How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos?
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
3.1. Substances Classified as DG for Transport by Air
3.2. Application of Air Transport DG Regulations
3.3. Training of Personnel
- 1.
- Shippers (including Categories 1 and 2 in Table 2), with responsibility for:
- Packing DG in accordance with the regulations (Section 3.5).
- Producing a ‘Shipper’s Declaration for Dangerous Goods’ document to accompany consignments of DG packages (Section 3.5).
- 2.
- Operators (including Categories 6, 8 and 10 in Table 2), with responsibility for:
- Receiving DG and accompanying documentation from the shipper, and checking they are intact and correct (Section 3.5).
- Producing an ‘Air Waybill’ document as a receipt for the goods accepted for carriage (done for all goods, not just if DG are involved) (Section 3.5).
- Loading DG onto the UAV (Section 3.6).
- Producing the ‘Notification to Captain (NOTOC)’ document listing all DG for signing by the pilot in command (PIC) (Section 3.5).
- Flying the UAV to the destination.
- Unloading DG off the UAV and delivering to the recipient (Section 3.6).
3.4. Operator Approval
- Open category operations apply to situations presenting a low risk to third parties, and can be conducted without NAA authorisation. The boundaries of the category are: UAV maximum take-off mass (MTOM) below 25 kg; within Visual Line Of Sight (VLOS) and below 120 m (400 ft) above ground level (agl); and not over assemblies of people (gatherings where people are unable to move away due to crowd density).
- Specific category operations apply to situations where any of the requirements of the open category are exceeded, and require the UAV operator to obtain an operational authorisation from the NAA, which involves the operator submitting a Risk Assessment (RA) for the proposed operation, for example using the Specific Operations Risk Assessment (SORA) methodology developed by the Joint Authorities for Rule-making on Unmanned Systems (JARUS). Alternatively, Standard Scenarios (STSs) and Pre-Defined Risk Assessments (PDRAs) are options available to operators (depending on the system adopted in the nation of operation) both designed to reduce the need for evidence of risk mitigation for pre-determined types of operation defined by prescriptive conditions (in essence ‘off-the-shelf’ RAs for particular operation types). (For example, UKPDRA01 published recently by the UK CAA is for an operation type defined by the following conditions: VLOS only, maximum 500 m horizontally from remote pilot; use of a UA observer situated next to the remote pilot, is permitted; maximum height not to exceed 120 m (400 ft) agl; flight permitted within 150 m of any Residential, Commercial, Industrial or Recreational Area for UAV; no flight within 50 m of any uninvolved person, except that during take-off and landing this distance may be reduced to 30 m; no flight within Flight Restriction Zones (FRZs) unless permitted by the relevant aerodrome; no flight over or within 150 m of open-air assemblies of more than 1000 persons; UAV mass of less than 25 kg (fixed wing or rotary wing to be defined); UAV equipped with a mechanism that makes it land in the event of loss or disruption of Command and Control (C2) Link; insurance cover to meet insurance regulatory requirements [35].) DG can be carried in specific category operations, unless they are assessed during the RA as presenting a high risk to third parties in the event of an accident. In the UK, applications for an operational authorisation and a DG approval must be submitted separately because they are processed by different teams within the CAA, but may be submitted at the same time.
- Certified category operations apply to situations presenting a high risk, specifically: flying over assemblies of people by UAVs with a characteristic dimension greater than 3 m; carrying passengers; or carrying DG assessed during the RA as presenting a high risk to third parties in the event of an accident. These operations present a risk equivalent to that of crewed aircraft operations, and are therefore subject to the same authorisations, namely certification of the aircraft, certification of the UAV operator, and licensing of the remote pilot. In the UK, regulations for certified category operations are still under development and not yet published, and therefore the principles from the relevant crewed aviation regulations will be used as the basis for regulation of this category in the meantime.
- Mitigation of risks unique to UAV operations.
- Adequate DG training to ensure personnel are competent, commensurate with their responsibilities.
- For incidents or accidents: an emergency response plan, procedures for communicating with appropriate authorities (when necessary) and with people who may be unfamiliar with labelling/marking of DG, and procedures for recording/reporting safety data.
