Do Babesia microti Hosts Share a Blood Group System Gene Ortholog, Which Could Generate an Erythrocyte Antigen That Is Essential for Parasite Invasion?
Abstract
1. Introduction
2. Materials and Methods
2.1. hBG System Genes and Orthologs
2.2. Literature Review
2.3. Animal Taxonomy and Total Number of Genes in NCBI
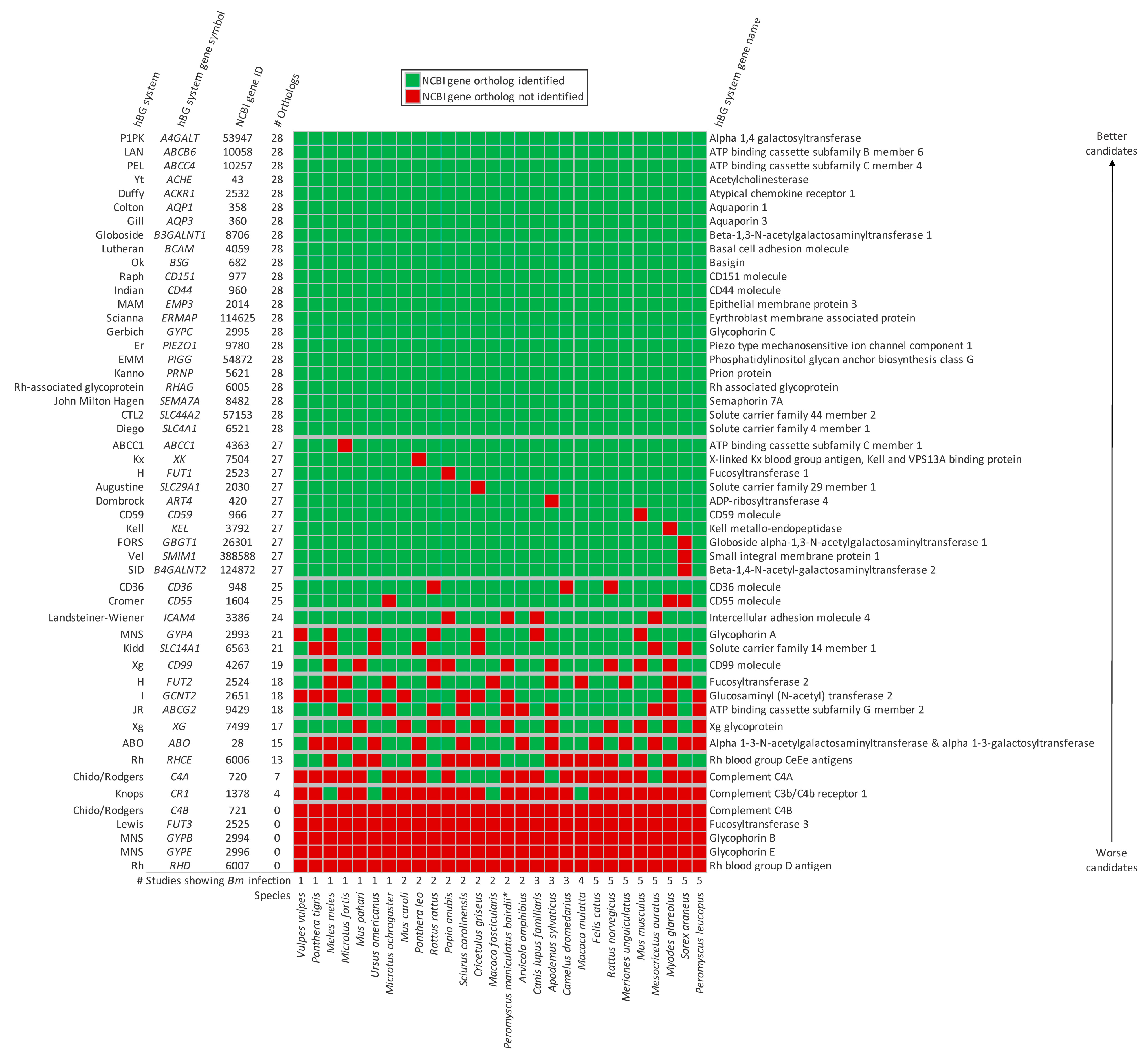
3. Results
| Species | Genbank Common Name | NCBI BLAST Name | NCBI Gene Entries | # Studies Showing Infection * |
|---|---|---|---|---|
| Peromyscus leucopus | white-footed mouse | rodents | 32,259 | 5 [7,8,45,46,53] |
| Sorex araneus | European shrew | insectivores | 28,675 | 5 [37,54,55,56,57] |
| Myodes glareolus | bank vole | rodents | 30,720 | 5 [37,58,59,60,61] |
| Mesocricetus auratus | golden hamster | rodents | 36,157 | 5 [62,63,64,65,66] |
| Mus musculus | house mouse | rodents | 107,992 | 5 [67,68,69,70,71] |
| Meriones unguiculatus | Mongolian gerbil | rodents | 34,732 | 5 [72,73,74,75,76] |
| Rattus norvegicus | Norway rat | rodents | 47,827 | 5 [67,70,77,78,79] |
| Felis catus | domestic cat | carnivores | 39,395 | 5 [51,80,81,82,83] |
| Macaca mulatta | Rhesus monkey | primates | 40,413 | 4 [65,84,85,86] |
| Camelus dromedarius | Arabian camel | even-toed ungulates | 37,476 | 3 [87,88,89] |
| Apodemus sylvaticus | European woodmouse | rodents | 34,663 | 3 [58,59,60] |
| Canis lupus familiaris | dog | carnivores | 50,757 | 3 [35,51,52] |
| Arvicola amphibius | Eurasian water vole | rodents | 28,375 | 2 [90,91] |
| Peromyscus maniculatus bairdii | prairie deer mouse | rodents | 36,461 | 2 [45,46] ^ |
| Macaca fascicularis | crab-eating macaque | primates | 35,716 | 2 [84,92] |
| Cricetulus griseus | Chinese hamster | rodents | 34,824 | 2 [38,39] |
| Sciurus carolinensis | gray squirrel | rodents | 37,368 | 2 [7,8] |
| Papio anubis | olive baboon | primates | 39,330 | 2 [36,93] |
| Rattus rattus | black rat | rodents | 32,124 | 2 [94,95] |
| Panthera leo | lion | carnivores | 32,109 | 2 [80,96] |
| Mus caroli | Ryukyu mouse | rodents | 32,457 | 2 [79,94] |
| Microtus ochrogaster | prairie vole | rodents | 26,434 | 1 [97] |
| Ursus americanus | American black bear | carnivores | 28,897 | 1 [98] |
| Mus pahari | shrew mouse | rodents | 29,520 | 1 [94] |
| Microtus fortis | reed vole | rodents | 29,738 | 1 [99] |
| Meles meles | Eurasian badger | carnivores | 31,234 | 1 [100] |
| Panthera tigris | tiger | carnivores | 33,598 | 1 [80] |
| Vulpes vulpes | red fox | carnivores | 29,062 | 1 [101] |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Christie, J.; Köster, L.; Du, A.; Yao, C. Emerging Human Babesiosis with “Ground Zero” in North America. Microorganisms 2021, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.B.; Herwaldt, B.L. Babesiosis Surveillance—United States, 2011–2015. MMWR. Surveill. Summ. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Holbrook, N.R.; Klontz, E.H.; Adams, G.C.; Schnittman, S.R.; Issa, N.C.; Bond, S.A.; Branda, J.A.; Lemieux, J.E. Babesia microti Variant with Multiple Resistance Mutations Detected in an Immunocompromised Patient Receiving Atovaquone Prophylaxis. Open Forum Infect. Dis. 2023, 10, ofad097. [Google Scholar] [CrossRef] [PubMed]
