Granular Bait Applications for Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System
Abstract
:1. Introduction
2. Materials and Methods
2.1. RPAAS
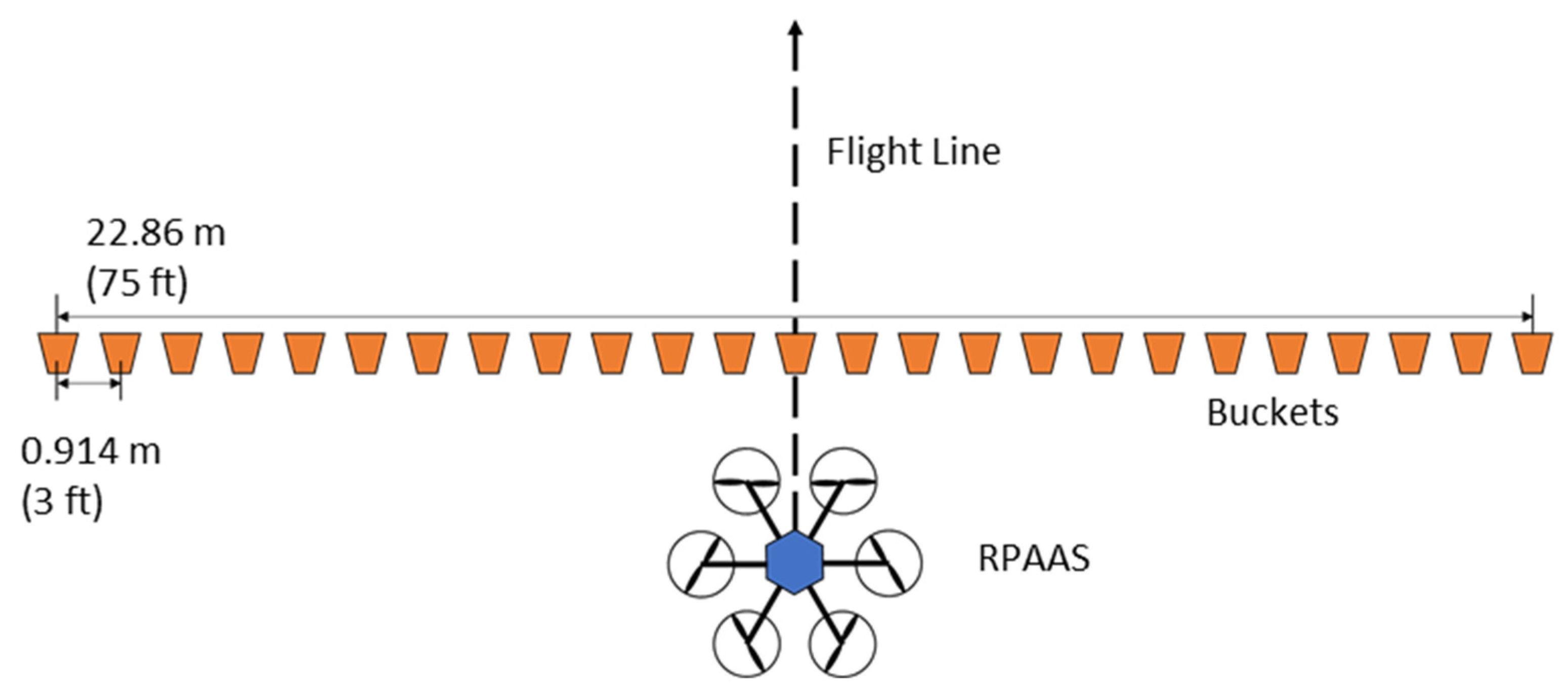
2.2. Bait Swath Measurement
2.3. Field Trials
3. Results
3.1. Bait Swath Measurement
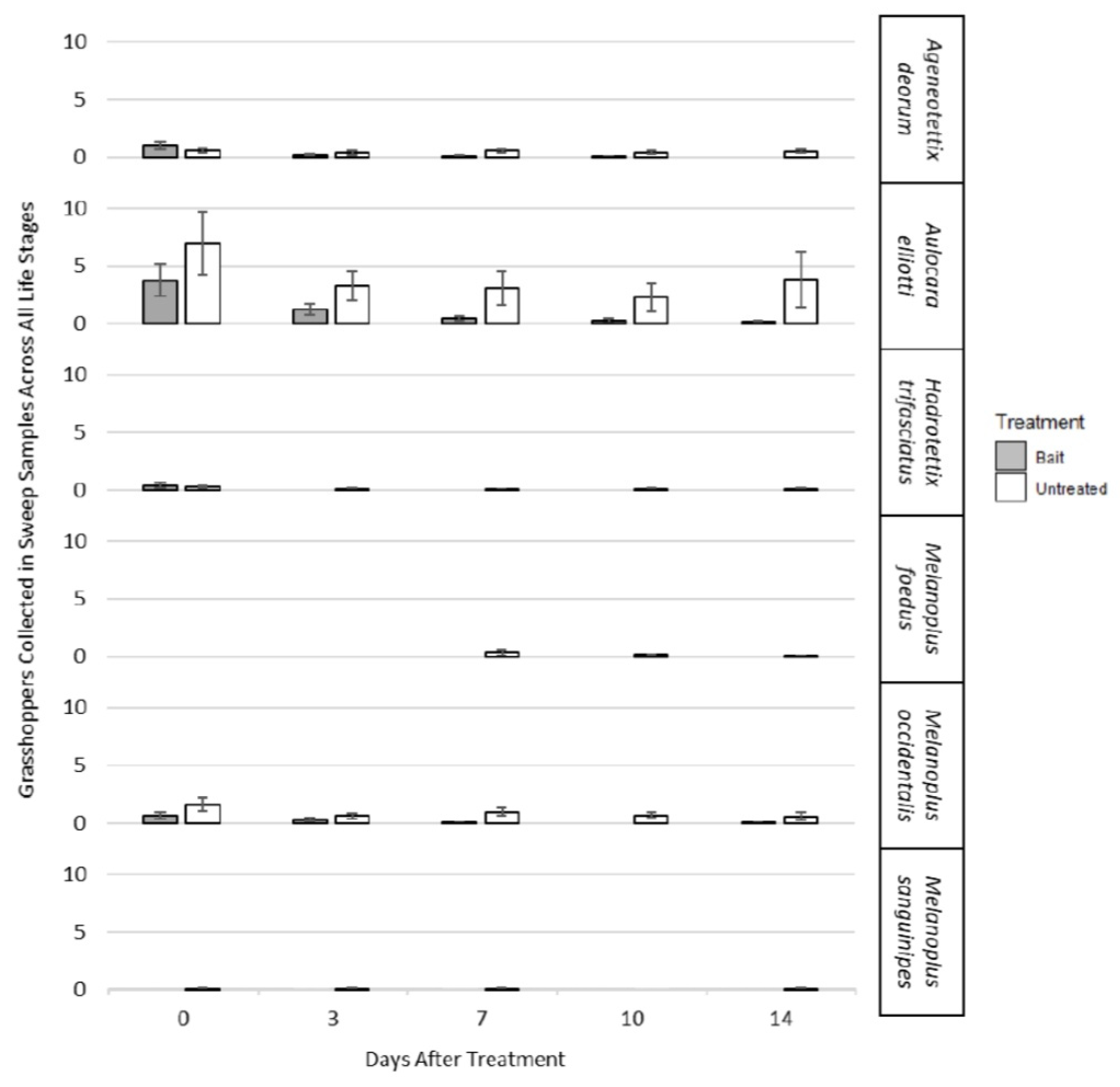
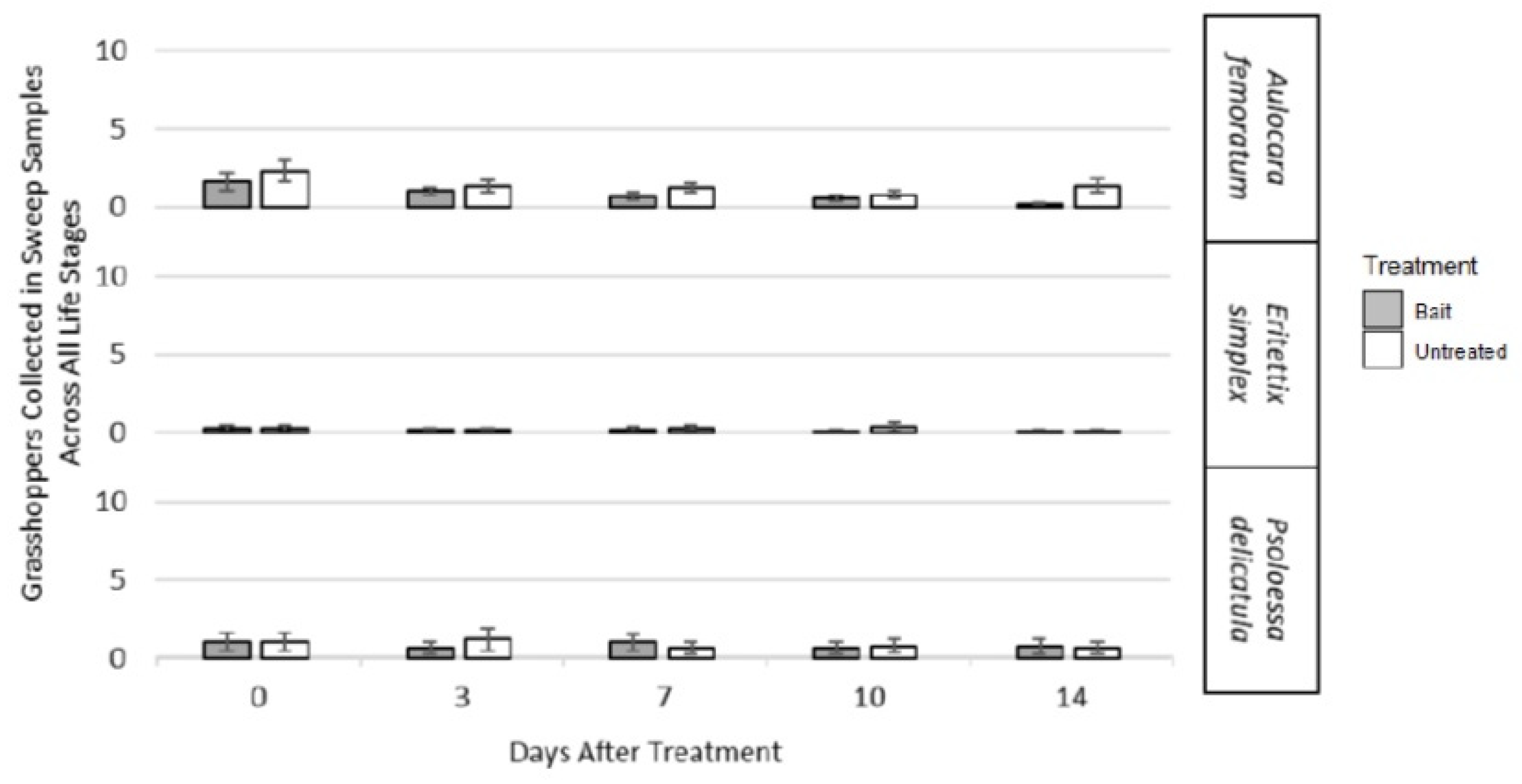
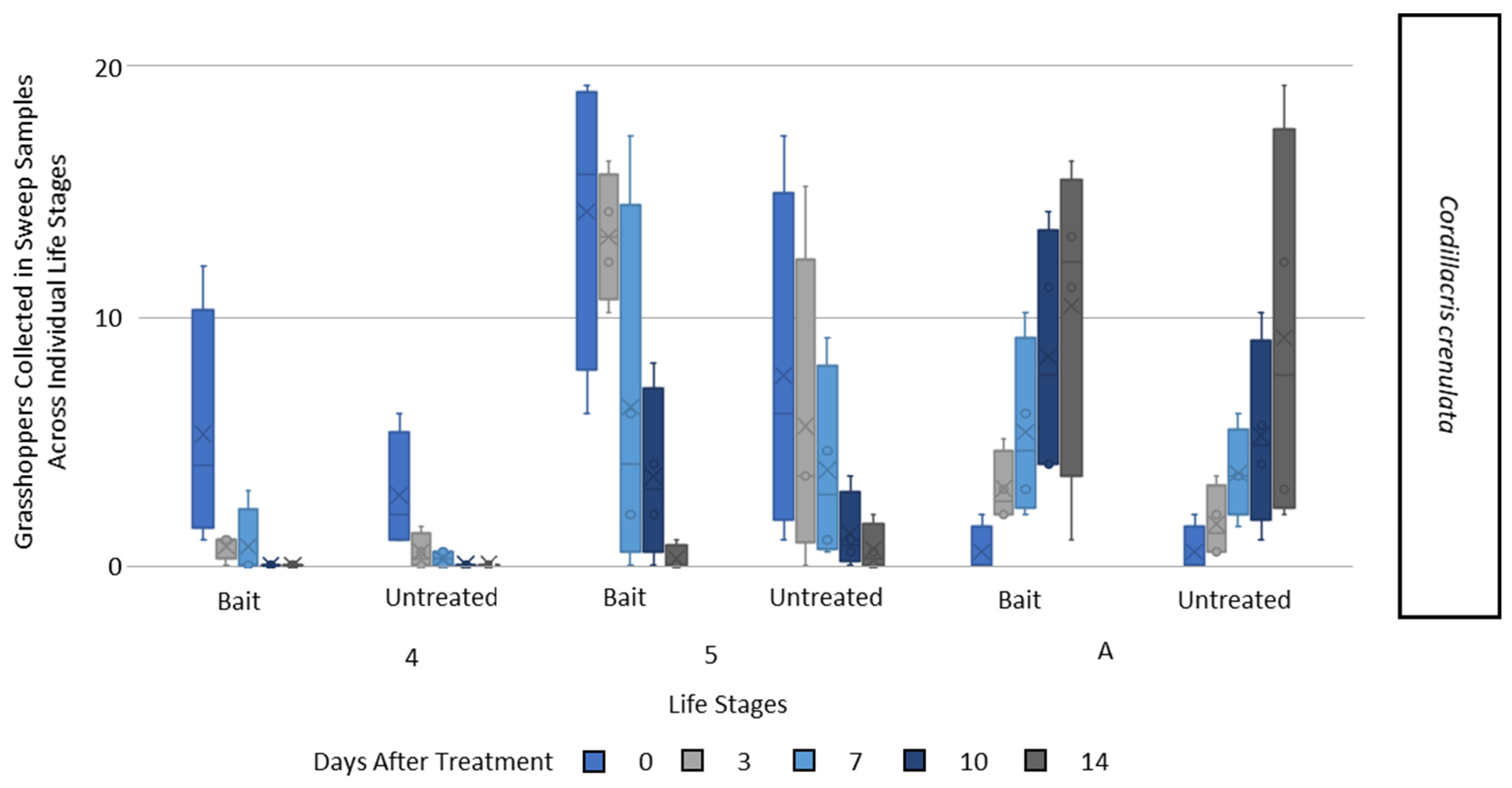
3.2. Bioassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfadt, R. Field Guide to Common Western Grasshoppers, 3rd ed.; Bulletin No. 912; University of Wyoming, Wyoming Agricultural Experiment Station: Laramie, WY, USA, 2002; pp. 1–288. [Google Scholar]
- Dysart, R.J. VI.6 Relative Importance of Rangeland Grasshoppers in Western North America: A Numerical Ranking from the Literature. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 1996–2000; pp. 1–20. [Google Scholar]
- Hewitt, G.; Onsager, J. Control of Grasshoppers on Rangeland in the United States—A Perspective. Rangel. Ecol. Manag. J. Range Manag. Arch. 1983, 36, 202–207. [Google Scholar] [CrossRef]
