A Novel Drone Sampling Method for Lower Atmospheric Fungal Spores
Abstract
:1. Introduction
2. Materials and Methods
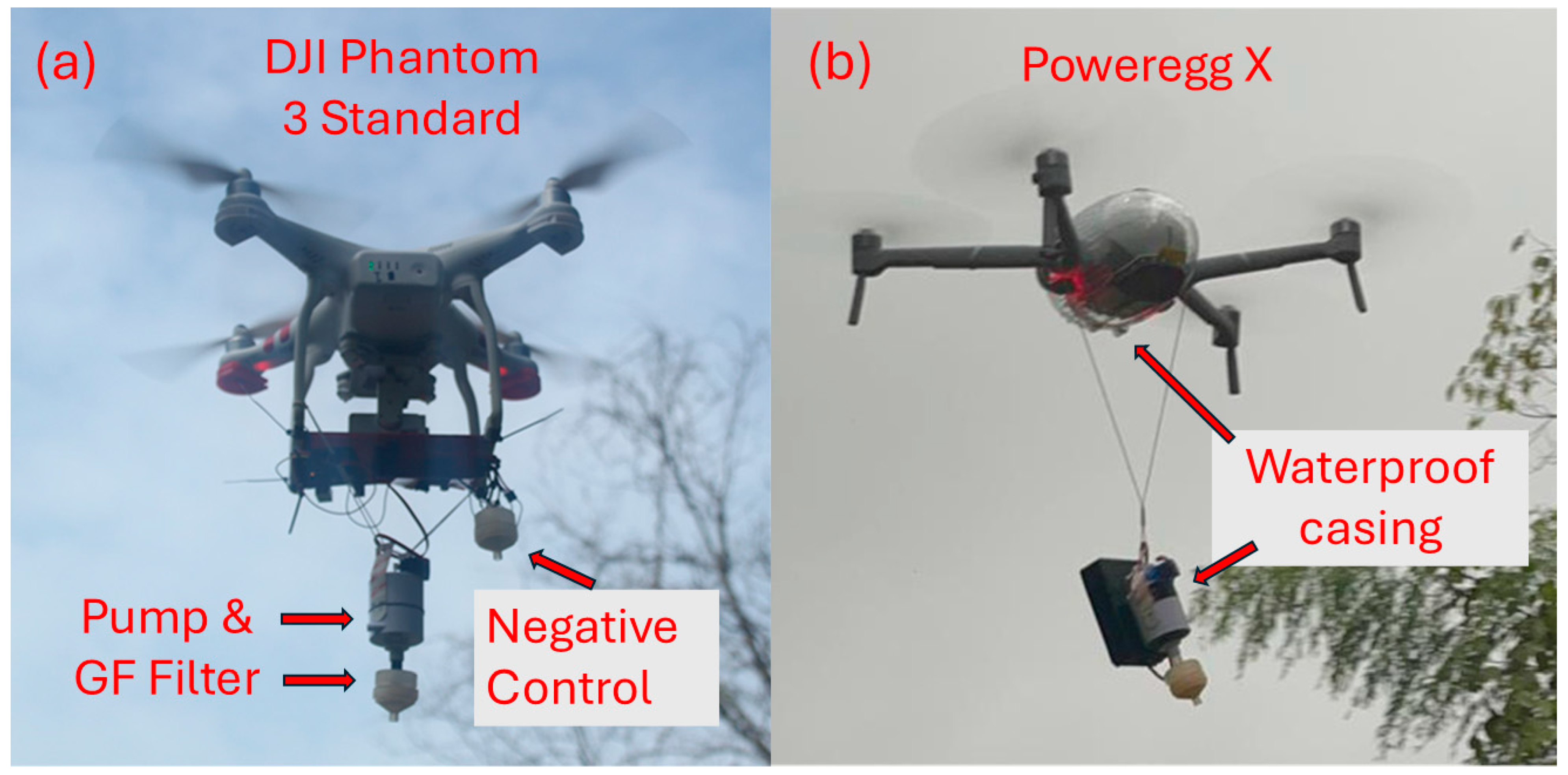
2.1. Pump Design and Mechanism
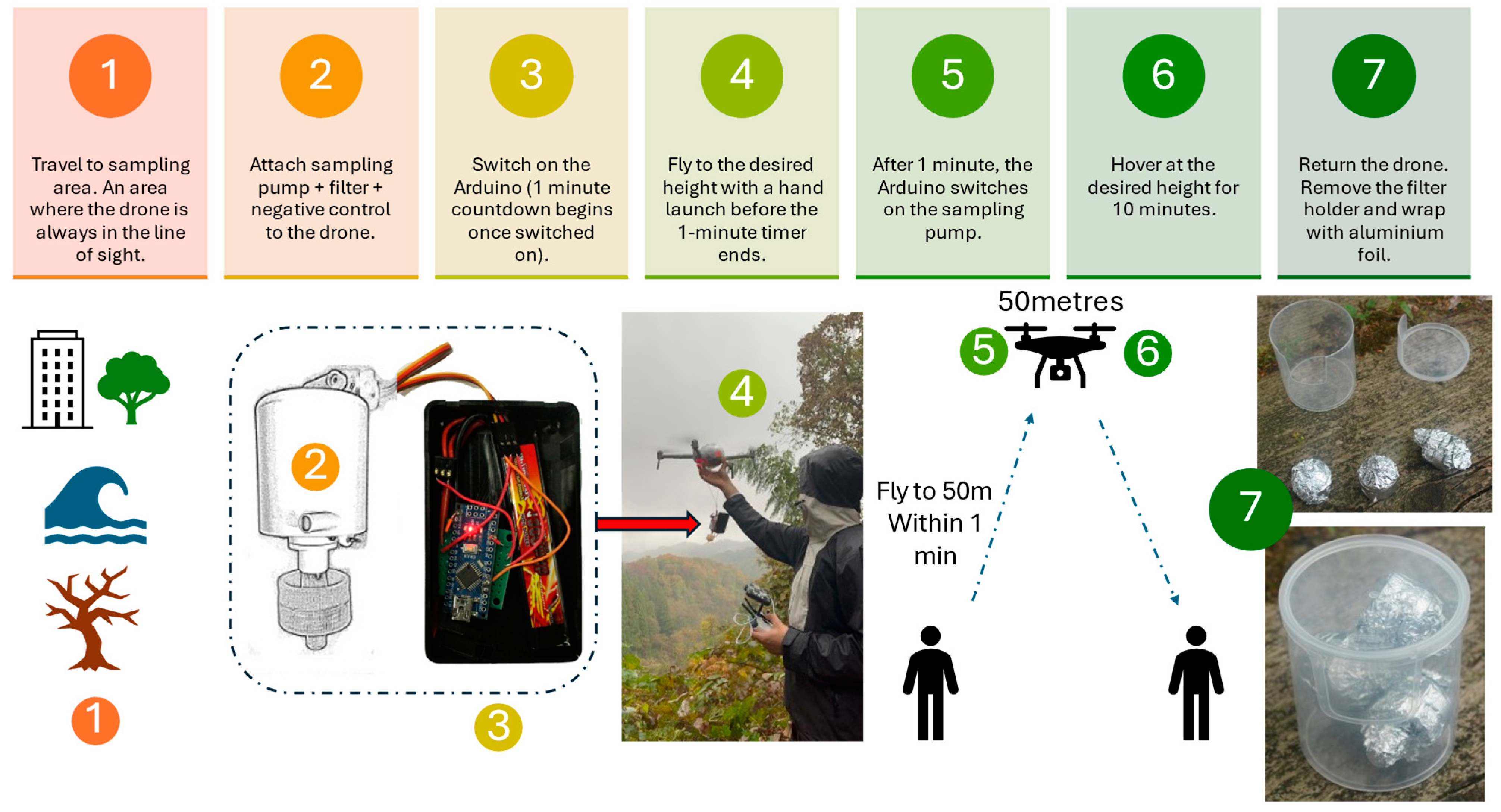
2.2. Drones
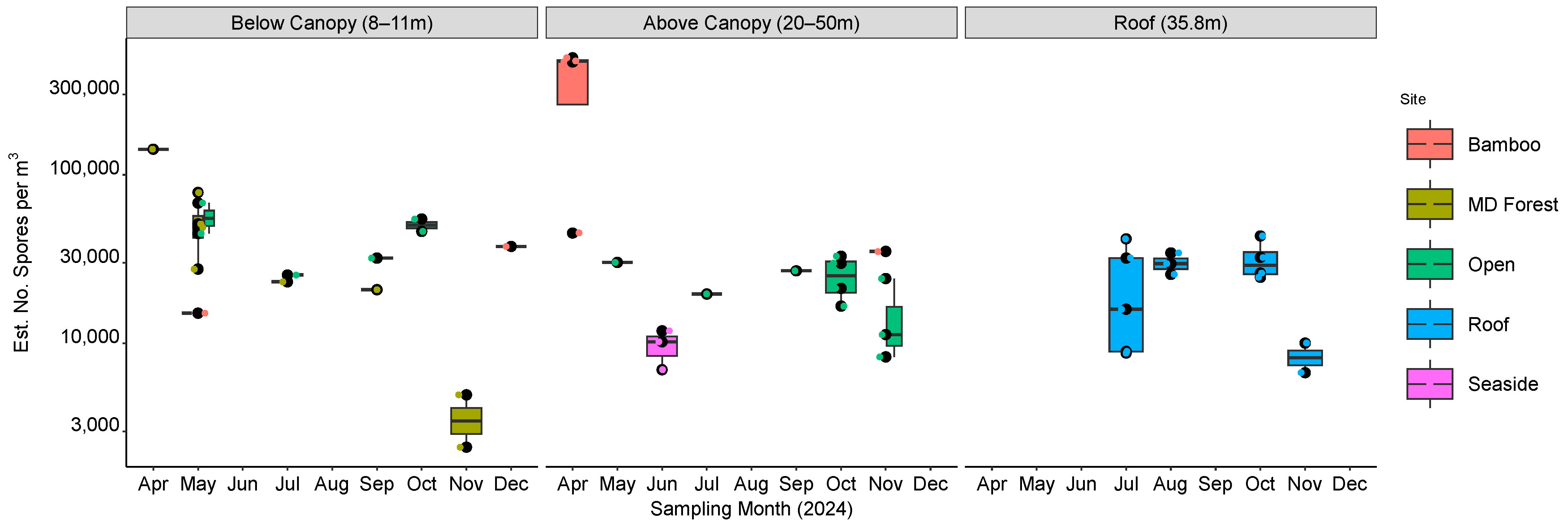
2.3. Study Site and Sampling
2.4. Collection Efficiency and Spore Quantification
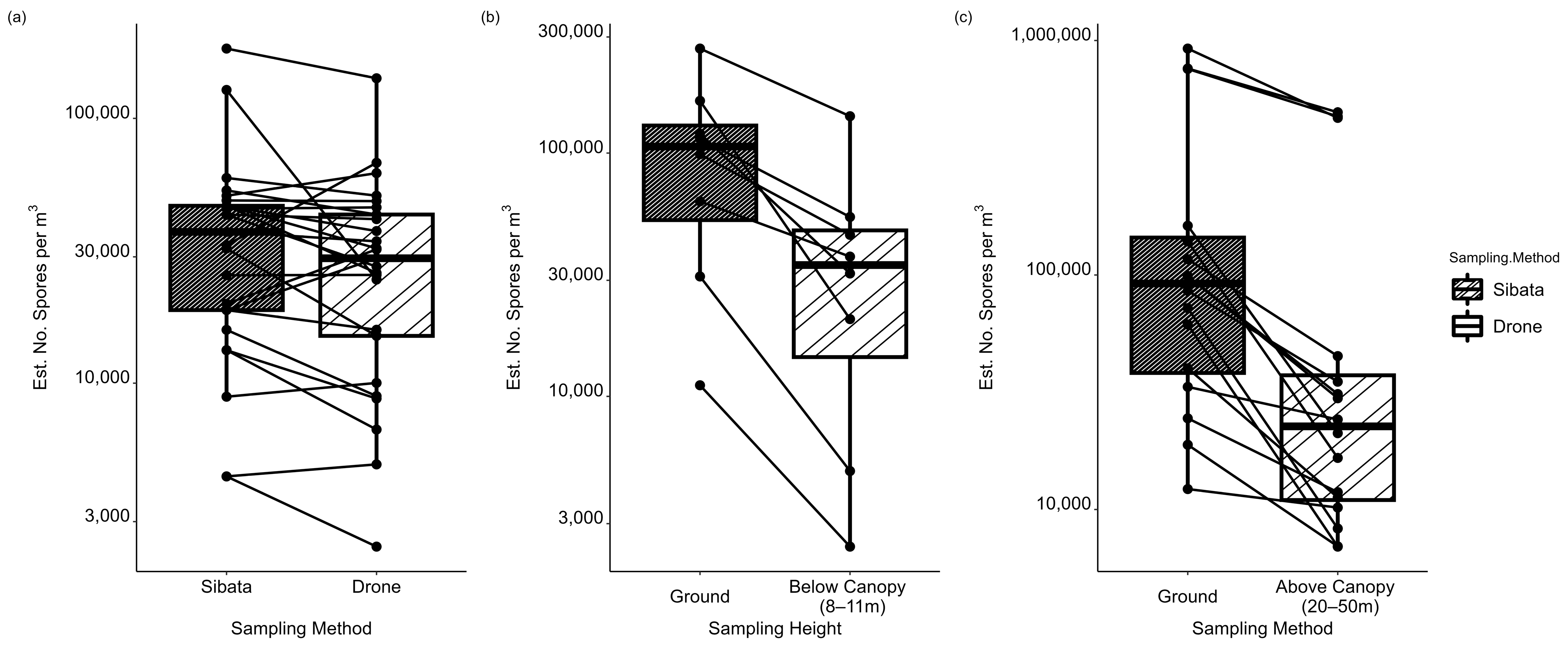
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAV | Unmanned Aerial Vehicle |
| GF | glass fiber |
| CFUs | colony forming units |
References
- Despres, V.; Huffman, J.; Burrows, S.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosols in the atmosphere: A review of observations and relevance. Tellus B 2012, 64, 15598. [Google Scholar] [CrossRef]