- Marcos, L.A.; Wormser, G.P. Relapsing Babesiosis With Molecular Evidence of Resistance to Certain Antimicrobials Commonly Used to Treat Babesia microti Infections. Open Forum Infect. Dis. 2023, 10, ofad391. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.; Krause, P.J.; Norris, A.M.; Ting, M.H.; Nagami, E.H.; Cilley, B.; Vannier, E. Broad Antimicrobial Resistance in a Case of Relapsing Babesiosis Successfully Treated With Tafenoquine. Clin. Infect. Dis. 2022, 76, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Rogers, R.; Shah, M.K.; Kang, H.; Parsonnet, J.; Kodama, R.; Vannier, E. Tafenoquine for Relapsing Babesiosis: A Case Series. Clin. Infect. Dis. 2024, 79, 130–137. [Google Scholar] [CrossRef]
- Hersh, M.H.; Tibbetts, M.; Strauss, M.; Ostfeld, R.S.; Keesing, F. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 2012, 18, 1951–1957. [Google Scholar] [CrossRef]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Can exchange transfusions using red blood cells from donors with Southeast Asian ovalocytosis prevent or ameliorate cerebral malaria in patients with multi-drug resistant Plasmodium falciparum? Transfus. Apher. Sci. 2017, 56, 865–866. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Can the Therapeutically-rational Exchange (T-REX) of Glucose-6-phosphate Dehydrogenase Deficient Red Blood Cells Reduce Plasmodium falciparum Malaria Morbidity and Mortality? J. Nepal. Health Res. Counc. 2018, 16, 108. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Can Therapeutically-Rational Exchange (T-REX) of Thalassemic Red Blood Cells Improve the Clinical Course of Plasmodium falciparum Malaria? Eurasian J. Med. 2018, 50, 215–216. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. To prevent or ameliorate severe Plasmodium falciparum malaria, why not evaluate the impact of exchange transfusions of sickle cell trait red blood cells? Transfus. Apher. Sci. 2018, 57, 63–64. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Can Exchange Transfusions Using Red Blood Cells from Donors with Hemoglobin E Trait Prevent or Ameliorate Severe Malaria in Patients with Multi-drug Resistant Plasmodium falciparum? Indian J. Hematol. Blood Transfus. 2018, 34, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Can therapeutically-rational exchange (T-REX) of type-O red blood cells (RBCs) benefit Plasmodium falciparum malaria patients? Transfus. Apher. Sci. 2019, 58, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Therapeutically-rational exchange (T-REX) of Gerbich-negative red blood cells can be evaluated in Papua New Guinea as “a rescue adjunct” for patients with Plasmodium falciparum malaria. Ther. Apher. Dial. 2021, 25, 242–247. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Wu, S.-C.; Jajosky, P.G.; Stowell, S.R. Plasmodium knowlesi (Pk) Malaria: A Review & Proposal of Therapeutically Rational Exchange (T-REX) of Pk-Resistant Red Blood Cells. Trop. Med. Infect. Dis. 2023, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. Optimizing exchange transfusion for patients with severe Babesia divergens babesiosis: Therapeutically-Rational Exchange (T-REX) of M antigen-negative and/or S antigen-negative red blood cells should be evaluated now. Transfus. Clin. Biol. 2018, 26, 76–79. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2023; WHO: Geneva, Switzerland, 2023.
