Bartonella Neuroretinitis with Initial Seronegativity and an Absent Macular Star: A Case Report and Literature Review
Abstract
:1. Background
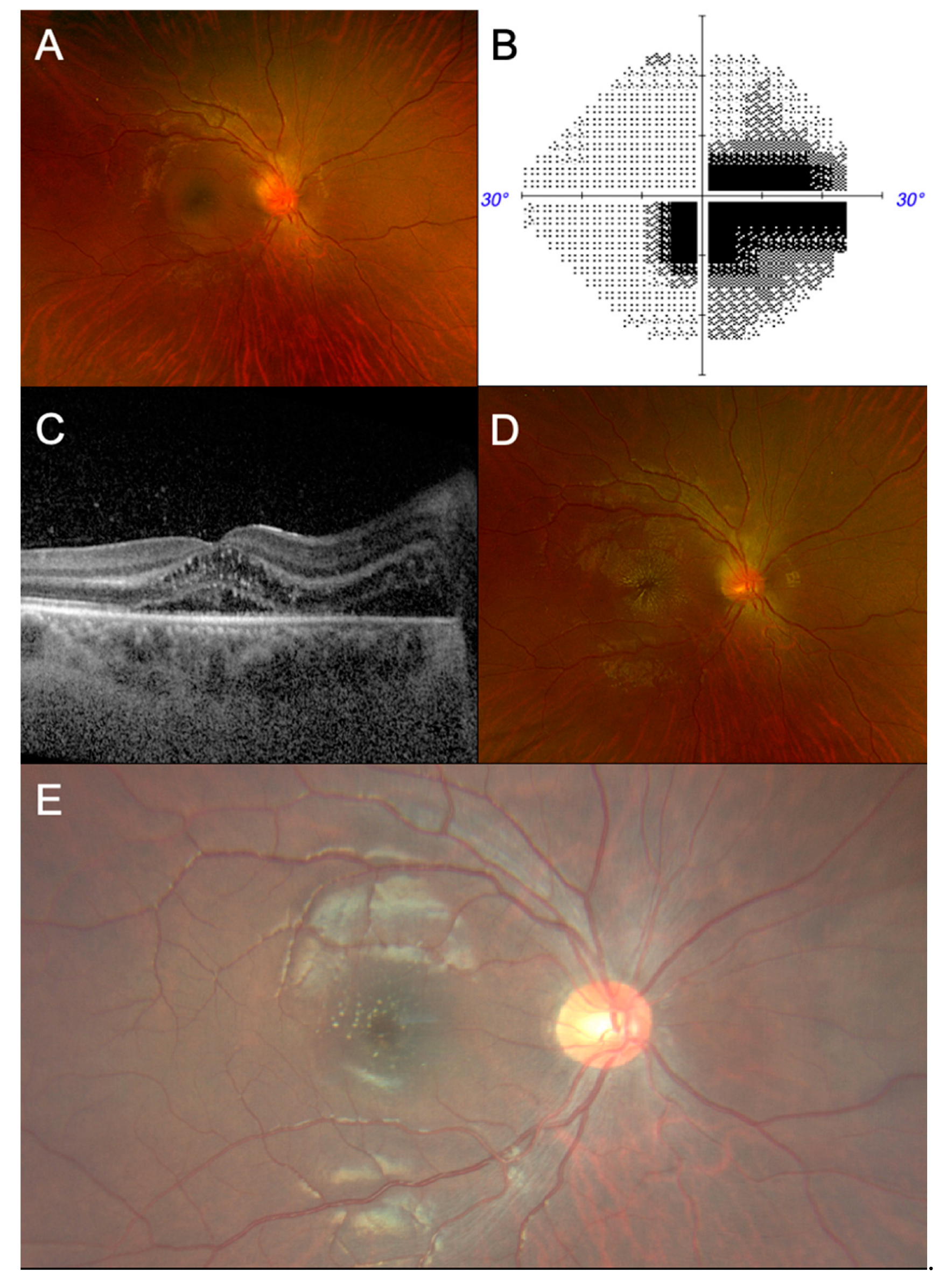
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Abdelhakim, A.; Rasool, N. Neuroretinitis: A review. Curr. Opin. Ophthalmol. 2018, 29, 514–519. [Google Scholar] [CrossRef]
- Joo, T.; Kim, T.G.; Moon, S.W. Incidental multiple myeloma in a patient with neuroretinitis: A case report. BMC Ophthalmol. 2023, 23, 495. [Google Scholar] [CrossRef]
- Kahloun, R.; Khairallah-Ksiaa, I.; Abroug, N.; Mahmoud, A.; Ben Yahia, S.; Zaouali, S.; Jelliti, B.; Khairallah, M. Final Diagnosis in Patients Referred with a Diagnosis of Neuroretinitis. Neuroophthalmology 2015, 39, 266–270. [Google Scholar] [CrossRef]
- Nasirudeen, A.M.; Thong, M.L. Prevalence of Bartonella henselae immunoglobulin G antibodies in Singaporean cats. Pediatr. Infect. Dis. J. 1999, 18, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Sanjay, S. Lyme neuroretinitis in Singapore: A diagnostic dilemma. Ann. Acad. Med. Singap. 2012, 41, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Gajjar, N.; Karnath, B.; Zhou, Y. Bartonella Neuroretinitis: There Is More to Cat Scratch Disease than Meets the Eye. Am. J. Case Rep. 2023, 24, e938380. [Google Scholar] [CrossRef]
- Purvin, V.; Sundaram, S.; Kawasaki, A. Neuroretinitis: Review of the Literature and New Observations. J. Neuro-Ophthalmol. 2011, 31, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Foo, R.; Yau, C.; Singhal, S.; Tow, S.; Loo, J.-L.; Tan, K.; Milea, D. Optic Neuritis in the Era of NMOSD and MOGAD: A Survey of Practice Patterns in Singapore. Asia-Pac. J. Ophthalmol. 2022, 11, 184–195. [Google Scholar] [CrossRef]
- Dreyer, R.F.; Hopen, G.; Gass, J.D.; Smith, J.L. Leber’s idiopathic stellate neuroretinitis. Arch. Ophthalmol. 1984, 102, 1140–1145. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Besada, E.; Woods, A.; Caputo, M. An Uncommon Presentation of Bartonella-Associated Neuroretinitis. Optom. Vis. Sci. 2002, 79, 479–488. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Klistorner, A.; Thie, J.; Graham, S.L. Latency Delay of Visual Evoked Potential Is a Real Measurement of Demyelination in a Rat Model of Optic Neuritis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6911–6918. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Yamamoto, S.; Hirayama, A.; Yotsukura, J.; Yamazaki, H. Pattern Visual Evoked Potentials in Eyes with Disc Swelling due to Cat Scratch Disease-associated Neuroretinitis. Doc. Ophthalmol. 2005, 110, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.K.; Kaliaperumal, S.; Srinivasan, R. Neuroretinitis, a great mimicker. Ann. Indian Acad. Neurol. 2008, 11, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Byon, I.S.; Kwon, H.J.; Park, S.W. Case series of neuroretinitis in Korea. BMC Ophthalmol. 