- Risks associated with unintentional release (i.e., leak/spill) of DG (e.g., infectious substances, toxic/corrosive substances).
- Risks associated with transport over populated, remote or environmentally sensitive areas.
- Risks associated with securing DG payloads by direct attachment to UAVs or as underslung loads.
- Risks associated with DG payloads being released by dropping from UAVs.
- Potential for dangerous reactions resulting from unintentional mixing of incompatible DG.
- Packing in accordance with the provisions of the DGR to the extent possible. Where deviation occurs, an equivalent level of safety must be established accounting for: UAV cargo compartment conditions (e.g., airflow, precipitation ingress, temperature, pressure, vibration), lowest volume DG containers necessary for intended purpose, measures to prevent leaks/spills, full and easily accessible DG documentation, and the effects on packing if DG are to be released by dropping.
3.5. Packing, Marking, Labelling and Documentation
- Packages to be marked with “Biological Substance, Category B”, the name/address of shipper and consignee, and name/telephone number of person responsible for the package (details of the person responsible can be provided on accompanying documentation instead);
- Requirements for accidents/incidents to be reported to NAAs (Section 3.7);
- Requirements for packages to be inspected for damage/leaks/spills prior to loading and after unloading (Section 3.7).
3.6. Loading
3.7. Non-Normal Procedures
- Accident is an occurrence in which: (a) a person is fatally or seriously injured; (b) the aircraft sustains damage or structural failure which adversely affects the structural strength, performance or flight characteristics of the aircraft, and would normally require major repair or replacement of the affected component; or (c) the aircraft is missing or is completely inaccessible.
- Incident is an occurrence, other than an accident, which affects or could affect the safety of operation.
- Serious incident is an incident involving circumstances indicating that there was a high probability of an accident.
- In the event of accidents or incidents (e.g., vehicle accident/breakdown, package with damage/leak/spill), inform the health service organisation without delay so that expert advice can be provided (e.g., by the designated Safety Officer) before packages are handled, assistance can be provided with any clean-up/disinfection operations necessary, and the occurrence can be recorded in the health service’s own risk management reporting/recording systems.
- In the event of any circumstances occurring during transport that could affect the integrity of the goods being transported (e.g., excessive delays, extremes of temperature), inform the health service organisation so they can take the appropriate action.
- Availability and use of spill-kits at origin and destination, including items such as PPE (e.g., gloves, apron, mask, over-shoes), water for irrigation, absorbing material, scoop, waste disposal bags and hand sanitiser.
- Cooperating with health service organisations in conducting temperature audits of vehicles by carrying in-vehicle temperature recording devices. However, this could present a challenge if the recording devices were powered by lithium batteries, which are subject to the DGR (Table 1). In addition, other devices could be used to monitor in-vehicle conditions. For example, 3-axis accelerometers can be positioned on the craft and/or payload in a similar arrangement to trials in the UK monitoring in-flight dynamics such as vibration [43], which can negatively impact on the stability of medical cargos (refer to Section 3.8). These could be integrated into flight systems such that live updates can be given to UAV operators.