- Moorthy, V.; Hamel, M.J.; Smith, P.G. Malaria vaccines for children: And now there are two. Lancet 2024, 403, 504–505. [Google Scholar] [CrossRef]
- Miller, L.H.; Mason, S.J.; Clyde, D.F.; McGinniss, M.H. The resistance factor to Plasmodium vivax in blacks: The Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 1976, 295, 302–304. [Google Scholar] [CrossRef]
- Miller, L.H.; Hudson, D.; Davidhaynes, J. Identification of Plasmodium knowlesi erythrocyte binding proteins. Mol. Biochem. Parasitol. 1988, 31, 217–222. [Google Scholar] [CrossRef]
- Wertheimer, S.P.; Barnwell, J.W. Plasmodium vivax interaction with the human Duffy blood group glycoprotein: Identification of a parasite receptor-like protein. Exp. Parasitol. 1989, 69, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Dickey, T.H.; Tolia, N.H. Designing an effective malaria vaccine targeting Plasmodium vivax Duffy-binding protein. Trends Parasitol. 2023, 39, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Silk, S.E.; Kalinga, W.F.; Salkeld, J.; Mtaka, I.M.; Ahmed, S.; Milando, F.; Diouf, A.; Bundi, C.K.; Balige, N.; Hassan, O.; et al. Blood-stage malaria vaccine candidate RH5.1/Matrix-M in healthy Tanzanian adults and children; an open-label, non-randomised, first-in-human, single-centre, phase 1b trial. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.; Wang, Y.; Ren, W.; Zhao, X.; Ji, F.; Zhu, Y.; Feng, F.; Gong, M.; Ju, X.; et al. Functional and genetic analysis of viral receptor ACE2 orthologs reveals a broad potential host range of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2025373118. [Google Scholar] [CrossRef]
- Maurer, K.J.; Quimby, F.W. Chapter 34—Animal Models in Biomedical Research. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1497–1534. [Google Scholar]
- Wanaguru, M.; Liu, W.; Hahn, B.H.; Rayner, J.C.; Wright, G.J. RH5–Basigin interaction plays a major role in the host tropism of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 20735–20740. [Google Scholar] [CrossRef]
- Salzberg, S.L. Open questions: How many genes do we have? BMC Biol. 2018, 16, 94. [Google Scholar] [CrossRef]
- Borggraefe, I.; Yuan, J.; Telford, S.R., 3rd; Menon, S.; Hunter, R.; Shah, S.; Spielman, A.; Gelfand, J.A.; Wortis, H.H.; Vannier, E. Babesia microti primarily invades mature erythrocytes in mice. Infect. Immun. 2006, 74, 3204–3212. [Google Scholar] [CrossRef]
- ISBT. Table of Blood Group Systems 2023. Available online: https://www.isbtweb.org/resource/tableofbloodgroupsystems.html (accessed on 1 January 2023).
- NCBI. NCBI Gene 2024. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 1 January 2023).
- NCBI. Command-Line Tools 2024. Available online: https://www.ncbi.nlm.nih.gov/datasets/docs/v2/download-and-install/ (accessed on 1 January 2024).
- NLM. How Are Orthologs Calculated? 2024. Available online: https://www.ncbi.nlm.nih.gov/kis/info/how-are-orthologs-calculated/#:~:text=With%20a%20few%20exceptions%2C%20ortholog,based%20on%20protein%20sequence%20similarity (accessed on 1 January 2023).
- Google. Google Scholar 2024. Available online: https://scholar.google.com/ (accessed on 1 January 2023).