- Belovsky, G.E.; Lockwood, J.A.; Winks, K. IV.8 Recognizing and Managing Potential Outbreak Conditions. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 1996; pp. 1–4. [Google Scholar]
- Branson, D.H.; Joern, A.; Sword, G.A. Sustainable Management of Insect Herbivores in Grassland Ecosystems: New Perspectives in Grasshopper Control. BioScience 2006, 56, 743–755. [Google Scholar] [CrossRef]
- Onsager, J.A. Suppression of Grasshoppers in the Great Plains through Grazing Management. Rangel. Ecol. Manag. J. Range Manag. Arch. 2000, 53, 592–602. [Google Scholar]
- Xiongkui, H.; Bonds, J.; Herbst, A.; Langenakens, J. Recent Development of Unmanned Aerial Vehicle for Plant Protection in East Asia. Int. J. Agric. Biol. Eng. 2017, 10, 18–30. [Google Scholar] [CrossRef]
- Shi, Y.; Thomasson, J.A.; Murray, S.C.; Pugh, N.A.; Rooney, W.L.; Shafian, S.; Rajan, N.; Rouze, G.; Morgan, C.L.; Neely, H.L.; et al. Unmanned Aerial Vehicles for High-Throughput Phenotyping and Agronomic Research. PLoS ONE 2016, 11, e0159781. [Google Scholar] [CrossRef]