3.8. Other Regulatory Considerations
3.9. Summary Guidance for UAV Operators Intending to Transport DG
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, K.; Praharaj, S.; Nanda, T. Unmanned Aerial Vehicles (UAVs): An Emerging Technology for Logistics. Int. J. Bus. Manag. Invent. 2016, 5, 86–92. [Google Scholar]
- Scott, J.E.; Scott, C.H. Drone Delivery Models for Healthcare. In Proceedings of the 50th Hawaii International Conference on System Sciences (HICSS), Waikoloa Village, HI, USA, 4–7 January 2017; University of Hawaii at Manoa: Waikoloa, HI, USA, 2017. [Google Scholar]
- Goodchild, A.; Toy, J. Delivery by drone: An evaluation of unmanned aerial vehicle technology in reducing CO2 emissions in the delivery service industry. Transp. Res. Part D Transp. Environ. 2018, 61, 58–67. [Google Scholar] [CrossRef]
- Lin, C.A.; Shah, K.; Mauntel, L.C.C.; Shah, S.A. Drone delivery of medications: Review of the landscape and legal considerations. Am. J. Health Syst. Pharm. 2018, 75, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Aurambout, J.-P.; Gkoumas, K.; Ciuffo, B. Last mile delivery by drones: An estimation of viable market potential and access to citizens across European cities. Eur. Transp. Res. Rev. 2019, 11, 1–21. [Google Scholar] [CrossRef]
- Sah, B.; Gupta, R.; Bani-Hani, D. Analysis of barriers to implement drone logistics. Int. J. Logist. Res. Appl. 2020, 1–20. [Google Scholar] [CrossRef]
- Darvishpoor, S.; Roshanian, J.; Raissi, A.; Hassanalian, M. Configurations, flight mechanisms, and applications of unmanned aerial systems: A review. Prog. Aerosp. Sci. 2020, 121, 100694. [Google Scholar] [CrossRef]
- Wright, C.; Rupani, S.; Nichols, K.; Chandani, Y.; Machagge, M. What Should You Deliver by Unmanned Aerial Systems? White Paper; JSI Research & Training Institute: Boston, MA, USA, 2018. [Google Scholar]
- Eichleay, M.; Evens, E.; Stankevitz, K.; Parkera, C. Using the Unmanned Aerial Vehicle Delivery Decision Tool to Consider Transporting Medical Supplies via Drone. Glob. Health Sci. Pract. 2019, 7, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, E.; Koziol, M. The Blood is Here—IEEE Spectrum Magazine; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2019. [Google Scholar]
- Apian. Dreadnought; [Online]; Apian: London, UK, 2021; Available online: https://www.apian.aero/projects.html (accessed on 11 February 2021).
- Skyports. NHS Drone Delivery Trial; [Online]; Skyports: London, UK, 2021; Available online: https://skyports.net/nhs-trials/ (accessed on 2 March 2021).
- Whalley, S. Personal communication to Grote M, 16 March 2021, RE: Paper on UAVs and DG regulations [email].
- O’Keeffe, D.T.; Johnson, K.; Maraka, S. Autonomous Drone Delivery of Insulin. J. Endocr. Soc. 2020, 4 (Suppl. 1), A445–A446. [Google Scholar] [CrossRef]
- O’Keeffe, D.T.; Johnson, K.; Maraka, S. Provision of Bidirectional Remote Patient Care With an Unmanned Aerial Vehicle. Mayo Clin. Proc. 2020, 95, 830. [Google Scholar] [CrossRef] [PubMed]
- International Civil Aviation Organization (ICAO). Annex 18 to the Convention on International Civil Aviation—The Safe Transport of Dangerous Goods by Air, 4th ed.; International Civil Aviation Organization: Montreal, QC, Canada, 2011. [Google Scholar]
- International Civil Aviation Organization (ICAO). Technical Instructions for the Safe Transport of Dangerous Goods by Air (Doc 9284), 2019–2020 Edition; International Civil Aviation Organization: Montreal, QC, Canada, 2018. [Google Scholar]
- International Air Transport Association (IATA). Dangerous Goods Regulations, Edition 61; International Air Transport Association: Montreal, QC, Canada, 2019. [Google Scholar]
- International Civil Aviation Organization (ICAO). Unmanned Aircraft Systems (UAS) for Humanitarian Aid and Emergency Response Guidance—U-AID; International Civil Aviation Organization: Montreal, QC, Canada, 2019. [Google Scholar]
- Medicines and Healthcare products Regulatory Agency (MHRA). Rules and Guidance for Pharmaceutical Distributors 2017 (The Green Guide), 10th ed.; Pharmaceutical Press: London, UK, 2017. [Google Scholar]
- LabChem. Material Safety Data Sheet: Arsenic Trioxide; LabChem Inc.: Zelienople, PA, USA, 2007. [Google Scholar]
- European Union Aviation Safety Agency (EASA). Acceptable Means of Compliance (AMC) and Guidance Material (GM) to Commission Implementing Regulation (EU) 2019/947 (Annex I to ED Decision 2019/021/R); European Union Aviation Safety Agency: Cologne, Germany, 2019. [Google Scholar]
- United Nations Economic Commission for Europe (UNECE). ADR—European Agreement Concerning the International Carriage of Dangerous Goods by Road (ECE/TRANS/257); United Nations Economic Commission for Europe: New York, NY, USA, 2017. [Google Scholar]
- Intergovernmental Organisation for International Carriage by Rail (OTIF). Convention concerning International Carriage by Rail (COTIF)—Appendix C—Regulations concerning the International Carriage of Dangerous Goods by Rail (RID); Intergovernmental Organisation for International Carriage by Rail: Berne, Switzerland, 2020. [Google Scholar]
- International Maritime Organization (IMO). IMDG Code (2020 Edition Including Amendment 40-20); International Maritime Organization: London, UK, 2020. [Google Scholar]
- Mohr, N. Personal Communication to Grote M, 9 February 2021, RE: Dangerous Goods by Air [email].