- Ohmori, T.; Uetsuka, K.; Nunoya, T. Experimental infection of dogs with Babesia microti. J. Protozool. Res. 2011, 21, 78–84. [Google Scholar] [CrossRef]
- Maamun, J.M.; Suleman, M.A.; Akinyi, M.; Ozwara, H.; Kariuki, T.; Carlsson, H.-E. Prevalence of Babesia microti in free-ranging baboons and african green monkeys. J. Parasitol. 2011, 97, 63–67. [Google Scholar] [CrossRef]
- Samokhvalov, M.V.; Kovalevskii, Y.V.; Korenberg, E.I.; Morozov, A.V.; Kuzikov, I.V.; Sheftel’, B.I. Small mammals as potential reservoir hosts of Babesia microti in the Middle Urals. Biol. Bull. 2010, 37, 748–752. [Google Scholar] [CrossRef]
- Ike, K.; Komatsu, T.; Murakami, T.; Kato, Y.; Takahashi, M.; Uchida, Y.; Imai, S. High susceptibility of Djungarian hamsters (Phodopus sungorus) to the infection with Babesia microti supported by hemodynamics. J. Veter. Med. Sci. 2005, 67, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ike, K.; Murakami, T.; Komatsu, T.; Uchida, Y.; Imai, S. Susceptibility of Chinese hamsters (Cricetulus griseus) to the infection of Babesia microti. J. Veter. Med. Sci. 2005, 67, 333–336. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Nadal, C. Experimental Infection of Ticks: An Essential Tool for the Analysis of Babesia Species Biology and Transmission. Pathogens 2021, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Coburn, L.A. Insights into the development of Ixodes scapularis: A resource for research on a medically important tick species. Parasites Vectors 2015, 8, 592. [Google Scholar] [CrossRef]
- Goethert, H.K. What Babesia microti Is Now. Pathogens 2021, 10, 1168. [Google Scholar] [CrossRef]
- NCBI. Taxonomy 2024. Available online: https://www.ncbi.nlm.nih.gov/taxonomy (accessed on 1 January 2024).
- NCBI. Datasets 2024. Available online: https://www.ncbi.nlm.nih.gov/datasets/ (accessed on 1 January 2024).
- Rocco, J.M.; Regan, K.M.; Larkin, J.L.; Eichelberger, C.; Wisgo, J.; Nealen, P.M.; Irani, V.R. Higher Prevalence of Babesia microti than Borrelia burgdorferi in Small Mammal Species in Central Pennsylvania, United States. Vector-Borne Zoonotic Dis. 2020, 20, 151–154. [Google Scholar] [CrossRef]
- Larson, R.T.; Bron, G.M.; Lee, X.; Zembsch, T.E.; Siy, P.N.; Paskewitz, S.M. Peromyscus maniculatus (Rodentia: Cricetidae): An overlooked reservoir of tick-borne pathogens in the Midwest, USA? Ecosphere 2021, 12, e03831. [Google Scholar] [CrossRef]
- Burbrink, F.T.; Crother, B.I.; Murray, C.M.; Smith, B.T.; Ruane, S.; Myers, E.A.; Pyron, R.A. Empirical and philosophical problems with the subspecies rank. Ecol. Evol. 2022, 12, e9069. [Google Scholar] [CrossRef] [PubMed]
- Fuller, L. Continuous in vitro propagation of Babesia microti. Infect. Immun. 2024, 92, e0048123. [Google Scholar] [CrossRef]
- Piesman, J.; Spielman, A.; Etkind, P.; Ruebush, T.K., 2nd; Juranek, D.D. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J. Med. Entomol. 1979, 15, 537–540. [Google Scholar] [CrossRef]
- Wu, J.; Cao, J.; Zhou, Y.; Zhang, H.; Gong, H.; Zhou, J. Evaluation on Infectivity of Babesia microti to Domestic Animals and Ticks Outside the Ixodes Genus. Front. Microbiol. 2017, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Akram, I.N.; Parveen, T.; Abrar, A.; Mehmood, A.K.; Iqbal, F. Molecular detection of Babesia microti in dogs and cat blood samples collected from Punjab (Pakistan). Trop. Biomed. 2019, 36, 304–309. [Google Scholar]
- Gabrielli, S.; Otašević, S.; Ignjatović, A.; Savić, S.; Fraulo, M.; Arsić-Arsenijević, V.; Momčilović, S.; Cancrini, G. Canine Babesioses in Noninvestigated Areas of Serbia. Vector-Borne Zoonotic Dis. 2015, 15, 535–538. [Google Scholar] [CrossRef]
- Tufts, D.M.; Diuk-Wasser, M.A. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasites Vectors 2018, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Girardi, M.; Cagnacci, F.; Devineau, O.; Tagliapietra, V. First Record of Hepatozoon spp. in Alpine Wild Rodents: Implications and Perspectives for Transmission Dynamics across the Food Web. Microorganisms 2022, 10, 712. [Google Scholar] [CrossRef]
