The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
Study Variables and Data Analysis
3. Results
3.1. Descriptive Characteristics of the Studies
3.2. Lyme Disease Vectors
3.3. Lyme Disease in Human Hosts
3.4. Lyme Disease in Animal Hosts
3.5. Lyme Disease Risk and Expansion
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC Lyme Disease. Available online: https://www.cdc.gov/lyme/index.html (accessed on 17 December 2022).
- World Health Organization Regional Office for Europe/European Centres for Disease Control: Lyme Borreliosis in Europe. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/healthtopics/vectors/world-health-day-2014/Documents/factsheet-lyme-borreliosis.pdf (accessed on 17 December 2022).
- Marques, A.R.; Strle, F.; Wormser, G.P. Comparison of Lyme Disease in the United States and Europe. Emerg. Infect. Dis. 2021, 27, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A Vector on the Rise. Parasites Vectors 2016, 9, 314. [Google Scholar] [CrossRef]
- Heglasová, I.; Rudenko, N.; Golovchenko, M.; Zubriková, D.; Miklisová, D.; Stanko, M. Ticks, Fleas and Rodent-Hosts Analyzed for the Presence of Borrelia miyamotoi in Slovakia: The First Record of Borrelia miyamotoi in a Haemaphysalis Inermis Tick. Ticks Tick Borne Dis. 2020, 11, 101456. [Google Scholar] [CrossRef] [PubMed]
- Del Cerro, A.; Oleaga, A.; Somoano, A.; Barandika, J.F.; García-Pérez, A.L.; Espí, A. Molecular Identification of Tick-Borne Pathogens (Rickettsia Spp., Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Coxiella burnetii and Piroplasms) in Questing and Feeding Hard Ticks from North-Western Spain. Ticks Tick Borne Dis. 2022, 13, 101961. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.K.; Singh, P.; Puri, S.; Jindal, S.; Choudhary, P.N. Dog Tick (Rhipecephalus) Causing Lyme Disease in an Adult Human. J. Fam. Med. Prim. Care 2022, 11, 4824–4826. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Jian, M.; Yue, P.; Cao, W.; Xu, X.; Zhang, Y.; Pan, Y.; Yang, J.; Chen, J.; Liu, M.; et al. Prevalence of Borrelia burgdorferi in Ixodidae Tick around Asia: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Skufca, J.; Vyse, A.; Pilz, A.; Begier, E.; Riera-Montes, M.; Gessner, B.D.; Stark, J.H. The Landscape of Lyme Borreliosis Surveillance in Europe. Vector Borne Zoonotic Dis. 2023, 23, 142–155. [Google Scholar] [CrossRef]
- ECDC Comment: European Commission Updates Communicable Disease Surveillance List—Lyme Neuroborreliosis Now under EU/EEA Surveillance. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-comment-european-commission-updates-communicable-disease-surveillance-list-lyme (accessed on 17 December 2022).
- Tick Maps. Available online: https://www.ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 25 May 2023).
- Lyme Borreliosis in Europe. Available online: https://climate-adapt.eea.europa.eu/en/metadata/publications/lyme-borreliosis-in-europe (accessed on 17 December 2022).
- Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures. Available online: https://www.who.int/publications/i/item/9789289022910 (accessed on 25 May 2023).
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public Health 2022, 19, 6516. [Google Scholar] [CrossRef] [PubMed]
- Wongnak, P.; Bord, S.; Jacquot, M.; Agoulon, A.; Beugnet, F.; Bournez, L.; Cèbe, N.; Chevalier, A.; Cosson, J.-F.; Dambrine, N.; et al. Meteorological and Climatic Variables Predict the Phenology of Ixodes ricinus Nymph Activity in France, Accounting for Habitat Heterogeneity. Sci. Rep. 2022, 12, 7833. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Giesen, C.; Roche, J.; Redondo-Bravo, L.; Ruiz-Huerta, C.; Gomez-Barroso, D.; Benito, A.; Herrador, Z. The Impact of Climate Change on Mosquito-Borne Diseases in Africa. Pathog. Glob. Health 2020, 114, 287–301. [Google Scholar] [CrossRef]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental Drivers, Climate Change and Emergent Diseases Transmitted by Mosquitoes and Their Vectors in Southern Europe: A Systematic Review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 May 2023).
- World Meteorological Organization. Available online: https://public.wmo.int/en (accessed on 14 July 2023).
- Linard, C.; Lamarque, P.; Heyman, P.; Ducoffre, G.; Luyasu, V.; Tersago, K.; Vanwambeke, S.O.; Lambin, E.F. Determinants of the Geographic Distribution of Puumala Virus and Lyme Borreliosis Infections in Belgium. Int. J. Health Geogr. 2007, 6, 15. [Google Scholar] [CrossRef]
- Heylen, D.; Lasters, R.; Adriaensen, F.; Fonville, M.; Sprong, H.; Matthysen, E. Ticks and Tick-Borne Diseases in the City: Role of Landscape Connectivity and Green Space Characteristics in a Metropolitan Area. Sci. Total Environ. 2019, 670, 941–949. [Google Scholar] [CrossRef]
- Keukeleire, M.D.; Robert, A.; Kabamba, B.; Dion, E.; Luyasu, V.; Vanwambeke, S.O. Individual and Environmental Factors Associated with the Seroprevalence of Borrelia burgdorferi in Belgian Farmers and Veterinarians. Infect. Ecol. Epidemiol. 2016, 6, 32793. [Google Scholar] [CrossRef]
- Barrios, J.M.; Verstraeten, W.W.; Maes, P.; Aerts, J.M.; Farifteh, J.; Coppin, P. Seasonal Vegetation Variables and Their Impact on the Spatio-Temporal Patterns of Nephropathia Epidemica and Lyme Borreliosis in Belgium. Appl. Geogr. 2013, 45, 230–240. [Google Scholar] [CrossRef]
- Tack, W.; Madder, M.; Baeten, L.; Vanhellemont, M.; Gruwez, R.; Verheyen, K. Local Habitat and Landscape Affect Ixodes ricinus Tick Abundances in Forests on Poor, Sandy Soils. For. Ecol. Manag. 2012, 265, 30–36. [Google Scholar] [CrossRef]
- Barrios, J.; Verstraeten, W.; Maes, P.; Aerts, J.-M.; Farifteh, J.; Coppin, P. Using the Gravity Model to Estimate the Spatial Spread of Vector-Borne Diseases. IJERPH 2012, 9, 4346–4364. [Google Scholar] [CrossRef]
- Barrios, J.M.; Verstraeten, W.W.; Maes, P.; Clement, J.; Aerts, J.M.; Farifteh, J.; Lagrou, K.; Van Ranst, M.; Coppin, P. Remotely Sensed Vegetation Moisture as Explanatory Variable of Lyme Borreliosis Incidence. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 1–12. [Google Scholar] [CrossRef]