- Duarte, F.; Ratti, C. The Impact of Autonomous Vehicles on Cities: A Review. J. Urban Technol. 2018, 25, 3–18. [Google Scholar] [CrossRef]
- Gavanas, N. Autonomous Road Vehicles: Challenges for Urban Planning in European Cities. Urban Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Engler, Á. Autonomous Technologies in the Transportation of Dangerous Goods. Hadmérnök 2018, 13, 89–95. [Google Scholar]
- Bhargava, K.; Choy, K.W.; Jennings, P.A.; Birrell, S.A.; Higgins, M.D. Traffic simulation of connected and autonomous freight vehicles (CAV-F) using a data-driven traffic model of a real-world road tunnel. Transp. Eng. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Sindi, S.; Woodman, R. Autonomous Goods Vehicles for Last-mile Delivery: Evaluation of Impact and Barriers. In Proceedings of the 23rd Intelligent Transportation Systems Conference (IEEE ITSC 2020), Rhodes, Greece, 20–23 September 2020. [Google Scholar]
- Pipeline and Hazardous Materials Safety Administration (PHMSA). Request for Information on Regulatory Challenges to Safely Transporting Hazardous Materials by Surface Modes in an Automated Vehicle Environment (Federal Register/Vol. 83, No. 61/Thursday, March 29, 2018); Office of the Federal Register: Washington, DC, USA, 2018. [Google Scholar]
- Devoid, W.; Middendorf, T.; Wass, C. Hazardous Material Transport with Unmanned Systems: Phase 1—Exploration (Draft Report CDTS-AL003-19-00200); A-P-T Research, Inc.: Huntsville, AL, USA, 2019. [Google Scholar]
- National Health Service (NHS). Invitation to Tender to Supply the Pathology Collection Network for The East and South East London NHS Pathology Partnership; National Health Service: London, UK, 2021. [Google Scholar]
- Civil Aviation Authority (CAA). Unmanned Aircraft System Operations in UK Airspace—Guidance (CAP 722); Civil Aviation Authority: Crawley, UK, 2020. [Google Scholar]
- European Union Aviation Safety Agency (EASA). Easy Access Rules for Unmanned Aircraft Systems (Regulations (EU) 2019/947 and (EU) 2019/945); European Union Aviation Safety Agency: Cologne, Germany, 2021. [Google Scholar]
- European Commission (EC). Commission Implementing Regulation (EU) 2019/947 of 24 May 2019 on the Rules and Procedures for the Operation of Unmanned Aircraft; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Holman, A.L. Warsaw Convention—Limited Liability—Air Waybill Requirements. J. Air Law Commer. 1970, 36, 771–780. [Google Scholar]
- Beck, S.; Bui, T.T.; Davies, A.; Courtney, P.; Brown, A.; Geudens, J.; Royall, P.G. An Evaluation of the Drone Delivery of Adrenaline Auto-Injectors for Anaphylaxis: Pharmacists’ Perceptions, Acceptance, and Concerns. Drones 2020, 4, 66. [Google Scholar] [CrossRef]