- Bown, K.J.; Lambin, X.; Telford, G.; Heyder-Bruckner, D.; Ogden, N.H.; Birtles, R.J. The common shrew (Sorex araneus): A neglected host of tick-borne infections? Vector-Borne Zoonotic Dis. 2011, 11, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.A.; Epikhina, T.I.; Livanova, N.N.; Panov, V.V. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology 2010, 138, 175–182. [Google Scholar] [CrossRef]
- Rar, V.A.; Epikhina, T.I.; Livanova, N.N.; Panov, V.V.; Pukhovskaya, N.M.; Vysochina, N.P.; Ivanov, L.I. Detection of Babesia DNA in small mammals and ixodid ticks in the North Urals, Western Siberia, and Far East of Russia. Mol. Genet. Microbiol. Virol. 2010, 25, 118–123. [Google Scholar] [CrossRef]
- Usluca, S.; Celebi, B.; Karasartova, D.; Gureser, A.S.; Matur, F.; Oktem, M.A.; Sozen, M.; Karatas, A.; Babur, C.; Mumcuoglu, K.Y.; et al. Molecular Survey of Babesia microti (Aconoidasida: Piroplasmida) in Wild Rodents in Turkey. J. Med. Entomol. 2019, 56, 1605–1609. [Google Scholar] [CrossRef]
- Zintl, A.; McManus, A.; Galan, M.; Diquattro, M.; Giuffredi, L.; Charbonnel, N.; Gray, J.; Holland, C.; Stuart, P. Presence and identity of Babesia microti in Ireland. Ticks Tick-Borne Dis. 2023, 14, 102221. [Google Scholar] [CrossRef]
- Azagi, T.; Jaarsma, R.I.; van Leeuwen, A.D.; Fonville, M.; Maas, M.; Franssen, F.F.J.; Kik, M.; Rijks, J.M.; Montizaan, M.G.; Groenevelt, M.; et al. Circulation of Babesia Species and Their Exposure to Humans through Ixodes ricinus. Pathogens 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Kallio, E.R.; Begon, M.; Birtles, R.J.; Bown, K.J.; Koskela, E.; Mappes, T.; Watts, P.C. First Report of Anaplasma phagocytophilum and Babesia microti in Rodents in Finland. Vector-Borne Zoonotic Dis. 2014, 14, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.D.; Winton, C.S.; Tonnetti, L.; Townsend, R.L.; Berardi, V.P.; Hewins, M.-E.; Weeks, K.E.; Dodd, R.Y.; Stramer, S.L. Screening for Babesia microti in the U.S. Blood Supply. N. Engl. J. Med. 2016, 375, 2236–2245. [Google Scholar] [CrossRef]
- Torianyk, I.I. Biological method for babesiosis detection: The unified version in vivo. Wiad Lek 2021, 74, 268–272. [Google Scholar] [CrossRef]
- Zamoto, A.; Tsuji, M.; Kawabuchi, T.; Wei, Q.; Asakawa, M.; Ishihara, C. US-Type Babesia microti isolated from small wild mammals in Eastern Hokkaido, Japan. J. Veter. Med. Sci. 2004, 66, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Gumber, S.; Nascimento, F.S.; Rogers, K.A.; Bishop, H.S.; Rivera, H.N.; Xayavong, M.V.; Devare, S.G.; Schochetman, G.; Amancha, P.K.; Qvarnstrom, Y.; et al. Experimental transfusion-induced Babesia microti infection: Dynamics of parasitemia and immune responses in a rhesus macaque model. Transfusion 2016, 56, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.E.; Tran, A.D.; Freimark, L.; Schaffner, S.F.; Goethert, H.; Andersen, K.G.; Bazner, S.; Li, A.; McGrath, G.; Sloan, L.; et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat. Microbiol. 2016, 1, 16079. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, S.; Xu, G.; Liu, W.; Han, T.; Liu, J.; Wang, J.; Deng, Y.; Xiao, F. Prevalence and phylogenetic analysis of Babesia parasites in reservoir host species in Fujian province, Southeast China. Zoonoses Public Health 2022, 69, 915–924. [Google Scholar] [CrossRef]
- Tołkacz, K.; Rodo, A.; Wdowiarska, A.; Bajer, A.; Bednarska, M. Impact of Babesia microti infection on the initiation and course of pregnancy in BALB/c mice. Parasites Vectors 2021, 14, 132. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, S.; Yang, C.; Zhao, Z.; Li, H.; Lu, Y.; Ai, L.; Chu, Y.; Shen, H.; Chen, J. Dynamics of routine blood tests in BALB/c mice with Babesia microti infection. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2018, 30, 300–306. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Wang, X.-M.; Wang, Z.-S.; Wang, Z.-H.; Guan, Z.-Z.; Zhang, L.-H.; Dou, X.-F.; Wang, H. High prevalence of Babesia microti in small mammals in Beijing. Infect. Dis. Poverty 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, C.; Wang, R.; Hu, X.; Liao, S.; Liu, W.; Du, A.; Ji, S.; Galon, E.M.; Li, H.; et al. Serum metabolomic profiles in BALB/c mice induced by Babesia microti infection. Front. Cell. Infect. Microbiol. 2023, 13, 1179967. [Google Scholar] [CrossRef]