- Heylen, D.; Adriaensen, F.; Van Dongen, S.; Sprong, H.; Matthysen, E. Ecological Factors That Determine Ixodes ricinus Tick Burdens in the Great Tit (Parus Major), an Avian Reservoir of Borrelia burgdorferi s.l. Int. J. Parasitol. 2013, 43, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hönig, V.; Svec, P.; Halas, P.; Vavruskova, Z.; Tykalova, H.; Kilian, P.; Vetiskova, V.; Dornakova, V.; Sterbova, J.; Simonova, Z.; et al. Ticks and Tick-Borne Pathogens in South Bohemia (Czech Republic)—Spatial Variability in Ixodes ricinus Abundance, Borrelia burgdorferi and Tick-Borne Encephalitis Virus Prevalence. Ticks Tick-Borne Dis. 2015, 6, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Materna, J.; Hönig, V.; Metelka, L.; Danielová, V.; Harčarik, J.; Kliegrová, S.; Grubhoffer, L. Vertical Distribution of the Tick Ixodes ricinus and Tick-Borne Pathogens in the Northern Moravian Mountains Correlated with Climate Warming (Jeseníky Mts., Czech Republic). Cent. Eur. J. Public Health 2009, 17, 139–145. [Google Scholar] [CrossRef]
- Daniel, M.; Kříž, B.; Valter, J.; Kott, I.; Danielová, V. The Influence of Meteorological Conditions of the Preceding Winter on the Incidences of Tick-Borne Encephalitis and Lyme Borreliosis in the Czech Republic. Int. J. Med. Microbiol. 2008, 298, 60–67. [Google Scholar] [CrossRef]
- Hubálek, Z. North Atlantic Weather Oscillation and Human Infectious Diseases in the Czech Republic, 1951–2003. Eur. J. Epidemiol. 2005, 20, 263–270. [Google Scholar] [CrossRef]
- Daniel, M.; Malý, M.; Danielová, V.; Kříž, B.; Nuttall, P. Abiotic Predictors and Annual Seasonal Dynamics of Ixodes ricinus, the Major Disease Vector of Central Europe. Parasites Vectors 2015, 8, 478. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J.; Juricová, Z. Host-Seeking Activity of Ixodid Ticks in Relation to Weather Variables. J. Vector Ecol. 2003, 28, 159–165. [Google Scholar]
- Tkadlec, E.; Václavík, T.; Široký, P. Rodent Host Abundance and Climate Variability as Predictors of Tickborne Disease Risk 1 Year in Advance. Emerg. Infect. Dis. 2019, 25, 1738–1741. [Google Scholar] [CrossRef]
- Jensen, P.M.; Jespersen, J.B. Five Decades of Tick–Man Interaction in Denmark—An Analysis. Exp. Appl. Acarol. 2005, 35, 131–146. [Google Scholar] [CrossRef]
- Jensen, P.M.; Hansen, H. Spatial Risk Assessment for Lyme Borreliosis in Denmark. Scand. J. Infect. Dis. 2000, 32, 545–550. [Google Scholar] [CrossRef]
- Jensen, P.M.; Frand, F. Temporal Risk Assessment for Lyme Borreliosis in Denmark. Scand. J. Infect. Dis. 2000, 32, 539–544. [Google Scholar] [CrossRef]
- Kjær, L.J.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Korslund, L.; Kjelland, V.; Slettan, A.; Stuen, S.; et al. Predicting and Mapping Human Risk of Exposure to Ixodes ricinus Nymphs Using Climatic and Environmental Data, Denmark, Norway and Sweden, 2016. Eurosurveillance 2019, 24, 1800101. [Google Scholar] [CrossRef] [PubMed]
- Kjær, L.J.; Soleng, A.; Edgar, K.S.; Lindstedt, H.E.H.; Paulsen, K.M.; Andreassen, Å.K.; Korslund, L.; Kjelland, V.; Slettan, A.; Stuen, S.; et al. Predicting the Spatial Abundance of Ixodes ricinus Ticks in Southern Scandinavia Using Environmental and Climatic Data. Sci. Rep. 2019, 9, 18144. [Google Scholar] [CrossRef] [PubMed]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of Global Changes on the Climatic Niche of the Tick Ixodes ricinus Inferred by Species Distribution Modelling. Parasites Vectors 2013, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Heyman, P.; Cochez, C.; Simons, L.; Vanwambeke, S.O. A Multi-Level Analysis of the Relationship between Environmental Factors and Questing Ixodes ricinus Dynamics in Belgium. Parasites Vectors 2012, 5, 149. [Google Scholar] [CrossRef]
- Li, S.; Gilbert, L.; Vanwambeke, S.O.; Yu, J.; Purse, B.V.; Harrison, P.A. Lyme Disease Risks in Europe under Multiple Uncertain Drivers of Change. Environ. Health Perspect. 2019, 127, 67010. [Google Scholar] [CrossRef]
- Fernández-Ruiz, N.; Estrada-Peña, A. Could Climate Trends Disrupt the Contact Rates between Ixodes ricinus (Acari, Ixodidae) and the Reservoirs of Borrelia burgdorferi s.l. PLoS ONE 2020, 15, e0233771. [Google Scholar] [CrossRef]
- Uusitalo, R.; Siljander, M.; Lindén, A.; Sormunen, J.J.; Aalto, J.; Hendrickx, G.; Kallio, E.; Vajda, A.; Gregow, H.; Henttonen, H.; et al. Predicting Habitat Suitability for Ixodes ricinus and Ixodes persulcatus Ticks in Finland. Parasites Vectors 2022, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Mariet, A.-S.; Retel, O.; Avocat, H.; Serre, A.; Schapman, L.; Schmitt, M.; Charron, M.; Monnet, E. Estimated Incidence of Erythema Migrans in Five Regions of France and Ecological Correlations with Environmental Characteristics. Vector-Borne Zoonotic Dis. 2013, 13, 666–673. [Google Scholar] [CrossRef]
- Vassallo, M.; Paul, R.E.L.; Pérez-Eid, C. Temporal distribution of the annual nymphal stock of Ixodes ricinus ticks. Exp. Appl. Acarol. 2000, 24, 941–949. [Google Scholar] [CrossRef]
- Goldstein, V.; Boulanger, N.; Schwartz, D.; George, J.-C.; Ertlen, D.; Zilliox, L.; Schaeffer, M.; Jaulhac, B. Factors Responsible for Ixodes ricinus Nymph Abundance: Are Soil Features Indicators of Tick Abundance in a French Region Where Lyme Borreliosis Is Endemic? Ticks Tick-Borne Dis. 2018, 9, 938–944. [Google Scholar] [CrossRef]
- Vourc’h, G.; Abrial, D.; Bord, S.; Jacquot, M.; Masséglia, S.; Poux, V.; Pisanu, B.; Bailly, X.; Chapuis, J.-L. Mapping Human Risk of Infection with Borrelia burgdorferi sensu lato, the Agent of Lyme Borreliosis, in a Periurban Forest in France. Ticks Tick-Borne Dis. 2016, 7, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.E.L.; Cote, M.; Le Naour, E.; Bonnet, S.I. Environmental Factors Influencing Tick Densities over Seven Years in a French Suburban Forest. Parasites Vectors 2016, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Halos, L.; Bord, S.; Cotté, V.; Gasqui, P.; Abrial, D.; Barnouin, J.; Boulouis, H.-J.; Vayssier-Taussat, M.; Vourc’h, G. Ecological Factors Characterizing the Prevalence of Bacterial Tick-Borne Pathogens in Ixodes ricinus Ticks in Pastures and Woodlands. Appl. Environ. Microbiol. 2010, 76, 4413–4420. [Google Scholar] [CrossRef] [PubMed]
- Boyard, C.; Barnouin, J.; Gasqui, P.; Vourc’H, G. Local Environmental Factors Characterizing Ixodes ricinus Nymph Abundance in Grazed Permanent Pastures for Cattle. Parasitology 2007, 134, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Bourdin, A.; Bord, S.; Durand, J.; Galon, C.; Moutailler, S.; Scherer-Lorenzen, M.; Jactel, H. Forest Diversity Reduces the Prevalence of Pathogens Transmitted by the Tick Ixodes ricinus. Front. Ecol. Evol. 2022, 10, 891908. [Google Scholar] [CrossRef]