- International Civil Aviation Organization (ICAO). Emergency Response Guidance for Aircraft Incidents Involving Dangerous Goods (Doc 9481), 2019–2020 Edition; International Civil Aviation Organization: Montreal, QC, Canada, 2018. [Google Scholar]
- International Civil Aviation Organization (ICAO). Annex 13 to the Convention on International Civil Aviation-Aircraft Accident and Incident Investigation, 10th ed.; International Civil Aviation Organization: Montreal, QC, Canada, 2010. [Google Scholar]
- Department for Transport (DfT). Transport of Infectious Substances UN2814, UN2900 and UN3373 (Guidance Note Number: 17/2012 [Rev.7]); Department for Transport: London, UK, 2012. [Google Scholar]
- Oakey, A.; Waters, T.; Zhu, W.; Royall, P.G.; Cherrett, T.; Courtney, P.; Majoe, D.; Jelev, N. Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed-Wing and Multi-Copter Drones. Drones 2021, 5, 22. [Google Scholar] [CrossRef]
- International Civil Aviation Organization (ICAO). Unmanned Aircraft Systems Traffic Management (UTM)—A Common Framework with Core Principles for Global Harmonization; International Civil Aviation Organization: Montreal, QC, Canada, 2020. [Google Scholar]
- Civil Aviation Authority (CAA). A Unified Approach to the Introduction of UAS Traffic Management (CAP 1868); Civil Aviation Authority: Crawley, UK, 2019. [Google Scholar]
- Branch, S.K. Guidelines from the International Conference on Harmonisation (ICH). J. Pharm. Biomed. Anal. 2005, 38, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability Testing of Pharmaceutical Products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Torisu, T.; Maruno, T.; Yoneda, S.; Hamaji, Y.; Honda, S.; Ohkubo, T.; Uchiyama, S. Friability Testing as a New Stress-Stability Assay for Biopharmaceuticals. J. Pharm. Sci. 2017, 106, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Amukele, T.K.; Hernandez, J.; Snozek, C.L.H.; Wyatt, R.G.; Douglas, M.; Amini, R.; Street, J. Drone Transport of Chemistry and Hematology Samples Over Long Distances. Am. J. Clin. Pathol. 2017, 148, 427–435. [Google Scholar] [CrossRef] [PubMed]
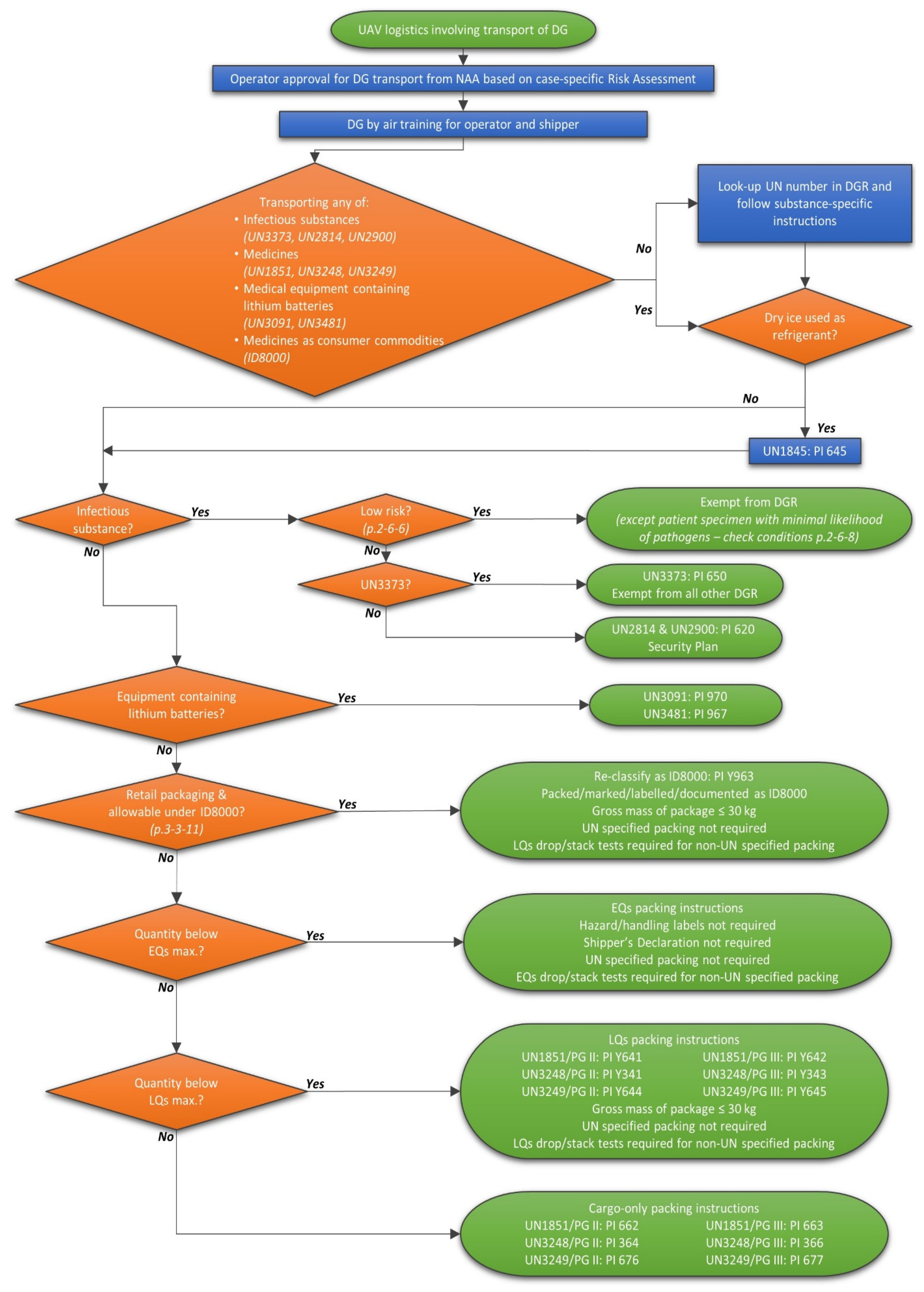
| UN No. PSN Class (Sub. Class) | PG | EQs Packing Instructions Max. Quantities | LQs Packing Instructions Max. Quantities | Pax/Cargo Aircraft Packing Instructions Max. Quantities | Cargo-Only Aircraft Packing Instructions Max. Quantities | Example Medical Goods |
|---|---|---|---|---|---|---|
| UN3373 Biological substance, Category B 6.2 | na | Not permitted as EQs | Not permitted as LQs | PI 650: −I ≤ 1 L/4 kg −O ≤ 4 L/4 kg | As for Pax/Cargo | Diagnostic specimens (lower risk) |
| UN2814 Infectious substance, affecting humans Category A 6.2 | na | Not permitted as EQs | Not permitted as LQs | PI 620: −I ≤ 50 mL/50 g −O ≤ 50 mL/50 g | PI 620: −I ≤ 4 L/4 kg −O ≤ 4 L/4 kg | Diagnostic specimens (high risk to humans) |
