A Drone Study of Sociality in the Finless Porpoise Neophocaena asiaeorientalis in the Ariake Sound, Japan
Abstract
:1. Introduction
2. Materials and Methods
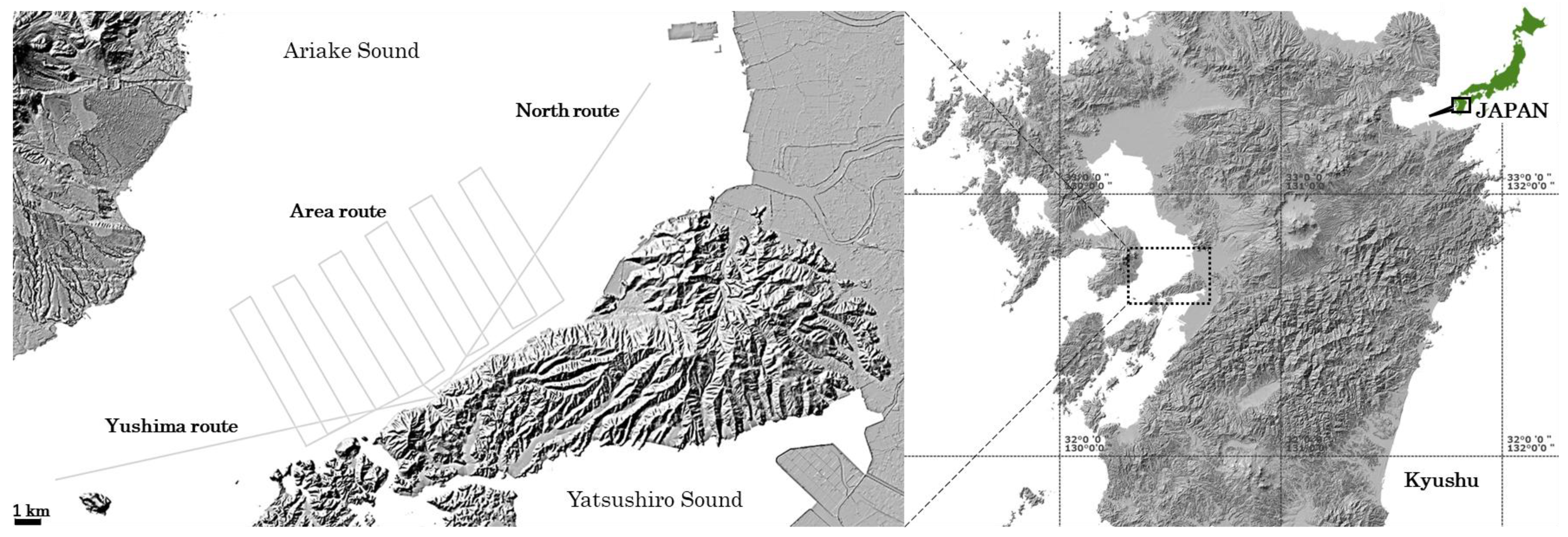
2.1. Study Area
2.2. Drone Use and Flight Routes
2.3. Data Analysis
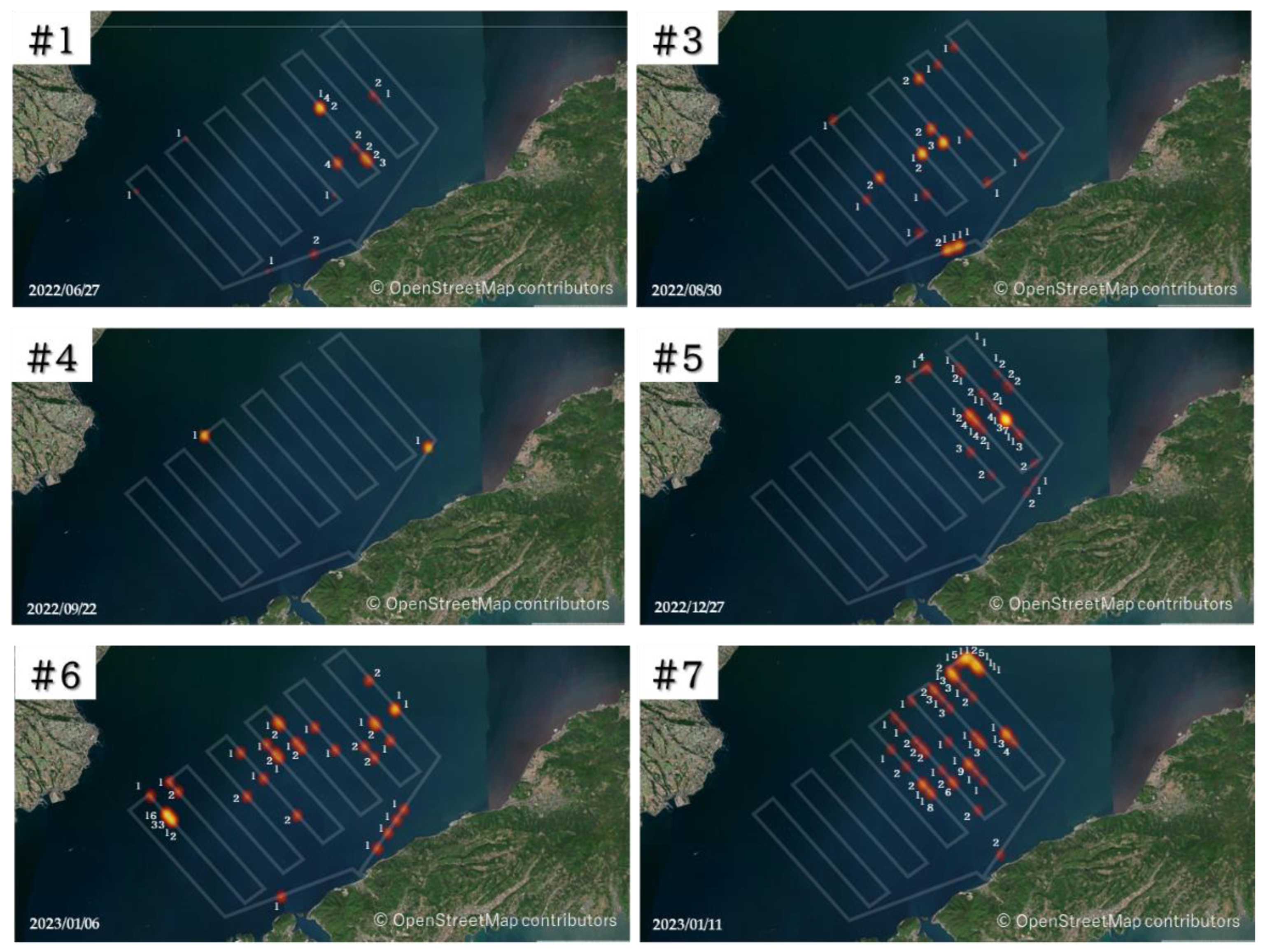
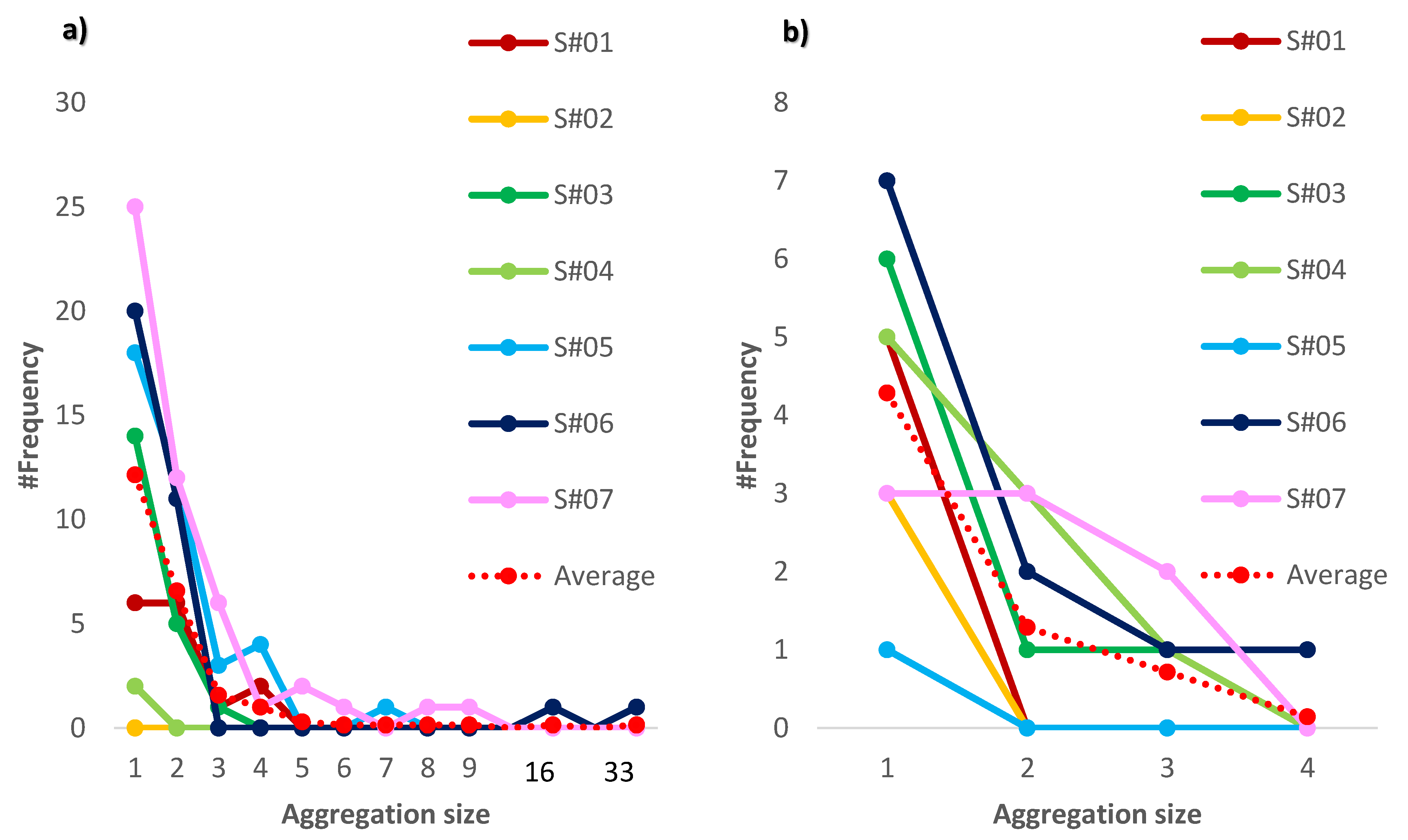
2.3.1. The Size of the Animal Aggregations
2.3.2. Distances across Solitaries and Aggregations
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roshier, D.A.; Doerr, V.A.; Doerr, E.D. Animal movement in dynamic landscapes: Interaction between behavioural strategies and resource distributions. Oecologia 2008, 156, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Schipper, J.; Chanson, J.S.; Chiozza, F.; Cox, N.A.; Hoffmann, M.; Katariya, V.; Lamoreux, J.; Rodrigues, A.S.L.; Stuart, S.N.; Temple, H.J.; et al. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 2008, 322, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Gutiérrez, A. Habitat use. In Encyclopedia of Marine Mammals; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 524–529. [Google Scholar]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin: Social relationships in a fission–fusion society. In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Conner, R.C., Tyack, P.L., Whitehead, H., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. [Google Scholar]
- Wittemyer, G.; Douglas-Hamilton, I.; Getz, W.M. The socioecology of elephants: Analysis of the processes creating multitiered social structures. Anim. Behav. 2005, 69, 1357–1371. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.B. Predicting fate from early connectivity in a social network. Proc. Natl. Acad. Sci. USA 2007, 104, 10910–10914. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.M.; Hazen, E.L.; Bograd, S.J.; Halpern, B.S.; Breed, G.A.; Nickel, B.; Teutschel, N.M.; Crowder, L.B.; Benson, S.; Dutton, P.H.; et al. Cumulative human impacts on marine predators. Nat. Commun. 