- Cornillot, E.; Dassouli, A.; Garg, A.; Pachikara, N.; Randazzo, S.; Depoix, D.; Carcy, B.; Delbecq, S.; Frutos, R.; Silva, J.C.; et al. Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PLoS ONE 2013, 8, e72657. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; von Stedingk, L.V.; Gürtelschmid, M.; Granström, M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 2002, 40, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Pichon, B.; Egan, D.; Rogers, M.; Gray, J. Detection and identification of pathogens and host DNA in unfed host-seeking Ixodes ricinus L. (Acari: Ixodidae). J. Med. Entomol. 2003, 40, 723–731. [Google Scholar] [CrossRef]
- Gray, J.S.; Pudney, M. Activity of atovaquone against Babesia microti in the Mongolian gerbil, Meriones unguiculatus. J. Parasitol. 1999, 85, 723. [Google Scholar] [CrossRef]
- Ruebush, T.K.; Contacos, P.G.; Steck, E.A. Chemotherapy of Babesia microti infections in Mongolian Jirds. Antimicrob. Agents Chemother. 1980, 18, 289–291. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Li, H.; Sun, Y.; Zhang, Y.-Y.; Jiang, J.-F.; Liu, W.; Cao, W.-C. Dual infection with Anaplasma phagocytophilum and Babesia microti in a Rattus norvegicus, China. Ticks Tick-Borne Dis. 2013, 4, 399–402. [Google Scholar] [CrossRef]
- de Cock, M.P.; de Vries, A.; Fonville, M.; Esser, H.J.; Mehl, C.; Ulrich, R.G.; Joeres, M.; Hoffmann, D.; Eisenberg, T.; Schmidt, K.; et al. Increased rat-borne zoonotic disease hazard in greener urban areas. Sci. Total Environ. 2023, 896, 165069. [Google Scholar] [CrossRef]
- Karnchanabanthoeng, A.; Morand, S.; Jittapalapong, S.; Carcy, B. Babesia Occurrence in Rodents in Relation to Landscapes of Mainland Southeast Asia. Vector-Borne Zoonotic Dis. 2018, 18, 121–130. [Google Scholar] [CrossRef]
- Bosman, A.-M. Detection of Babesia Species in Domestic and Wild Southern African Felids by Means of DNA Probes 2010. Available online: https://repository.up.ac.za/bitstream/handle/2263/23149/dissertation.pdf?sequence=1&isAllowed=y (accessed on 1 January 2023).
- Spada, E.; Proverbio, D.; Galluzzo, P.; Perego, R.; De Giorgi, G.B.; Roggero, N.; Caracappa, S. Frequency of Piroplasms Babesia microti and Cytauxzoon felis in Stray Cats from Northern Italy. BioMed Res. Int. 2014, 2014, 943754. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.-M.; Penzhorn, B.L.; Brayton, K.A.; Schoeman, T.; Oosthuizen, M.C. A novel Babesia sp. associated with clinical signs of babesiosis in domestic cats in South Africa. Parasites Vectors 2019, 12, 138. [Google Scholar] [CrossRef]
- Muz, M.N.; Erat, S.; Mumcuoglu, K.Y. Protozoan and Microbial Pathogens of House Cats in the Province of Tekirdag in Western Turkey. Pathogens 2021, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yang, Y.C.; Chen, Z.P.; Shi, Y.L. Infection of Plasmodium knowlesi and Babesia microti in farmed monkeys in Guangxi. Chin. J. Parasitol. Parasit. Dis. 2019, 37, 494–496. [Google Scholar]
- van Duivenvoorde, L.M.; der Wel, A.V.-V.; van der Werff, N.M.; Braskamp, G.; Remarque, E.J.; Kondova, I.; Kocken, C.H.M.; Thomas, A.W. Suppression of Plasmodium cynomolgi in Rhesus Macaques by Coinfection with Babesia microti. Infect. Immun. 2010, 78, 1032–1039. [Google Scholar] [CrossRef]
- Ruebush, T.K.; Warren, M.; Spielman, A.; Collins, W.E.; Piesman, J. Tick transmission of Babesia microti to rhesus monkeys (Macaca mulatta). Am. J. Trop. Med. Hyg. 1981, 30, 555–559. [Google Scholar] [CrossRef]
- Rizk, M.A. Molecular detection of Babesia microti in one-humped camel (Camelus dromedarius) in Halayeb and Shalateen, Halayeb, Egypt. Egypt. Veter. Med. Soc. Parasitol. J. (EVMSPJ) 2021, 17, 109–119. [Google Scholar] [CrossRef]
- Ashour, R.; Hamza, D.; Kadry, M.; Sabry, M.A. Molecular detection of Babesia microti in dromedary camels in Egypt. Trop. Anim. Health Prod. 2023, 55, 91. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.M.; Galon, E.M.; Soliman, A.M.; Do, T.; Zafar, I.; Ma, Y.; Li, H.; Ji, S.; Mohanta, U.K.; Xuan, X. Molecular detection of tick-borne piroplasmids in camel blood samples collected from Cairo and Giza governorates, Egypt. Acta Trop. 2024, 256, 107252. [Google Scholar] [CrossRef]
- Gelling, M.; Macdonald, D.W.; Telfer, S.; Jones, T.; Bown, K.; Birtles, R.; Mathews, F. Parasites and pathogens in wild populations of water voles (Arvicola amphibius) in the UK. Eur. J. Wildl. Res. 2011, 58, 615–619. [Google Scholar] [CrossRef]