| UN2900 Infectious substance, affecting animals only 6.2 | na | Not permitted as EQs | Not permitted as LQs | PI 620: −I ≤ 50 mL/50 g −O ≤ 50 mL/50 g | PI 620: −I ≤ 4 L/4 kg −O ≤ 4 L/4 kg | Diagnostic specimens (high risk to animals) |
| UN1851 Medicine, liquid, toxic, n.o.s. 6.1 | II | E4: −I ≤ 1 mL −O ≤ 500 mL | PI Y641: −I ≤ 0.1 L −O ≤ 1 L | PI 654: −I ≤ 1 L (m: 2.5 L) −O ≤ 5 L | PI 662: −I ≤ 2.5 L (m: 5 L) −O ≤ 60 L | Liquid cytotoxic (Chemotherapy) drugs |
| UN1851 Medicine, liquid, toxic, n.o.s. 6.1 | III | E1: −I ≤ 30 mL −O ≤ 1 L | PI Y642: −I ≤ 0.5 L −O ≤ 2 L | PI 655: −I ≤ 2.5 L (m: 5 L) −O ≤ 60 L | PI 663: −I ≤ 5 L (m: 10 L) −O ≤ 220 L | Liquid cytotoxic (Chemotherapy) drugs |
| UN3248 Medicine, liquid, flammable, toxic, n.o.s. 3 (6.1) | II | E2 −I ≤ 30 mL −O ≤ 500 mL | PI Y341: −I ≤ 0.5 L −O ≤ 1 L | PI 352: −I ≤ 1 L −O ≤ 1 L | PI 364: −I ≤ 2.5 L (p: 5 L, m: 10 L) −O ≤ 60 L | Topical sprays |
| UN3248 Medicine, liquid, flammable, toxic, n.o.s. 3 (6.1) | III | E1 −I ≤ 30 mL −O ≤ 1 L | PI Y343: −I ≤ 1 L −O ≤ 2 L | PI 355: −I ≤ 2.5 L (p/m: 10 L) −O ≤ 60 L | PI 366: −I ≤ 5 L (p: 10 L, m: 25 L) −O ≤ 220 L | Topical sprays |
| UN3249 Medicine, solid, toxic, n.o.s. 6.1 | II | E4 −I ≤ 1 g −O ≤ 500 g | PI Y644: −I ≤ 0.5 kg −O ≤ 1 kg | PI 669: −I ≤ 1 kg (p/m: 2.5 kg) −O ≤ 25 kg | PI 676: −I ≤ 2.5 kg (p/m: 5 kg) −O ≤ 100 kg | Powders for solution for injection |
| UN3249 Medicine, solid, toxic, n.o.s. 6.1 | III | E1 −I ≤ 30 g −O ≤ 1 kg | PI Y645: −I ≤ 1 kg −O ≤ 10 kg | PI 670: −I ≤ 5 kg (p/m: 10 kg) −O ≤ 100 kg | PI 677: −I ≤ 5 kg (p/m: 10 kg) −O ≤ 200 kg | Powders for solution for injection |
| UN3091 Lithium metal batteries contained in equipment 9 | na | Not permitted as EQs | Not permitted as LQs | PI 970: −I ≤ 5 kg −O ≤ 5 kg | PI 970: −I ≤ 35 kg −O ≤ 35 kg | Defibrillator |
| UN3481 Lithium ion batteries contained in equipment 9 | na | Not permitted as EQs | Not permitted as LQs | PI 967: −I ≤ 5 kg −O ≤ 5 kg | PI 967: −I ≤ 35 kg −O ≤ 35 kg | Defibrillator |
| ID8000 Consumer commodity 9 | na | na | PI Y963: −I ≤ 0.5 L/0.5 kg −O ≤ 30 kg (G) | As for LQs | As for LQs | Drugs packaged for retail sale to patients |
| UN1845 Dry Ice 9 | na | Not permitted as EQs | Not permitted as LQs | PI 954: −I ≤ 200 kg −O ≤ 200 kg | As for Pax/Cargo | Refrigerant for diagnostic specimens |
| Aspect of DG Required in Training Course Syllabus | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General philosophy | x | x | x | x | x | x | x | x | x | x | x | x |
| Limitations | x | x | x | x | x | x | x | x | x | x | x | |
| General requirements for shippers | x | x | x | |||||||||
| Classification | x | x | x | x | x | |||||||
| List of DG | x | x | x | x | x | |||||||
| Packing requirements | x | x | x | x | ||||||||
| Labelling and marking | x | x | x | x | x | x | x | x | x | x | x | x |