- Perez, G.; Bastian, S.; Agoulon, A.; Bouju, A.; Durand, A.; Faille, F.; Lebert, I.; Rantier, Y.; Plantard, O.; Butet, A. Effect of Landscape Features on the Relationship between Ixodes ricinus Ticks and Their Small Mammal Hosts. Parasites Vectors 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Liira, J.; Gärtner, S.; Hansen, K.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; Decocq, G.; De Frenne, P.; De Smedt, P.; et al. Environmental Drivers of Ixodes ricinus Abundance in Forest Fragments of Rural European Landscapes. BMC Ecol. 2017, 17, 31. [Google Scholar] [CrossRef]
- Ehrmann, S.; Ruyts, S.C.; Scherer-Lorenzen, M.; Bauhus, J.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; Decocq, G.; De Frenne, P.; De Smedt, P.; et al. Habitat Properties Are Key Drivers of Borrelia burgdorferi (s.l.) Prevalence in Ixodes ricinus Populations of Deciduous Forest Fragments. Parasites Vectors 2018, 11, 23. [Google Scholar] [CrossRef]
- Brugger, K.; Walter, M.; Chitimia-Dobler, L.; Dobler, G.; Rubel, F. Forecasting next Season’s Ixodes ricinus Nymphal Density: The Example of Southern Germany 2018. Exp. Appl. Acarol. 2018, 75, 281–288. [Google Scholar] [CrossRef]
- Nolzen, H.; Brugger, K.; Reichold, A.; Brock, J.; Lange, M.; Thulke, H.-H. Model-Based Extrapolation of Ecological Systems under Future Climate Scenarios: The Example of Ixodes ricinus Ticks. PLoS ONE 2022, 17, e0267196. [Google Scholar] [CrossRef]
- Răileanu, C.; Silaghi, C.; Fingerle, V.; Margos, G.; Thiel, C.; Pfister, K.; Overzier, E. Borrelia burgdorferi Sensu Lato in Questing and Engorged Ticks from Different Habitat Types in Southern Germany. Microorganisms 2021, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.; Krücken, J.; McKay-Demeler, J.; Pachnicke, S.; Krieger, K.; von Samson-Himmelstjerna, G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity Patterns and Associated Pathogens. Ticks Tick-Borne Dis. 2019, 10, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Brugger, K.; Walter, M.; Chitimia-Dobler, L.; Dobler, G.; Rubel, F. Seasonal Cycles of the TBE and Lyme Borreliosis Vector Ixodes ricinus Modelled with Time-Lagged and Interval-Averaged Predictors. Exp. Appl. Acarol. 2017, 73, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, D.; Brugger, K.; Pfäffle, M.; Sebastian, P.; Norra, S.; Petney, T.; Oehme, R.; Littwin, N.; Lebl, K.; Raith, J.; et al. Estimating Ixodes ricinus Densities on the Landscape Scale. Int. J. Health Geogr. 2015, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Maier, W.A.; Kistemann, T.; Kampen, H. Analysis of the Distribution of the Tick Ixodes ricinus L. (Acari: Ixodidae) in a Nature Reserve of Western Germany Using Geographic Information Systems. Int. J. Hyg. Environ. Health 2009, 212, 87–96. [Google Scholar] [CrossRef]
- Vollack, K.; Sodoudi, S.; Névir, P.; Müller, K.; Richter, D. Influence of Meteorological Parameters during the Preceding Fall and Winter on the Questing Activity of Nymphal Ixodes ricinus Ticks. Int. J. Biometeorol. 2017, 61, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Mahling, M.; Pfister, K. Abundance and Seasonal Activity of Questing Ixodes ricinus Ticks in Their Natural Habitats in Southern Germany in 2011. J. Vector Ecol. 2014, 39, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gethmann, J.; Hoffmann, B.; Kasbohm, E.; Süss, J.; Habedank, B.; Conraths, F.J.; Beer, M.; Klaus, C. Research Paper on Abiotic Factors and Their Influence on Ixodes ricinus Activity—Observations over a Two-Year Period at Several Tick Collection Sites in Germany. Parasitol. Res. 2020, 119, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, R.; Wells, K.; O’Hara, R.B.; Kalko, E.K.V.; Renner, S.C. Variable Strength of Forest Stand Attributes and Weather Conditions on the Questing Activity of Ixodes ricinus Ticks over Years in Managed Forests. PLoS ONE 2013, 8, e55365. [Google Scholar] [CrossRef]
- Hauck, D.; Springer, A.; Chitimia-Dobler, L.; Strube, C. Two-Year Monitoring of Tick Abundance and Influencing Factors in an Urban Area (City of Hanover, Germany). Ticks Tick-Borne Dis. 2020, 11, 101464. [Google Scholar] [CrossRef]
- Trájer, A.; Bobvos, J.; Páldy, A.; Krisztalovics, K. Association between Incidence of Lyme Disease and Spring-Early Summer Season Temperature Changes in Hungary—1998–2010. Ann. Agric. Environ. Med. 2013, 20, 245–251. [Google Scholar] [PubMed]
- Trájer, A.; Bede-Fazekas, Á.; Hufnagel, L.; Bobvos, J.; Páldy, A. The Paradox of the Binomial Ixodes ricinus Activity and the Observed Unimodal Lyme Borreliosis Season in Hungary. Int. J. Environ. Health Res. 2014, 24, 226–245. [Google Scholar] [CrossRef]
- Hornok, S.; Mulvihill, M.; Szőke, K.; Gönczi, E.; Sulyok, K.M.; Gyuranecz, M.; Hofmann-Lehmann, R. Impact of a Freeway on the Dispersal of Ticks and Ixodes ricinus-Borne Pathogens: Forested Resting Areas May Become Lyme Disease Hotspots. Acta Vet. Hung. 2017, 65, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks Climb the Mountains: Ixodid Tick Infestation and Infection by Tick-Borne Pathogens in the Western Alps. Ticks Tick-Borne Dis. 2020, 11, 101489. [Google Scholar] [CrossRef] [PubMed]
- Rosà, R.; Pugliese, A.; Ghosh, M.; Perkins, S.E.; Rizzoli, A. Temporal Variation of Ixodes ricinus Intensity on the Rodent Host Apodemus flavicollis in Relation to Local Climate and Host Dynamics. Vector-Borne Zoonotic Dis. 2007, 7, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Merler, S.; Furlanello, C.; Genchi, C. Geographical Information Systems and Bootstrap Aggregation (Bagging) of Tree-Based Classifiers for Lyme Disease Risk Prediction in Trentino, Italian Alps. J. Med. Entomol. 2002, 39, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zanzani, S.A.; Rimoldi, S.G.; Manfredi, M.; Grande, R.; Gazzonis, A.L.; Merli, S.; Olivieri, E.; Giacomet, V.; Antinori, S.; Cislaghi, G.; et al. Lyme Borreliosis Incidence in Lombardy, Italy (2000–2015): Spatiotemporal Analysis and Environmental Risk Factors. Ticks Tick-Borne Dis. 2019, 10, 101257. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, A.; Boemo, B.; Mignozzi, K.; Bandi, M.; Floris, R.; Menardi, G.; Cinco, M. Spatial Lyme Borreliosis Risk Assessment in North-Eastern Italy. Int. J. Med. Microbiol. 2008, 298, 125–128. [Google Scholar] [CrossRef]
- Bisanzio, D.; Amore, G.; Ragagli, C.; Tomassone, L.; Bertolotti, L.; Mannelli, A. Temporal Variations in the Usefulness of Normalized Difference Vegetation Index as a Predictor for Ixodes ricinus (Acari: Ixodidae) in a Borrelia lusitaniae Focus in Tuscany, Central Italy. J. Med. Entomol. 2008, 45, 547–555. [Google Scholar] [CrossRef]
- Tagliapietra, V.; Rosà, R.; Arnoldi, D.; Cagnacci, F.; Capelli, G.; Montarsi, F.; Hauffe, H.C.; Rizzoli, A. Saturation Deficit and Deer Density Affect Questing Activity and Local Abundance of Ixodes ricinus (Acari, Ixodidae) in Italy. Vet. Parasitol. 2011, 183, 114–124. [Google Scholar] [CrossRef]