- Giles, D.; Billing, R.; Singh, W. Performance Results, Economic Viability and Outlook for Remotely Piloted Aircraft for Agricultural Spraying. Asp. Appl. Biol. 2016, 132, 15–21. [Google Scholar]
- Yan, X.; Zhou, Y.; Liu, X.; Yang, D.; Yuan, H. Minimizing Occupational Exposure to Pesticide and Increasing Control Efficacy of Pests by Unmanned Aerial Vehicle Application on Cowpea. Appl. Sci. 2021, 11, 9579. [Google Scholar] [CrossRef]
- Martin, D.E.; Rodriguez, R.; Woller, D.A.; Reuter, K.C.; Black, L.R.; Latheef, M.A.; Taylor, M.; López Colón, K.M. Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones 2022, 6, 239. [Google Scholar] [CrossRef]
- Onsager, J.A.; Foster, R.N.; Jech, L. II.12 Bait Acceptance by Different Grasshopper Species and Instars. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 1996; pp. 1–5. [Google Scholar]
- Foster, R.N.; Onsager, J.A. II.3 Sprays versus Baits. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 1996; pp. 1–3. [Google Scholar]
- Xunwei, W.; Zhiyan, Z.; Boqian, C.; Jinfeng, Z.; Xiaolong, F.; Hewitt, A. Distribution Uniformity Improvement Methods of a Large Discharge Rate Disc Spreader for UAV Fertilizer Application. Comput. Electron. Agric. 2024, 220, 108928. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Chen, L.; Chang, K.; Qian, S.; Chen, W.; Lan, Y. Design and Test of Aerial Broadcast Device for Agricultural Granular Materials. Int. J. Precis. Agric. Aviat. 2020, 3, 44–50. [Google Scholar] [CrossRef]
- Yan, X.; Yuan, H.; Chen, Y.; Shi, X.; Liu, X.; Wang, Z.; Liu, Y.; Yang, D. Broadcasting of Tiny Granules by Drone to Mimic Liquid Spraying for the Control of Fall Armyworm (Spodoptera frugiperda). Pest Manag. Sci. 2022, 78, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, Z.; Wang, X.; Odhiambo, M.O.; Sun, G. Fertilization Strategy and Application Model Using a Centrifugal Variable-Rate Fertilizer Spreader. Int. J. Agric. Biol. Eng. 2018, 11, 41–48. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Zhao, C.; Meng, Z.; Chen, L. Design and Experiment of Variable Rate Fertilizer Spreader with Conveyor Chain. Trans. Chin. Soc. Agric. Eng. 2012, 28, 20–25. [Google Scholar]