| DG transport document and other relevant documentation | x | x | x | x | x | |||||||
| Acceptance procedures | x | |||||||||||
| Recognition of undeclared DG | x | x | x | x | x | x | x | x | x | x | x | x |
| Storage and loading procedures | x | x | x | x | ||||||||
| Pilots’ notification | x | x | x | |||||||||
| Provisions for passengers and crew | x | x | x | x | x | x | x | x | x | x | x | x |
| Emergency procedures | x | x | x | x | x | x | x | x | x | x | x | x |
| Issue Encountered |
|---|
| An important part of the DG approvals process is the assessment by the CAA of the Quality Assurance (QA) procedures an operator has in-place to ensure on-going compliance with the DGR (or permitted deviations therefrom) once CAA approval has been granted. |
| Operators can expect to be audited for compliance with the DGR by the CAA before approval is granted (i.e., prior to commencement of operations), which can delay the approvals process somewhat, and then further auditing on a regular basis thereafter. There is currently no specific approach for the audit of UAVs in this regard and the CAA will need to develop a process which improves on the current system used for conventional crewed aviation which has been described as overly cumbersome and not fit-for-purpose. |
| The CAA’s Dangerous Goods Office (DGO) processes DG approvals, and is separate from the department within the CAA responsible for processing operational authorisations. This can lead to uncertainty because it is not clear whether the DGO has sight of all documents submitted by UAV operators (including the entirety of operations manuals and RAs), or just the parts relating specifically to DG approvals. |
| Historically, DG approvals have been granted for aircraft that are type certified (i.e., airworthiness certification as would normally be the case for crewed aircraft) and there is likely to be some residual cautiousness over granting DG approvals for UAVs because there is no regime for UAV airworthiness certification. The separation within the CAA between the teams processing operational authorisations (including the UAVs involved) and DG approvals may mean that the DGO stray into questions concerning UAV reliability and safety, which should not be a concern if the UAV has been assessed as airworthy during the operational authorisation. This could delay the DG approval process until the DGO become accustomed to granting approvals when aircraft are not type certified. |
| In addition to meeting Category 8 requirements for DG training (Table 2), any health service staff involved in loading/unloading UAVs will also need to be trained in all the UAV operator’s procedures for carriage of DG, which adds to training burdens and limits operational freedoms and opportunities for cost saving. |