- Rosà, R.; Andreo, V.; Tagliapietra, V.; Baráková, I.; Arnoldi, D.; Hauffe, H.; Manica, M.; Rosso, F.; Blaňarová, L.; Bona, M.; et al. Effect of Climate and Land Use on the Spatio-Temporal Variability of Tick-Borne Bacteria in Europe. Int. J. Environ. Res. Public Health 2018, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martí, I.; Zurita-Milla, R.; Swart, A.; van den Wijngaard, K.C.; van Vliet, A.J.H.; Bennema, S.; Harms, M. Identifying Environmental and Human Factors Associated With Tick Bites Using Volunteered Reports and Frequent Pattern Mining. Trans. GIS 2017, 21, 277–299. [Google Scholar] [CrossRef]
- Swart, A.; Ibañez-Justicia, A.; Buijs, J.; van Wieren, S.E.; Hofmeester, T.R.; Sprong, H.; Takumi, K. Predicting Tick Presence by Environmental Risk Mapping. Front. Public Health 2014, 2, 238. [Google Scholar] [CrossRef]
- Qviller, L.; Grøva, L.; Viljugrein, H.; Klingen, I.; Mysterud, A. Temporal Pattern of Questing Tick Ixodes ricinus Density at Differing Elevations in the Coastal Region of Western Norway. Parasites Vectors 2014, 7, 179. [Google Scholar] [CrossRef]
- Kiewra, D.; Szymanowski, M.; Zalewska, G.; Dobracka, B.; Dobracki, W.; Klakočar, J.; Czułowska, A.; Plewa-Tutaj, K. Seroprevalence of Borrelia burgdorferi in Forest Workers from Inspectorates with Different Forest Types in Lower Silesia, SW Poland: Preliminary Study. Int. J. Environ. Health Res. 2018, 28, 502–510. [Google Scholar] [CrossRef]
- Buczek, A.; Ciura, D.; Bartosik, K.; Zając, Z.; Kulisz, J. Threat of Attacks of Ixodes ricinus Ticks (Ixodida: Ixodidae) and Lyme Borreliosis within Urban Heat Islands in South-Western Poland. Parasites Vectors 2014, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Dyczko, D.; Kiewra, D.; Kolanek, A.; Błażej, P. The Influence of Local Environmental Factors in Southwestern Poland on the Abundance of Ixodes ricinus and Prevalence of Infection with Borrelia burgdorferi s.l. and B. miyamotoi. Parasitol. Res. 2022, 121, 1575–1585. [Google Scholar] [CrossRef]
- Kiewra, D.; Kryza, M.; Szymanowski, M. Influence of Selected Meteorological Variables on the Questing Activity of Ixodes ricinus Ticks in Lower Silesia, SW Poland. J. Vector Ecol. 2014, 39, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Domșa, C. Modeling the Distribution of Ixodes ricinus in Romania. North-West. J. Zool. 2018, 14, 25–29. [Google Scholar]
- Pangrácová, L.; Derdáková, M.; Pekárik, L.; Hviščová, I.; Víchová, B.; Stanko, M.; Hlavatá, H.; Peťko, B. Ixodes ricinus Abundance and Its Infection with the Tick-Borne Pathogens in Urban and Suburban Areas of Eastern Slovakia. Parasites Vectors 2013, 6, 238. [Google Scholar] [CrossRef]
- Kazimírová, M.; Hamšíková, Z.; Kocianová, E.; Marini, G.; Mojšová, M.; Mahríková, L.; Berthová, L.; Slovák, M.; Rosá, R. Relative Density of Host-Seeking Ticks in Different Habitat Types of South-Western Slovakia. Exp. Appl. Acarol. 2016, 69, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Donša, D.; Grujić, V.J.; Pipenbaher, N.; Ivajnšič, D. The Lyme Borreliosis Spatial Footprint in the 21st Century: A Key Study of Slovenia. Int. J. Environ. Res. Public Health 2021, 18, 12061. [Google Scholar] [CrossRef] [PubMed]
- Knap, N.; Durmiši, E.; Saksida, A.; Korva, M.; Petrovec, M.; Avšič-Županc, T. Influence of Climatic Factors on Dynamics of Questing Ixodes ricinus Ticks in Slovenia. Vet. Parasitol. 2009, 164, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Fernández-de-Mera, I.G.; Acevedo, P.; Gortázar, C.; de la Fuente, J. Factors Driving the Abundance of Ixodes ricinus Ticks and the Prevalence of Zoonotic I. ricinus-Borne Pathogens in Natural Foci. Appl. Environ. Microbiol. 2012, 78, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. Distribution, Abundance, and Habitat Preferences of Ixodes ricinus (Acari: Ixodidae) in Northern Spain. J. Med. Entomol. 2001, 38, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Carné, J.; García-Martín, A.; Estrada-Peña, A. Modelling the Phenological Relationships of Questing Immature Ixodes ricinus (Ixodidae) Using Temperature and NDVI Data. Zoonoses Public Health 2016, 63, 40–52. [Google Scholar] [CrossRef]
- Barandika, J.F.; Berriatua, E.; Barral, M.; Juste, R.A.; Anda, P.; Garcia-Perez, A.L. Risk Factors Associated with Ixodid Tick Species Distributions in the Basque Region in Spain. Med. Vet. Entomol. 2006, 20, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Bennet, L.; Halling, A.; Berglund, J. Increased Incidence of Lyme Borreliosis in Southern Sweden Following Mild Winters and during Warm, Humid Summers. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Eisen, L.; Comstedt, P.; Mejlon, H.A.; Lindgren, E.; Bergström, S.; Olsen, B. Risk Indicators for the Tick Ixodes ricinus and Borrelia burgdorferi Sensu Lato in Sweden. Med. Vet. Entomol. 2009, 23, 226–237. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Lindgren, E. The Range of Ixodes ricinus and the Risk of Contracting Lyme Borreliosis Will Increase Northwards When the Vegetation Period Becomes Longer. Ticks Tick-Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Keith, K.; Årestedt, K.; Tjernberg, I. The Relationship between the Laboratory Diagnosis of Lyme Neuroborreliosis and Climate Factors in Kalmar County Sweden—An Overview between 2008 and 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A.; Jaenson, T.G.T. Distribution of the Common Tick, Ixodes ricinus (Acari: Ixodidae), in Different Vegetation Types in Southern Sweden. J. Med. Entomol. 2003, 40, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, M.; Schötta, A.-M.; Höss, D.; Kundi, M.; Schray, C.; Stockinger, H.; Stanek, G. Infections with Tickborne Pathogens after Tick Bite, Austria, 2015–2018. Emerg. Infect. Dis. 2021, 27, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Petrulionienė, A.; Radzišauskienė, D.; Ambrozaitis, A.; Čaplinskas, S.; Paulauskas, A.; Venalis, A. Epidemiology of Lyme Disease in a Highly Endemic European Zone. Medicina 2020, 56, 115. [Google Scholar] [CrossRef]
- van den Wijngaard, C.C.; Hofhuis, A.; Simões, M.; Rood, E.; van Pelt, W.; Zeller, H.; Van Bortel, W. Surveillance Perspective on Lyme Borreliosis across the European Union and European Economic Area. Eurosurveillance 2017, 22, 30569. [Google Scholar] [CrossRef] [PubMed]
- Main Climates of Europe—European Environment Agency. Available online: https://www.eea.europa.eu/data-and-maps/figures/climate (accessed on 20 June 2023).