- Zhang, L.; Yuan, W.; Jin, C.; Feng, Y.; Liu, G.; Hu, Y. Research Progress on Key Mechanical Components of the Pneumatic Centralized Fertilizer Discharge System. Appl. Sci. 2024, 14, 3884. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, R.; Zhou, Z.; Song, C.; Luo, X.; Bao, R.; Lyu, Z.; Huang, J.; Lin, J. Discharge Rate Consistency of Each Channel for UAV-Based Pneumatic Granular Fertilizer Spreader. Int. J. Agric. Biol. Eng. 2023, 16, 20–28. [Google Scholar] [CrossRef]
- Morley, C.G.; Solaris, P.; Quinn, G.O.; Ross, K.E.; Peterson, B.J. Precision Pest Control Using Purpose-Built Uncrewed Aerial System (UAS) Technology and a Novel Bait Pod System. Drone Syst. Appl. 2024, 12, 1–13. [Google Scholar] [CrossRef]
- Yu, Q.; Xiao, N.; Yang, S.; Han, S. Deworming of Stray Dogs and Wild Canines with Praziquantel-Laced Baits Delivered by an Unmanned Aerial Vehicle in Areas Highly Endemic for Echinococcosis in China. Infect. Dis. Poverty 2017, 6, 80–85. [Google Scholar] [CrossRef]
- Johnston, M.; McCaldin, G.; Rieker, A. Assessing the Availability of Aerially Delivered Baits to Feral Cats through Rainforest Canopy Using Unmanned Aircraft. J. Unmanned Veh. Sys. 2016, 4, 276–281. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Tessmann, A.; Quinn, G.; Lawton, F. Quantification of Flight Times of Aerial Treatments Targeting Invasive Species: The Interplay of Helicopter or Drone with Bait-Delivery Systems, Flight Speed and Bait Form. Pest Manag. Sci. 2023, 79, 2050–2055. [Google Scholar] [CrossRef]
- Li, S.; Cui, C. The Effect of Bait Air Broadcasting by Unmanned Aerial Vehicles on the Ant Community Diversity. J. Appl. Entomol. 2023, 147, 55–62. [Google Scholar] [CrossRef]
- Quinn, M.A.; Foster, R.N.; Reuter, K.C. II.14 Effect of Multiple Concentrations and Rates of Carbaryl–Bran Bait. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 1996; pp. 1–4. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://Orthoptera.SpeciesFile.org (accessed on 12 December 2022).