- Mackenzie, L.S.; Lambin, X.; Bryce, E.; Davies, C.L.; Hassall, R.; Shati, A.A.M.; Sutherland, C.; Telfer, S.E. Patterns and drivers of vector-borne microparasites in a classic metapopulation. Parasitology 2023, 150, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Peng, H.; Zhu, H.-M.; Li, J.; Xue, S.-L. Investigation of two blood parasitic protozoa infection in farmed Macaca fascicularis in Guangxi Zhuang Autonomous Region. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2016, 28, 141–145. [Google Scholar] [CrossRef]
- Ezzelarab, M.; Yeh, P.; Wagner, R.; Cooper, D.K.C. Babesia as a complication of immunosuppression following pig-to-baboon heart transplantation. Xenotransplantation 2007, 14, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-R.; Ye, L.; Fan, J.-W.; Li, C.; Tang, F.; Liu, W.; Ren, L.-Z.; Bai, J.-Y. Detection of Kobe-type and Otsu-type Babesia microti in wild rodents in China’s Yunnan province. Epidemiol. Infect. 2017, 145, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Occhibove, F.; Capizzi, D.; Fratini, S.; Giannini, F.; Hoida, A.D.; Sposimo, P.; Valentini, F.; Ferroglio, E. Zoonotic Microparasites in Invasive Black Rats (Rattus rattus) from Small Islands in Central Italy. Animals 2023, 13, 3279. [Google Scholar] [CrossRef] [PubMed]
- Broughton, H.M. Infectious Diseases of the Felidae: Parasite Communities from Miniature to Massive: Oregon State University. 2017. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/cj82kd043 (accessed on 1 January 2023).
- Burkot, T.R.; Schneider, B.S.; Pieniazek, N.J.; Happ, C.M.; Rutherford, J.S.; Slemenda, S.B.; Hoffmeister, E.; O Maupin, G.; Zeidner, N.S. Babesia microti and Borrelia bissettii transmission by Ixodes spinipalpis ticks among prairie voles, Microtus ochrogaster, in Colorado. Parasitology 2000, 121 Pt 6, 595–599. [Google Scholar] [CrossRef]
- Zolnik, C.P.; Makkay, A.M.; Falco, R.C.; Daniels, T.J. American Black Bears as Hosts of Blacklegged Ticks (Acari: Ixodidae) in the Northeastern United States. J. Med. Entomol. 2015, 52, 1103–1110. [Google Scholar] [CrossRef]
- Fan, D.L.M.; Xu, H.; Hu, M.; Zhang, J.; Sun, Y. The situation of mice and ticks infected by Babesia microti. Chin. J. Hyg. Insect Equip. 2012, 18, 48–50. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20123361212 (accessed on 1 January 2023).
- Hong, S.-H.; Kim, H.-J.; Jeong, Y.-I.; Cho, S.-H.; Lee, W.-J.; Kim, J.-T.; Lee, S.-E. Serological and Molecular Detection of Toxoplasma gondii and Babesia microti in the Blood of Rescued Wild Animals in Gangwon-do (Province), Korea. Korean J. Parasitol. 2017, 55, 207–212. [Google Scholar] [CrossRef]
- Karbowiak, G.; Majláthová, V.; Hapunik, J.; Pet’ko, B.; Wita, I. Apicomplexan parasites of red foxes (Vulpes vulpes) in northeastern Poland. Acta Parasitol. 2010, 55, 210–214. [Google Scholar] [CrossRef]
- Jajosky, R.P.; O’bryan, J.; Spichler-Moffarah, A.; Jajosky, P.G.; Krause, P.J.; Tonnetti, L. The impact of ABO and RhD blood types on Babesia microti infection. PLoS Neglected Trop. Dis. 2023, 17, e0011060. [Google Scholar] [CrossRef]
- Baneth, G.; Cardoso, L.; Brilhante-Simões, P.; Schnittger, L. Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasites Vectors 2019, 12, 129. [Google Scholar] [CrossRef]
- Baniecki, M.L.; Moon, J.; Sani, K.; Lemieux, J.E.; Schaffner, S.F.; Sabeti, P.C. Development of a SNP barcode to genotype Babesia microti infections. PLoS Neglected Trop. Dis. 2019, 13, e0007194. [Google Scholar] [CrossRef]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef]
- Miller, L.H.; Mason, S.J.; Dvorak, J.A.; McGinniss, M.H.; Rothman, I.K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 1975, 189, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Jajosky, R.P.; Patel, S.R.; Wu, S.-C.; Patel, K.R.; Covington, M.L.; Vallecillo-Zúniga, M.L.; Ayona, D.; Bennett, A.; Luckey, C.J.; E Hudson, K.; et al. Prior Immunization to an Intracellular Antigen Enhances Subsequent Red Blood Cell Alloimmunization in Mice. Blood 2023, 141, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.L.; Jajosky, R.P.; Patel, S.R.; Verkerke, H.P.; Fuller, M.D.; Allen, J.W.; Zerra, P.E.; Fasano, R.M.; Chonat, S.; Josephson, C.D.; et al. Storage differentially impacts alloimmunization to distinct red cell antigens following transfusion in mice. Transfusion 2023, 63, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Gibb, D.R.; Girard-Pierce, K.; Zhou, X.; Rodrigues, L.C.; Arthur, C.M.; Bennett, A.L.; Jajosky, R.P.; Fuller, M.; Maier, C.L.; et al. Marginal Zone B Cells Induce Alloantibody Formation Following RBC Transfusion. Front. Immunol. 2018, 9, 2516. [Google Scholar] [CrossRef]