- Köhler, C.F.; Holding, M.L.; Sprong, H.; Jansen, P.A.; Esser, H.J. Biodiversity in the Lyme-Light: Ecological Restoration and Tick-Borne Diseases in Europe. Trends Parasitol. 2023, 39, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Dobson, A.D.M.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.M.; Fish, D.; et al. Lyme Disease Ecology in a Changing World: Consensus, Uncertainty and Critical Gaps for Improving Control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef]
- Tjernberg, I.; Lager, M.; Furset Jensen, G.; Eikeland, R.; Nyman, D.; Brudin, L.; Henningsson, A.J. Identification of Potential Biomarkers in Active Lyme Borreliosis. PLoS ONE 2023, 18, e0287586. [Google Scholar] [CrossRef]
- Nowakowski, J.; Schwartz, I.; Liveris, D.; Wang, G.; Rosenfeld, M.E.A.; Girao, G.; McKenna, D.; Nadelman, R.B.; Cavaliere, L.F.; Wormser, G.P.; et al. Laboratory Diagnostic Techniques for Patients with Early Lyme Disease Associated with Erythema Migrans: A Comparison of Different Techniques. Clin. Infect. Dis. 2001, 33, 2023–2027. [Google Scholar] [CrossRef]
- Ferguson, T.; Curtis, R.; Fraysse, F.; Olds, T.; Dumuid, D.; Brown, W.; Esterman, A.; Maher, C. Weather Associations with Physical Activity, Sedentary Behaviour and Sleep Patterns of Australian Adults: A Longitudinal Study with Implications for Climate Change. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 30. [Google Scholar] [CrossRef]
- How Ticks Spread Disease. Available online: https://www.cdc.gov/ticks/life_cycle_and_hosts.html (accessed on 20 June 2023).
- European Summer 2023: A Season of Contrasting Extremes—Copernicus. Available online: https://climate.copernicus.eu/european-summer-2023-season-contrasting-extremes (accessed on 7 March 2024).
- Randolph, S.E. Evidence That Climate Change Has Caused “emergence” of Tick-Borne Diseases in Europe? Int. J. Med. Microbiol. Suppl. 2004, 293, 5–15. [Google Scholar] [CrossRef]
- Matuschka, F.R.; Heiler, M.; Eiffert, H.; Fischer, P.; Lotter, H.; Spielman, A. Diversionary Role of Hoofed Game in the Transmission of Lyme Disease Spirochetes. Am. J. Trop. Med. Hyg. 1993, 48, 693–699. [Google Scholar] [CrossRef]
- Sala, V.; De Faveri, E. Epidemiology of Lyme Disease in Domestic and Wild Animals. Open Dermatol. J. 2016, 10, 15–26. [Google Scholar] [CrossRef]
- Dobson, A.D.M.; Randolph, S.E. Modelling the Effects of Recent Changes in Climate, Host Density and Acaricide Treatments on Population Dynamics of Ixodes ricinus in the UK: Ixodes ricinus Population Change Model. J. Appl. Ecol. 2011, 48, 1029–1037. [Google Scholar] [CrossRef]
- Bouchard, C.; Dumas, A.; Baron, G.; Bowser, N.; Leighton, P.A.; Lindsay, L.R.; Milord, F.; Ogden, N.H.; Aenishaenslin, C. Integrated Human Behavior and Tick Risk Maps to Prioritize Lyme Disease Interventions Using a “One Health” Approach. Ticks Tick-Borne Dis. 2023, 14, 102083. [Google Scholar] [CrossRef]
- AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/ar6/syr/ (accessed on 23 July 2023).

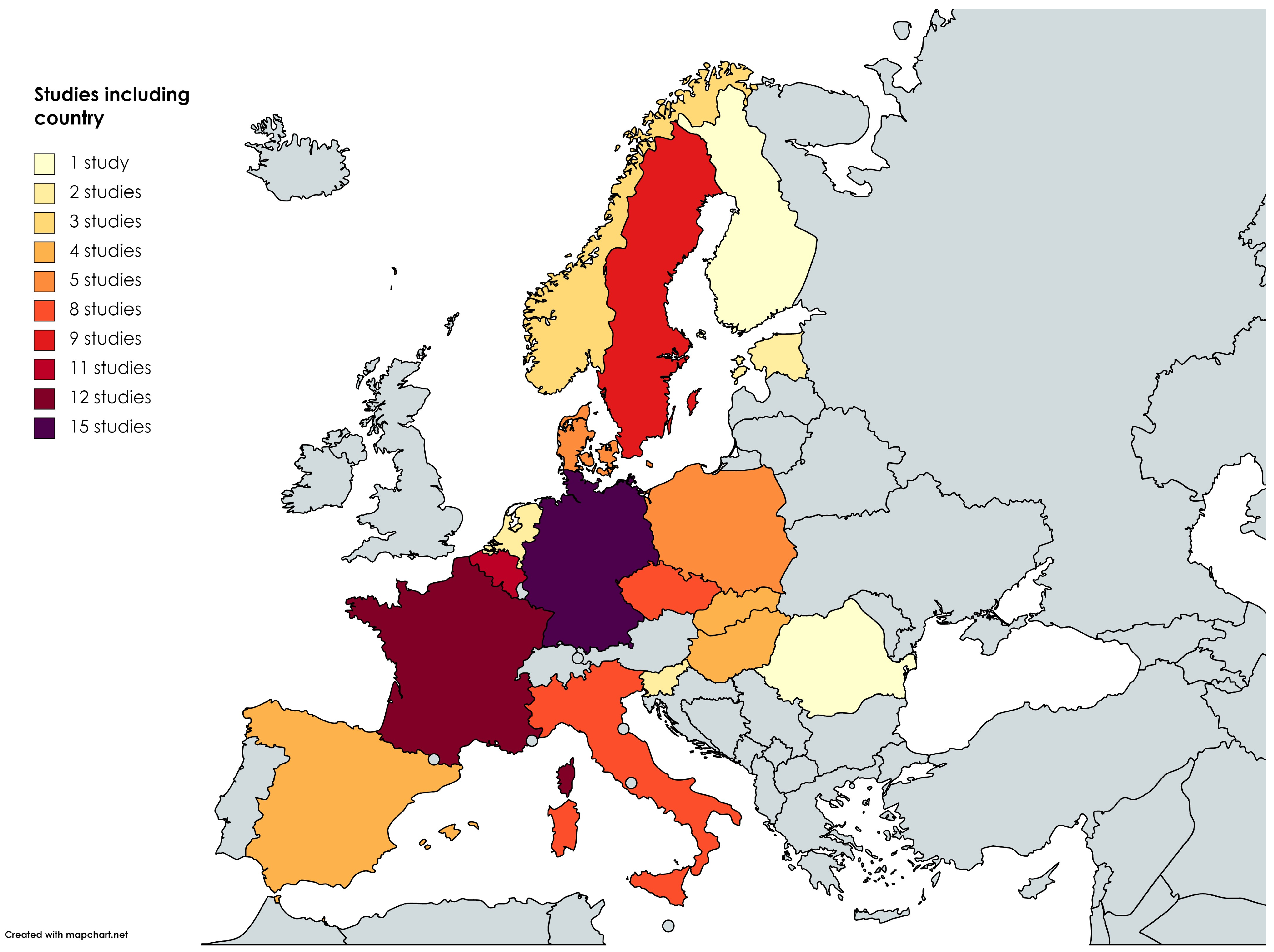
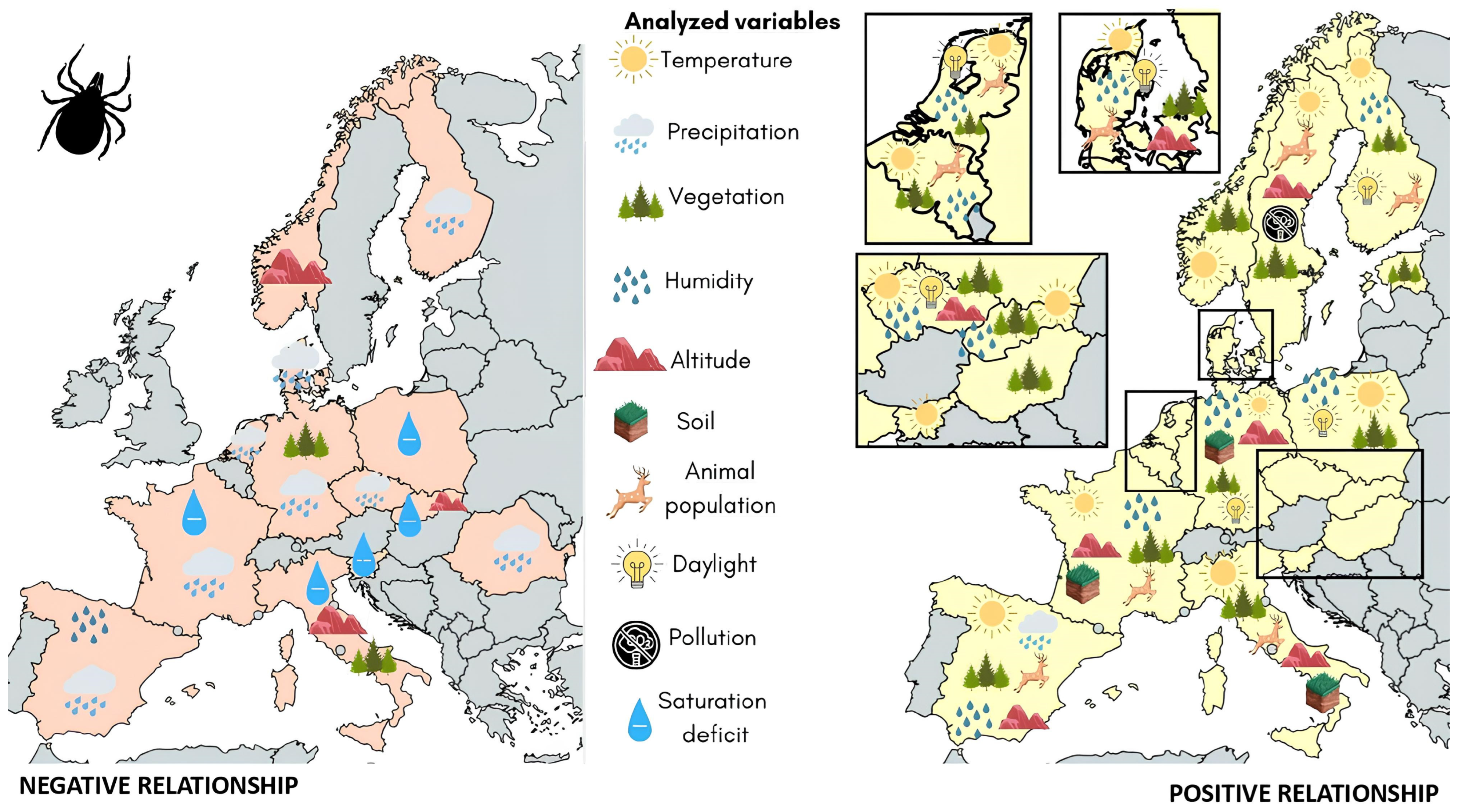
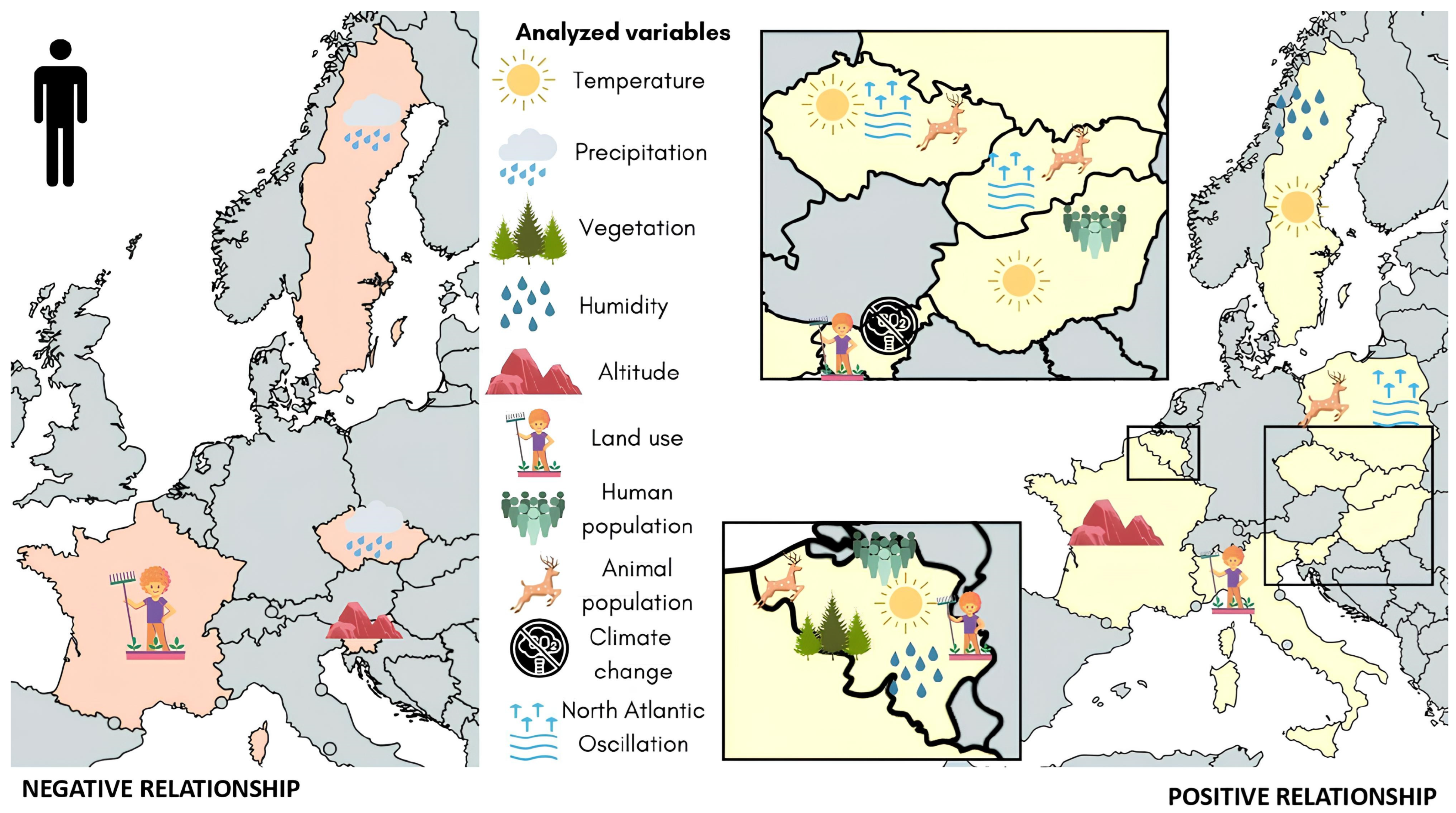
| First Author | Year of Publication | Analyzed Countries | Analyzed Vector (Species) | Analyzed Reservoirs and Hosts | Borrelia Species | Study Object | Analytical Approach | Scored Points at Quality Assessment |
|---|---|---|---|---|---|---|---|---|
| Linard C [21] | 2007 | Belgium | ND | Humans and animals | B. burgdorferi s.l. | Human cases | AM + SM | 12 |
| Heylen D [22] | 2019 | Belgium | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM | 11 |
| Keukeleire MD [23] | 2016 | Belgium | ND | Humans and animals | B. burgdorferi s.l. | Human cases | AM | 12 |