2013, 4, 2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisson-Curadeau, E.; Bird, D.; Burke, C.; Fifield, D.A.; Pace, P.; Sherley, R.B.; Elliott, K.H. Seabird species vary in behavioural response to UAV census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, A.; Avens, L.; Ballorain, K.; Bevan, E.; Broderick, A.; Carthy, R.; Christianen, M.; Duclos, G.; Heithaus; Johnston, D.; et al. The potential of unmanned aerial systems for sea turtle research and conservation: A review and future directions. Endanger. Species Res. 2018, 35, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Sánchez, C.A.; Acevedo-Whitehouse, K.A.; Gendron, D. Effect of drone-based blow sampling on blue whale (Balaenoptera musculus) behavior. Mar. Mammal Sci. 2018, 34, 841–850. [Google Scholar] [CrossRef]
- Subhan, B.; Arafat, D.; Santoso, P.; Pahlevi, K.; Prabowo, B.; Taufik, M.; Kusumo, B.S.; Awak, K.; Khaerudi, D.; Ohoiulun, H.; et al. Development of observing dolphin population method using Small Vertical Take-off and Landing (VTOL) Unmanned Aerial System (AUV). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 278, p. 012074. [Google Scholar]
- Fiori, L.; Doshi, A.; Martinez, E.; Orams, M.B.; Bollard-Breen, B. The use of unmanned aerial systems in marine mammal research. Remote Sens. 2017, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Fettermann, T.; Fiori, L.; Gillman, L.; Stockin, K.A.; Bollard, B. Drone surveys are more accurate than boat-based surveys of bottlenose dolphins (Tursiops truncatus). Drones 2022, 6, 82. [Google Scholar] [CrossRef]
- Fiori, L.; Martinez, E.; Bader, M.K.F.; Orams, M.B.; Bollard, B. Insights into the use of an unmanned aerial vehicle (UAV) to investigate the behavior of humpback whales (Megaptera novaeangliae) in Vava’u, Kingdom of Tonga. Mar. Mammal Sci. 2020, 36, 209–223. [Google Scholar] [CrossRef]
- Wang, J.Y.; Reeves, R. Neophocaena asiaeorientalis. IUCN Red List Threat. Species 2017, e.T41754A50381766. [Google Scholar] [CrossRef]
- Kasuya, T. Finless porpoise Neophocaena phocaenoides (G. Cuvier, 1829). In Handbook of Marine Mammals.; Ridgway, S.H., Harrison, R., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 411–442. [Google Scholar]
- Morimura, N.; Mori, Y. Social responses of travelling finless porpoises to boat traffic risk in Misumi West Port, Ariake Sound, Japan. PLoS ONE 2019, 14, e0208754. [Google Scholar] [CrossRef]