| LUC holders with an operational authorisation based on a STS/PDRA that provides DG approval could (potentially) self-authorise operations involving DG carriage. However, it is possible that there may be mismatches between the requirement to comply with the DGR (or deviations therefrom) for every operation and the operational freedoms/limitations specified in the STS/PDRA, leading to uncertainty for operators over appropriate compliance. |
| The way in which regulations for UAV operations in the certified category are likely to develop is that the DGR will apply without UAV-specific deviations either: (1) until ICAO adapts their safety management Standard and Recommended Practices (SARPs, provided to assist nations in managing aviation safety risks) and/or provides specific guidance for DG carriage by UAVs in the certified category; or (2) operators of UAVs in the certified category can produce an acceptable Alternative Method of Compliance (AMoC). |
| Packing Instruction Category | Test Type | Procedure |
|---|---|---|
| EQs | Drop | Five sample packages dropped from a height of 1.8 m, one sample dropped in each of the following orientations:
|
| EQs | Stack | Force applied to top of sample package for 24 h equivalent to being the bottom package in a 3 m stack. |
| LQs | Drop | One sample package dropped from a height of 1.2 m in the orientation likely to cause most damage (usually onto a corner). |
| LQs | Stack | Force applied to top of sample package for 24 h equivalent to being the bottom package in a 3 m stack. |
| Issue to Consider |
|---|
Substances Classified as DG for Transport by Air (Section 3.1)
|
Application of Air Transport DG Regulations (Section 3.2)
|
Training of Personnel (Section 3.3)
|
Operator Approval (Section 3.4)
|
Packing, Marking, Labelling and Documentation (Section 3.5)
|
Loading (Section 3.6)
|
Non-Normal Procedures (Section 3.7)
|
Other Regulatory Considerations (Section 3.8)
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grote, M.; Cherrett, T.; Oakey, A.; Royall, P.G.; Whalley, S.; Dickinson, J. How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos? Drones 2021, 5, 38. https://doi.org/10.3390/drones5020038
Grote M, Cherrett T, Oakey A, Royall PG, Whalley S, Dickinson J. How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos? Drones. 2021; 5(2):38. https://doi.org/10.3390/drones5020038
Chicago/Turabian StyleGrote, Matt, Tom Cherrett, Andrew Oakey, Paul G. Royall, Simon Whalley, and Janet Dickinson. 2021. "How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos?" Drones 5, no. 2: 38. https://doi.org/10.3390/drones5020038
APA StyleGrote, M., Cherrett, T., Oakey, A., Royall, P. G., Whalley, S., & Dickinson, J. (2021). How Do Dangerous Goods Regulations Apply to Uncrewed Aerial Vehicles Transporting Medical Cargos? Drones, 5(2), 38. https://doi.org/10.3390/drones5020038