2024, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Fhun, L.C.; Tai, E.L.; Abdul Gani, N.H.; Muhammed, J.; Tuan Jaafar, T.N.; Ahmad Tajudin, L.S.; Wan Hitam, W.H. Clinical Profile and Visual Outcome of Ocular Bartonellosis in Malaysia. J. Trop. Med. 2017, 2017, 7946123. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.J.; Herremans, M.; Verbakel, H.; Bergmans, A.M.; Roord, J.J.; van Dijken, P.J.; Peeters, M.F. Serological testing for Bartonella henselae infections in The Netherlands: Clinical evaluation of immunofluorescence assay and ELISA. Clin. Microbiol. Infect. 2007, 13, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Olson, J.G.; Perkins, B.A.; Bibb, W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 1992, 339, 1443–1445. [Google Scholar] [CrossRef]
- Szelc-Kelly, C.M.; Goral, S.; Perez-Perez, G.I.; Perkins, B.A.; Regnery, R.L.; Edwards, K.M. Serologic responses to Bartonella and Afipia antigens in patients with cat scratch disease. Pediatrics 1995, 96, 1137–1142. [Google Scholar] [CrossRef]
- Goaz, S.; Rasis, M.; Binsky Ehrenreich, I.; Shapira, L.; Halutz, O.; Graidy-Varon, M.; Leibovitch, C.; Maisler, N.; Glikman, D.; Ephros, M.; et al. Molecular Diagnosis of Cat Scratch Disease: A 25-Year Retrospective Comparative Analysis of Various Clinical Specimens and Different PCR Assays. Microbiol. Spectr. 2022, 10, e0259621. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Qian, Z.; Tao, Y. Application of metagenomic next-generation sequencing in the diagnosis of Bartonella neuroretinitis: A case report and literature review. J. Ophthalmic Inflamm. Infect. 2024, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.J.; Mandell, A.M.; Otis, J.A.; Holmuhamedova, M.; Perloff, M.D. Suspecting Optic Neuritis, Diagnosing Bartonella Cat Scratch Disease. Arch. Neurol. 2011, 68, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, Y.B.; Xu, X.F.; Pan, Y.; Lu, C.Y.; Zhu, X.L.; Li, Q.H.; Yu, R.S. Lymphadenitis associated with cat-scratch disease simulating a neoplasm: Imaging findings with histopathological associations. Oncol. Lett. 2018, 15, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Z.; Kruskal, J.; Kane, R.A.; Trey, G. Cat-scratch disease simulating lymphoma. J. Comput. Assist. Tomogr. 1996, 20, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Bernard, L.; Bayou, E.; Bani-Sadr, F.; Vallée, C.; Perronne, C. Bartonella henselae infection mimicking a splenic lymphoma. Scand. J. Infect. Dis. 2001, 33, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Ianas, V.; Elliott, S.P. Cat-scratch Disease. Am. Fam. Physician 2011, 83, 152–155. [Google Scholar] [PubMed]