- Johnson, D.D.; Kays, R.; Blackwell, P.G.; Macdonald, D.W. Does the resource dispersion hypothesis explain group living? Trends Ecol. Evol. 2002, 17, 563–570. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J.; Wrangham, R.W. Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behav. Ecol. Sociobiol. 1995, 36, 59–70. [Google Scholar] [CrossRef]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Front. Mar. Sci. 2016, 3, 277. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 September 2020).
- Lehmann, J.; Korstjens, A.H.; Dunbar, R.I. Group size, grooming and social cohesion in primates. Anim. Behav. 2007, 74, 1617–1629. [Google Scholar] [CrossRef] [Green Version]
- Ansmann, I.C.; Parra, G.J.; Chilvers, B.L.; Lanyon, J.M. Dolphins restructure social system after reduction of commercial fisheries. Anim. Behav. 2012, 84, 575–581. [Google Scholar] [CrossRef]
- Elliser, C.R.; MacIver, K.H.; Green, M. Group characteristics, site fidelity, and photo-identification of harbor porpoises, Phocoena phocoena, in Burrows Pass, Fidalgo Island, Washington. Mar. Mammal Sci. 2018, 34, 365–384. [Google Scholar] [CrossRef]
- Terada, T.; Morisaka, T.; Wakabayashi, I.; Yoshioka, M. Communication sounds produced by captive narrow-ridged finless porpoises (Neophocaena asiaeorientalis). J. Ethol. 2022, 40, 245–256. [Google Scholar] [CrossRef]
- Connor, R.C. Dolphin social intelligence: Complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 587–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, L. Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav. Evol. 2002, 59, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Connor, R.C.; Tyack, P.L.; Whitehead, H. Cetacean Societies: Field Studies of Dolphins and Whales; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Fox, K.C.; Muthukrishna, M.; Shultz, S. The social and cultural roots of whale and dolphin brains. Nat. Ecol. Evol. 2017, 1, 1699. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.P.; Wich, S.A. Dawn of drone ecology: Low-cost autonomous aerial vehicles for conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Watts, A.C.; Ambrosia, V.G.; Hinkley, E.A. Unmanned aircraft systems in remote sensing and scientific research: Classification and considerations of use. Remote Sens. 2012, 4, 1671–1692. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned aerial vehicles (UAVs) for surveying marine fauna: A dugong case study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef] [Green Version]
- Pomeroy, P.; O’connor, L.; Davies, P. Assessing use of and reaction to unmanned aerial systems in gray and harbor seals during breeding and molt in the UK. J. Unmanned Veh. Syst. 2015, 3, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Isabelle, D.A.; Westerlund, M. A review and categorization of artificial intelligence-based opportunities in wildlife, ocean and land conservation. Sustainability 2022, 14, 1979. [Google Scholar] [CrossRef]
- Herbert-Read, J.E.; Thornton, A.; Amon, D.J.; Birchenough, S.N.; Côté, I.M.; Dias, M.P.; Godley, B.J.; Keith, S.A.; McKinley, E.; Peck, L.S.; et al. A global horizon scan of issues impacting marine and coastal biodiversity conservation. Nat. Ecol. Evol. 2022, 6, 1262–1270. [Google Scholar] [CrossRef]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 592, 397–402. [Google Scholar] [CrossRef]
- Gill, P.C.; Pirzl, R.; Morrice, M.G.; Lawton, K. Cetacean diversity of the continental shelf and slope off southern Australia. J. Wildl. Manag. 2015, 79, 672–681. [Google Scholar] [CrossRef]
- Dunham, D. The Social Instinct: How Cooperation Shaped the World; Random House: New York, NY, USA, 2021. [Google Scholar]
- Allen, J.A. Community through culture: From insects to whales: How social learning and culture manifest across diverse animal communities. BioEssays 2019, 41, 1900060. [Google Scholar] [CrossRef] [PubMed]




| A | B | Frequency of Aggregation Sizes (#Individuals) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey Type -Session# | Start Time-Date | Route Length (km) | #Individuals | Detection Effort (A/B; km) | Mean Aggregation Size | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #16 | #33 |
| Area #01 | 10:56, 27 June 2022 | 95.0 | 29 | 3.3 | 1.9 | 6 | 6 | 1 | 2 | |||||||
| Area #02 | 10:56, 4 August 2022 | 95.0 | 0 | n/a | n/a | |||||||||||