| Barrios JM [24] | 2013 | Belgium | ND | Humans | B. burgdorferi s.l. | Human cases | PM | 9 |
| Tack W [25] | 2012 | Belgium | I. ricinus | ND | B. burgdorferi s.l. | Tick abundance | AM | 12 |
| Barrios JM [26] | 2012 | Belgium | ND | Humans | B. burgdorferi s.l. | Human cases | SM | 10 |
| Barrios JM [27] | 2012 | Belgium | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 10 |
| Heylen D [28] | 2013 | Belgium | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | PM | 11 |
| Hönig V [29] | 2015 | Czech Republic | I. ricinus | ND | B. afzelii, B. garinii, B. burgdorferi s.s., B. valaisiana, B. lusitaniae, B. spielmanii | Tick abundance | AM | 11 |
| Daniel M [30] | 2009 | Czech Republic | I. ricinus | ND | B. afzelii, B. garinii, B. burgdorferi s.s., B. valaisiana | Tick abundance | AM | 11 |
| Daniel M [31] | 2008 | Czech Republic | I. ricinus | Humans | B. burgdorferi s.l. | Human cases, tick abundance | AM | 11 |
| Hubálek Z [32] | 2005 | Czech Republic | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 12 |
| Daniel M [33] | 2015 | Czech Republic | I. ricinus | ND | ND | Tick abundance | PM | 12 |
| Hubálek Z [34] | 2003 | Czech Republic | I. ricinus, H. concinna, D. reticulatus | ND | ND | Tick abundance | AM | 11 |
| Tkadlec E [35] | 2019 | Czech Republic, Slovakia, Poland | ND | Humans and animals | B. burgdorferi s.l. | Human cases | AM | 12 |
| Jensen PM [36] | 2005 | Denmark | I. ricinus | Animals | ND | Tick density | AM | 11 |
| Jensen PM [37] | 2000 | Denmark | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM | 10 |
| Jensen PM [38] | 2000 | Denmark | I. ricinus | ND | ND | Tick abundance | PM | 10 |
| Kjær LJ [39] | 2019 | Denmark, Norway, Sweden | I. ricinus | ND | ND | Tick abundance | PM | 11 |
| Kjær LJ [40] | 2019 | Denmark, Norway, Sweden | I. ricinus | ND | ND | Tick abundance | PM | 12 |
| Porretta D [41] | 2013 | Europe | I. ricinus | ND | ND | Tick abundance | PM + SM | 9 |
| Li S [42] | 2012 | Europe | I. ricinus | ND | B. afzelii, B. garinii | Tick abundance | PM | 12 |
| Li S [43] | 2019 | Europe | I. ricinus | Animals | B. burgdorferi s.l. | Human LD risk | PM | 10 |
| Fernández-Ruiz N [44] | 2020 | Europe | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Uusitalo R [45] | 2022 | Finland | I. ricinus, I. persulcatus | Animals | B. burgdorferi s.l. | Tick abundance | PM | 12 |
| Mariet AS [46] | 2013 | France | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 11 |
| Vassalo M [47] | 2000 | France | I. ricinus | ND | ND | Tick density | AM | 12 |
| Goldstein V [48] | 2018 | France | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Vourc’h G [49] | 2016 | France | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM + SM | 12 |
| Paul REL [50] | 2016 | France | I. ricinus | ND | B. burgdorferi s.l., B. miyamotoi | Tick density | AM | 12 |
| Halos L [51] | 2010 | France | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM | 12 |
| Wongnak P [15] | 2022 | France | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Boyard C [52] | 2007 | France | I. ricinus | ND | ND | Tick abundance | PM | 12 |
| Bourdin A [53] | 2022 | France | I. ricinus | ND | B. afzelii, B. burgdorferi s.l., B. burgdorferi s.s., B. garinii, B. lusitaniae, B. valaisiana | Tick abundance | AM | 12 |
| Perez G [54] | 2016 | France | I. ricinus | Animals | ND | Tick abundance | PM | 12 |
| Ehrmann S [55] | 2017 | France, Belgium, Germany, Sweden, Estonia | I. ricinus | ND | ND | Tick abundance | AM | 10 |
| Ehrmann S [56] | 2018 | France, Belgium, Germany, Sweden, Estonia | I. ricinus | ND | B. burgdorferi s.l. | Tick abundance | PM | 10 |