- Connin, R.; Kuitert, L. Control of the American grasshopper with organic insecticides in Florida. J. Econ. Entomol. 1952, 45, 684–687. [Google Scholar] [CrossRef]
- Ku, H.H. Notes on the use of propagation of error formulas. J. Res. Natl. Bur. Stand. 1966, 70, 263–273. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Latchininsky, A.V.; van Dyke, K.A. Grasshopper and Locust Control with Poisoned Baits: A Renaissance of the Old Strategy? Outlooks Pest Manag. 2006, 17, 105–111. [Google Scholar] [CrossRef]
- Dakhel, W.H.; Jaronski, S.T.; Schell, S. Control of Pest Grasshoppers in North America. Insects 2020, 11, 566. [Google Scholar] [CrossRef]



| Expected Susceptibility to Bait | Species |
|---|---|
| Highly Sensitive (70–85%) | Ageneotettix deorum (Scudder, 1876) |
| Highly Sensitive (70–85%) | Aulocara elliotti (Thomas, 1870) |
| Highly Sensitive (70–85%) | Hadrotettix trifasciatus (Say, 1825) |
| Highly Sensitive (70–85%) | Melanoplus foedus Scudder, 1878 |
| Highly Sensitive (70–85%) | Melanoplus sanguinipes (Fabricius, 1798) |
| Sensitive (55–70%) | Melanoplus occidentalis (Thomas, 1872) |
| Highly Vulnerable (42–72%) | Eritettix simplex (Scudder, 1869) |
| Highly Vulnerable (42–72%) | Psoloessa delicatula (Scudder, 1876) |
| Vulnerable (12–42%) | Aulocara femoratum Scudder, 1899 |
| Nonsusceptible (15–30%) | Amphitornus coloradus (Thomas, 1873) |
| Nonsusceptible (15–30%) | Cordillacris crenulata (Bruner, 1889) |
| Nonsusceptible (15–30%) | Cordillacris occipitalis (Thomas, 1873) |
| Nonsusceptible (15–30%) | Metator pardalinus (Saussure, 1884) |
| Highly Nonsusceptible (0–15%) | Phlibostroma quadrimaculatum (Thomas, 1871) |
| Unknown | Heliaula rufa (Scudder, 1899) |
| Unknown | Melanoplus regalis (Dodge, 1876) |
| Unknown | Xanthippus corallipes (Haldeman, 1852) |
| Plot | Wind Direction | Wind Velocity (ms−1/mph) | Temperature (°C/°F) | Relative Humidity (%) |
|---|---|---|---|---|
| A | SSW | 0.16/0.36 | 27.0/80.6 | 33.1 |
| B | S | 1.41/3.15 | 30.5/86.9 | 26.0 |
| C | NE | 2.32/5.19 | 26.9/80.4 | 30.6 |
| D | NW | 1.23/2.75 | 28.6/83.5 | 29.1 |
| Effect | df | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|---|
| Treatment | 1 | 53 | 53.11 | 10.717 | 0.001 |
| Time | 4 | 177 | 44.33 | 8.944 | <0.001 |
| Species | 16 | 2014 | 125.86 | 25.397 | <0.001 |
| Treatment:Time | 4 | 7 | 1.63 | 0.330 | 0.858 |
| Treatment:Species | 16 | 496 | 30.98 | 6.251 | <0.001 |
| Time:Species | 64 | 509 | 7.95 | 1.605 | 0.002 |
| Treatment:Time:Species | 64 | 71 | 1.11 | 0.233 | 1.000 |
| Residuals | 3910 | 19,378 | 4.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, R.; Woller, D.A.; Martin, D.E.; Reuter, K.C.; Black, L.R.; Latheef, M.A.; Colón, K.M.L.; Taylor, M. Granular Bait Applications for Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones 2024, 8, 535. https://doi.org/10.3390/drones8100535
Rodriguez R, Woller DA, Martin DE, Reuter KC, Black LR, Latheef MA, Colón KML, Taylor M. Granular Bait Applications for Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones. 2024; 8(10):535. https://doi.org/10.3390/drones8100535
Chicago/Turabian StyleRodriguez, Roberto, Derek A. Woller, Daniel E. Martin, K. Chris Reuter, Lonnie R. Black, Mohamed A. Latheef, Kiara M. López Colón, and Mason Taylor. 2024. "Granular Bait Applications for Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System" Drones 8, no. 10: 535. https://doi.org/10.3390/drones8100535