- Zerra, P.E.; Patel, S.R.; Jajosky, R.P.; Arthur, C.M.; McCoy, J.W.; Allen, J.W.L.; Chonat, S.; Fasano, R.M.; Roback, J.D.; Josephson, C.D.; et al. Marginal zone B cells mediate a CD4 T-cell–dependent extrafollicular antibody response following RBC transfusion in mice. Blood 2021, 138, 706–721. [Google Scholar] [CrossRef]
- Mener, A.; Patel, S.R.; Arthur, C.M.; Chonat, S.; Wieland, A.; Santhanakrishnan, M.; Liu, J.; Maier, C.L.; Jajosky, R.P.; Girard-Pierce, K.; et al. Complement serves as a switch between CD4+ T cell–independent and –dependent RBC antibody responses. J. Clin. Investig. 2018, 3, e121631. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Patel, K.R.; Allen, J.W.L.; Zerra, P.E.; Chonat, S.; Ayona, D.; Maier, C.L.; Morais, D.; Wu, S.-C.; Luckey, C.J.; et al. Antibody-mediated antigen loss switches augmented immunity to antibody-mediated immunosuppression. Blood 2023, 142, 1082–1098. [Google Scholar] [CrossRef]
- Magid-Bernstein, J.; Beaman, C.B.; Carvalho-Poyraz, F.; Boehme, A.; Hod, E.A.; Francis, R.O.; Elkind, M.S.V.; Agarwal, S.; Park, S.; Claassen, J.; et al. Impacts of ABO-incompatible platelet transfusions on platelet recovery and outcomes after intracerebral hemorrhage. Blood 2021, 137, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.M.; Stowell, S.R. The Development and Consequences of Red Blood Cell Alloimmunization. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 537–564. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L.; Pirenne, F.; Fasano, R.M.; Habibi, A.; Bartolucci, P.; Chonat, S.; Hendrickson, J.E.; Stowell, S.R. Hemolytic transfusion reactions in sickle cell disease: Underappreciated and potentially fatal. Haematologica 2020, 105, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Avent, N.D.; Reid, M.E. The Rh blood group system: A review. Blood 2000, 95, 375–387. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Lum, M.D.; Kalogeropoulos, A.; Spitzer, E.; Marcos, L.A. 270. RhD negative Blood Type is Associated with Higher Levels of Babesia microti Parasitemia and May Be a Useful Point-of-Care Biomarker in Human Babesiosis. Open Forum Infect. Dis. 2023, 10. [Google Scholar] [CrossRef]
- Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G. The Centers for Disease Control and Prevention and State Health Departments should include Blood-Type Variables in their Babesiosis Case Reports. Transfus. Apher. Sci. 2020, 59, 102824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jajosky, R.P.; Jajosky, A.N.; Jajosky, P.G.; Stowell, S.R. Do Babesia microti Hosts Share a Blood Group System Gene Ortholog, Which Could Generate an Erythrocyte Antigen That Is Essential for Parasite Invasion? Trop. Med. Infect. Dis. 2024, 9, 195. https://doi.org/10.3390/tropicalmed9090195
Jajosky RP, Jajosky AN, Jajosky PG, Stowell SR. Do Babesia microti Hosts Share a Blood Group System Gene Ortholog, Which Could Generate an Erythrocyte Antigen That Is Essential for Parasite Invasion? Tropical Medicine and Infectious Disease. 2024; 9(9):195. https://doi.org/10.3390/tropicalmed9090195
Chicago/Turabian StyleJajosky, Ryan P., Audrey N. Jajosky, Philip G. Jajosky, and Sean R. Stowell. 2024. "Do Babesia microti Hosts Share a Blood Group System Gene Ortholog, Which Could Generate an Erythrocyte Antigen That Is Essential for Parasite Invasion?" Tropical Medicine and Infectious Disease 9, no. 9: 195. https://doi.org/10.3390/tropicalmed9090195
APA StyleJajosky, R. P., Jajosky, A. N., Jajosky, P. G., & Stowell, S. R. (2024). Do Babesia microti Hosts Share a Blood Group System Gene Ortholog, Which Could Generate an Erythrocyte Antigen That Is Essential for Parasite Invasion? Tropical Medicine and Infectious Disease, 9(9), 195. https://doi.org/10.3390/tropicalmed9090195