| Area #03 | 10:40, 30 August 2022 | 95.0 | 27 | 3.5 | 1.4 | 14 | 5 | 1 | ||||||||
| Area #04 | 10:57, 22 September 2022 | 95.0 | 2 | 47.5 | 1.0 | 2 | ||||||||||
| Area #05 | 10:17, 27 December 2022 | 95.0 | 74 | 1.3 | 1.9 | 18 | 12 | 3 | 4 | 1 | ||||||
| Area #06 | 10:23, 6 January 2023 | 95.0 | 91 | 1.0 | 2.8 | 20 | 11 | 1 | 1 | |||||||
| Area #07 | 10:20, 11 January 2023 | 95.0 | 104 | 0.9 | 2.1 | 25 | 12 | 6 | 1 | 2 | 1 | 1 | 1 | |||
| Line #01 | 9:39 and 12:56, 27 June 2022 | 56.7 | 5 | 11.3 | 1.0 | 5 | ||||||||||
| Line #02 | 9:41 and 12:56, 4 August 2022 | 56.7 | 3 | 18.9 | 1.0 | 3 | ||||||||||
| Line #03 | 9:43 and 12:36, 30 August 2022 | 56.7 | 11 | 5.2 | 1.4 | 6 | 1 | 1 | ||||||||
| Line #04 | 9:50 and 12:54, 22 September 2022 | 56.7 | 14 | 4.1 | 1.6 | 5 | 3 | 1 | ||||||||
| Line #05 | 9:35 and 12:21, 27 December 2022 | 44.4 | 1 | 44.4 | 1.0 | 1 | ||||||||||
| Line #06 | 9:38 and 12:15, 6 January 2023 | 44.4 | 18 | 2.5 | 1.6 | 7 | 2 | 1 | 1 | |||||||
| Line #07 | 9:34 and 12:28, 11 January 2023 | 44.4 | 15 | 3.0 | 1.9 | 3 | 3 | 2 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimura, N.; Itahara, A.; Brooks, J.; Mori, Y.; Piao, Y.; Hashimoto, H.; Mizumoto, I. A Drone Study of Sociality in the Finless Porpoise Neophocaena asiaeorientalis in the Ariake Sound, Japan. Drones 2023, 7, 422. https://doi.org/10.3390/drones7070422
Morimura N, Itahara A, Brooks J, Mori Y, Piao Y, Hashimoto H, Mizumoto I. A Drone Study of Sociality in the Finless Porpoise Neophocaena asiaeorientalis in the Ariake Sound, Japan. Drones. 2023; 7(7):422. https://doi.org/10.3390/drones7070422
Chicago/Turabian StyleMorimura, Naruki, Akihiro Itahara, James Brooks, Yusuke Mori, Yige Piao, Hiroki Hashimoto, and Itsuki Mizumoto. 2023. "A Drone Study of Sociality in the Finless Porpoise Neophocaena asiaeorientalis in the Ariake Sound, Japan" Drones 7, no. 7: 422. https://doi.org/10.3390/drones7070422
APA StyleMorimura, N., Itahara, A., Brooks, J., Mori, Y., Piao, Y., Hashimoto, H., & Mizumoto, I. (2023). A Drone Study of Sociality in the Finless Porpoise Neophocaena asiaeorientalis in the Ariake Sound, Japan. Drones, 7(7), 422. https://doi.org/10.3390/drones7070422






