic | 0.3991 |
| Peri-domestic vs. Sylvatic | 0.2531 |
| Inside the village vs. Outside the Village | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khogali, A.; Elnaiem, D.-E.A.; Díaz-Regañón, R.; Jibreel, T.; Nour, B.Y.M.; Abdelrahman, S.H.; Molina, R.; Jiménez, M. Infection of Leishmania donovani in Phlebotomus orientalis Sand Flies at Different Microhabitats of a Kala-Azar Endemic Village in Eastern Sudan. Trop. Med. Infect. Dis. 2024, 9, 40. https://doi.org/10.3390/tropicalmed9020040
Khogali A, Elnaiem D-EA, Díaz-Regañón R, Jibreel T, Nour BYM, Abdelrahman SH, Molina R, Jiménez M. Infection of Leishmania donovani in Phlebotomus orientalis Sand Flies at Different Microhabitats of a Kala-Azar Endemic Village in Eastern Sudan. Tropical Medicine and Infectious Disease. 2024; 9(2):40. https://doi.org/10.3390/tropicalmed9020040
Chicago/Turabian StyleKhogali, Altayeb, Dia-Eldin A. Elnaiem, Ramón Díaz-Regañón, Tayseer Jibreel, Bakri Y. M. Nour, Samira Hamid Abdelrahman, Ricardo Molina, and Maribel Jiménez. 2024. "Infection of Leishmania donovani in Phlebotomus orientalis Sand Flies at Different Microhabitats of a Kala-Azar Endemic Village in Eastern Sudan" Tropical Medicine and Infectious Disease 9, no. 2: 40. https://doi.org/10.3390/tropicalmed9020040
APA StyleKhogali, A., Elnaiem, D.-E. A., Díaz-Regañón, R., Jibreel, T., Nour, B. Y. M., Abdelrahman, S. H., Molina, R., & Jiménez, M. (2024). Infection of Leishmania donovani in Phlebotomus orientalis Sand Flies at Different Microhabitats of a Kala-Azar Endemic Village in Eastern Sudan. Tropical Medicine and Infectious Disease, 9(2), 40. https://doi.org/10.3390/tropicalmed9020040