- Bhatti, M.T.; Asif, R.; Bhatti, L.B. Macular Star in Neuroretinitis. Arch. Neurol. 2001, 58, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Cekiç, O.; Bozkurt, E.; Pekel, G.; Yazici, A.T.; Yilmaz, O.F. Combined intravitreal bevacizumab and triamcinolone acetonide injection for idiopathic neuroretinitis. Ocul. Immunol. Inflamm. 2009, 17, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.B.; Scales, D.K.; Wong, M.T.; Lattuada, C.P., Jr.; Dolan, M.J.; Schwab, I.R. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology 1998, 105, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Margileth, A.M. Antibiotic therapy for cat-scratch disease: Clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr. Infect. Dis. J. 1992, 11, 474–478. [Google Scholar] [CrossRef] [PubMed]
| Localisation | Intracranial | Retrobulbar | Ocular (Retina, Choroid, Optic Nerve Head) |
|---|---|---|---|
| Causes | Space occupying lesion Malignant hypertension Venous sinus thrombosis Idiopathic intracranial hypertension Meningitis | Inflammatory/demyelinating (e.g., optic neuritis, neuroretinitis) Ischaemic Compressive Others (e.g., traumatic, nutritional, hereditary) | Central retinal vein occlusion Infiltration Inflammatory (e.g., neuroretinitis, uveitis) Hypotony (decompression) |
| Symptoms | Headache Nausea and vomiting Diplopia Transient vision loss | Blurring of vision (typically progressive) Loss of colour vision Pain on eye movement | Blurring of vision, floaters Eye pain/redness |
| Ocular signs | Unilateral/bilateral disc swelling False localising sixth nerve palsy Macular star * | Reduced colour vision Relative afferent pupillary defect (if asymmetrical) Proptosis | Redness Cells in anterior chamber, vitritis Retinal vasculitis Retinal haemorrhages Macular star * |
| Systemic signs | Raised blood pressure Neck stiffness Fever | Focal neurological signs (e.g., numbness, weakness) Normal examination | Cervical lymphadenopathy Skin changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.T.; Yong, D.W.W.; Lin, H.A. Bartonella Neuroretinitis with Initial Seronegativity and an Absent Macular Star: A Case Report and Literature Review. Trop. Med. Infect. Dis. 2024, 9, 186. https://doi.org/10.3390/tropicalmed9080186
Pan JT, Yong DWW, Lin HA. Bartonella Neuroretinitis with Initial Seronegativity and an Absent Macular Star: A Case Report and Literature Review. Tropical Medicine and Infectious Disease. 2024; 9(8):186. https://doi.org/10.3390/tropicalmed9080186
Chicago/Turabian StylePan, Jason Timothy, Dayna Wei Wei Yong, and Hazel Anne Lin. 2024. "Bartonella Neuroretinitis with Initial Seronegativity and an Absent Macular Star: A Case Report and Literature Review" Tropical Medicine and Infectious Disease 9, no. 8: 186. https://doi.org/10.3390/tropicalmed9080186
APA StylePan, J. T., Yong, D. W. W., & Lin, H. A. (2024). Bartonella Neuroretinitis with Initial Seronegativity and an Absent Macular Star: A Case Report and Literature Review. Tropical Medicine and Infectious Disease, 9(8), 186. https://doi.org/10.3390/tropicalmed9080186