| Brugger K [57] | 2018 | Germany | I. ricinus | Animals | ND | Tick density | PM | 9 |
| Nolzen H [58] | 2022 | Germany | I. ricinus | ND | ND | Tick abundance | PM + SM | 11 |
| Răileanu C [59] | 2021 | Germany | I. ricinus | Humans and animals | B. afzelii, B. burgdorferi s.s., B. garinii, B. valaisiana, B. spielmanii, B. bavariensis | Tick abundance, tick and host infection | AM | 12 |
| Kohn M [60] | 2019 | Germany | D. reticulatus | ND | B. miyamotoi, B. afzelii | Tick abundance | AM | 11 |
| Brugger K [61] | 2017 | Germany | I. ricinus | Animals | ND | Tick abundance | AM | 12 |
| Boehnke D [62] | 2015 | Germany | I. ricinus | ND | ND | Tick density | SM | 12 |
| Schwarz A [63] | 2009 | Germany | I. ricinus | ND | ND | Tick abundance | AM + SM | 12 |
| Vollack K [64] | 2017 | Germany | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Schulz M [65] | 2014 | Germany | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Gethmann J [66] | 2020 | Germany | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Lauterbach R [67] | 2013 | Germany | I. ricinus | ND | ND | Tick density | PM | 12 |
| Hauck D [68] | 2020 | Germany | I. ricinus, I. inopinatus, I. frontalis, I. hexagonus | ND | ND | Tick abundance | AM | 12 |
| Trájer A [69] | 2013 | Hungary | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 12 |
| Trájer A [70] | 2014 | Hungary | ND | Humans | B. burgdorferi s.l. | Human cases | PM | 11 |
| Hornok S [71] | 2017 | Hungary | I. ricinus, D. reticulatus, D. marginatus, H. inermis, H. concinna | ND | B. burgdorferi s.l. | Tick abundance | AM | 12 |
| Garcia-Vozmediano A [72] | 2020 | Italy | I. ricinus, D. marginatus | Animals | B. burgdorferi s.l., B. miyamotoi | Tick abundance | AM | 9 |
| Rosà R [73] | 2007 | Italy | I. ricinus | Animals | ND | Tick abundance | PM | 12 |
| Rizzoli A [74] | 2002 | Italy | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | PM + SM | 12 |
| Zanzani SA [75] | 2019 | Italy | ND | Humans | B. burgdorferi s.l. | Human cases | SM | 12 |
| Altobelli A [76] | 2008 | Italy | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM + SM | 11 |
| Bisanzio D [77] | 2008 | Italy | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Tagliapietra V [78] | 2011 | Italy | I. ricinus | Animals | ND | Tick abundance | AM | 12 |
| Rosà R [79] | 2018 | Italy, Germany, Czech Republic, Slovakia, Hungary | I. ricinus | ND | B. burgdorferi s.l. | Tick abundance | AM | 12 |
| Garcia-Martí I [80] | 2017 | Netherlands | I. ricinus | ND | ND | Tick bites | AM + SM | 12 |
| Swart A [81] | 2014 | Netherlands | I. ricinus | Animals | ND | Tick abundance | PM + SM | 10 |
| Qviller L [82] | 2014 | Norway | I. ricinus | ND | ND | Tick density | AM | 12 |
| Kiewra D [83] | 2018 | Poland | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 12 |
| Buczek A [84] | 2014 | Poland | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Dyczko D [85] | 2022 | Poland | I. ricinus | Animals | B. afzelii, B. garinii, B. valaisiana, B. lusitaniae, B. miyamotoi | Tick abundance | AM | 12 |
| Kiewra D [86] | 2014 | Poland | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Domşa C [87] | 2018 | Romania | I. ricinus | ND | ND | Tick abundance | PM | 12 |
| Pangrácová L [88] | 2013 | Slovakia | I. ricinus | ND | ND | Tick abundance | AM | 11 |
| Kazimírová M [89] | 2016 | Slovakia | I. ricinus | Animals | ND | Tick density | AM | 12 |
| Donša D [90] | 2021 | Slovenia | ND | Humans and animals | B. burgdorferi s.l. | Human cases | PM + SM | 12 |
| Knap N [91] | 2009 | Slovenia | I. ricinus | ND | ND | Tick abundance | AM | 12 |
| Ruiz-Fons F [92] | 2012 | Spain | I. ricinus | Animals | B. burgdorferi s.l. | Tick abundance | AM + SM | 12 |
| Estrada-Peña A [93] | 2001 | Spain | I. ricinus | ND | ND | Tick abundance | AM | 10 |
| Alonso-Carné J [94] | 2016 | Spain | I. ricinus | ND | ND | Tick abundance | AM | 11 |
| Barandika JF [95] | 2006 | Spain | I. ricinus, H. punctata | Animals | ND | Tick abundance | AM | 12 |
| Bennet L [96] | 2006 | Sweden | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 12 |
| Jaenson TG [97] | 2009 | Sweden | I. ricinus | Animals | ND | Tick density | AM | 10 |
| Jaenson TG [98] | 2011 | Sweden | I. ricinus | ND | ND | Tick abundance | AM + SM + PM | 12 |
| Keith K [99] | 2022 | Sweden | ND | Humans | B. burgdorferi s.l. | Human cases | AM | 12 |
| Lindström A [100] | 2003 | Sweden | I. ricinus | ND | ND | Tick abundance | AM | 10 |
| Analyzed Species | Countries |
|---|---|
| Analyzed Borrelia species | |
| B. afzelii | Czech Republic [29,30], Germany [59,60], Poland [85] |
| B. bavariensis | Germany [59] |
| B. burgdorferi s.l. | Belgium [21,22,23,24,25,26,27,28,56], Czech Republic [31,32,35,79], Denmark [37], Estonia [56], Finland [45], France [46,49,50,51,53,56], Germany [56,79], Hungary [69,70,71,79], Italy [72,74,75,76,79], Poland [35,83], Slovakia [35,79], Slovenia [90], Spain [92], Sweden [56,96,99] |
| B. burgdorferi s.s. | Czech Republic [29,30], France [53], Germany [59] |
| B. garinii | Czech Republic [29,30], Germany [59], Poland [85] |
| B. lusitaniae | Czech Republic [29], Poland [85] |
| B. miyamotoi | France [50], Germany [60], Italy [72], Poland [85] |
| B. spielmanii | Czech Republic [29], Germany [59] |
| B. valaisiana | Czech Republic [29,30], Germany [59], Poland [85] |
| Analyzed vector species | |
| D. marginatus | Hungary [71], Italy [72] |
| D. reticulatus | Czech Republic [34], Germany [60], Hungary [71] |
| H. concinna | Czech Republic [34], Hungary [71] |
| H. inermis | Hungary [71] |
| H. punctata | Spain [95] |
| I. frontalis | Germany [68] |
| I. hexagonus | Germany [68] |
| I. inopinatus | Germany [68] |
| I. persulcatus | Finland [45] |
| I. ricinus | Belgium [22,25,28,55,56], Czech Republic [29,30,31,33,34,79], Denmark [36,37,38,39,40], Estonia [55,56], Finland [45], France [15,47,48,49,50,51,52,53,54,55,56], Germany [55,56,57,58,59,61,62,63,64,65,66,67,68,79], Hungary [71,79], Italy [72,73,74,76,77,78,79], Netherlands [80,81], Norway [39,40,82], Poland [84,85,86], Romania [87], Slovakia [79,88,89], Slovenia [91], Spain [92,93,94,95], Sweden [39,40,55,56,97,98,100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giesen, C.; Cifo, D.; Gomez-Barroso, D.; Estévez-Reboredo, R.M.; Figuerola, J.; Herrador, Z. The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review. Trop. Med. Infect. Dis. 2024, 9, 113. https://doi.org/10.3390/tropicalmed9050113
Giesen C, Cifo D, Gomez-Barroso D, Estévez-Reboredo RM, Figuerola J, Herrador Z. The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review. Tropical Medicine and Infectious Disease. 2024; 9(5):113. https://doi.org/10.3390/tropicalmed9050113
Chicago/Turabian StyleGiesen, Christine, Daniel Cifo, Diana Gomez-Barroso, Rosa M. Estévez-Reboredo, Jordi Figuerola, and Zaida Herrador. 2024. "The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review" Tropical Medicine and Infectious Disease 9, no. 5: 113. https://doi.org/10.3390/tropicalmed9050113
APA StyleGiesen, C., Cifo, D., Gomez-Barroso, D., Estévez-Reboredo, R. M., Figuerola, J., & Herrador, Z. (2024). The Role of Environmental Factors in Lyme Disease Transmission in the European Union: A Systematic Review. Tropical Medicine and Infectious Disease, 9(5), 113. https://doi.org/10.3390/tropicalmed9050113