- Mainelis, G. Bioaerosol sampling: Classical approaches, advances, and perspectives. Aerosol Sci. Technol. 2020, 54, 496–519. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bracero, M.; Markey, E.; Hourihane Clancy, J.; McGillicuddy, E.; Sewell, G.; O’Connor, D. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 308. [Google Scholar] [CrossRef]
- Anees-Hill, S.; Douglas, P.; Pashley, C.; Hansell, A.; Marczylo, E. A systematic review of outdoor airborne fungal spore seasonality across Europe and the implications for health. Sci. Total Environ. 2021, 818, 151716. [Google Scholar] [CrossRef] [PubMed]
- Helfer, S. Rust fungi and global change. New Phytol. 2014, 201, 770–780. [Google Scholar] [CrossRef]
- Iwata, A.; Imura, M.; Hama, M.; Maki, T.; Tsuchiya, N.; Kunihisa, R.; Matsuki, A. Release of Highly Active Ice Nucleating Biological Particles Associated with Rain. Atmosphere 2019, 10, 605. [Google Scholar] [CrossRef]
- Maki, T.; Hara, K.; Kobayashi, F.; Kurosaki, Y.; Kakikawa, M.; Matsuki, A.; Chen, B.; Shi, G.; Hasegawa, H.; Iwasaka, Y. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 2015, 119, 282–293. [Google Scholar] [CrossRef]
- Damialis, A.; Kaimakamis, E.; Konoglou, M.; Akritidis, I.; Traidl-Hoffmann, C.; Gioulekas, D. Estimating the abundance of airborne pollen and fungal spores at variable elevations using an aircraft: How high can they fly? Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Khattab, A.; Levetin, E. Effect of Sampling Height on the Concentration of Airborne Fungal Spores. Ann. Allergy Asthma Immunol. 2008, 101, 529–534. [Google Scholar] [CrossRef]
- Janssen, R.H.; Heald, C.L.; Steiner, A.L.; Perring, A.E.; Huffman, J.A.; Robinson, E.S.; Twohy, C.H.; Ziemba, L.D. Drivers of the fungal spore bioaerosol budget: Observational analysis and global modeling. Atmos. Chem. Phys. 2021, 21, 4381–4401. [Google Scholar] [CrossRef]
- Adkins, K.A.; Li, K.; Blasko, M.N.; Cabrera, J.L.; Neal, B.H.; James, T.Y.; Hajian-Forooshani, Z.; Brines, S.; Perfecto, I. A simple mechanism for uncrewed aircraft bioaerosol sampling in the lower atmosphere. Landsc. Ecol. 2024, 39, 133. [Google Scholar] [CrossRef]
- Mantoani, M.C.; Emygdio, A.P.; Degobbi, C.; Sapucci, C.R.; Guerra, L.C.; Dias, M.A.; Dias, P.L.; Zanetti, R.H.; Rodrigues, F.; Araujo, G.G.; et al. Rainfall effects on vertical profiles of airborne fungi over a mixed land-use context at the Brazilian Atlantic Forest biodiversity hotspot. Agric. For. Meteorol. 2023, 331, 109352. [Google Scholar] [CrossRef]
- Löcken, H.; Fischer, O.; Selz, J.; Boppré, M. “Drone-Netting” for Sampling Live Insects. J. Insect Sci. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, R.; Jacquemin, S.J.; Birbeck, J.A.; Westrick, J.A.; Harb, C.; Gruszewski, H.; Ault, A.P.; Scott, D.; Foroutan, H.; Ross, S.D.; et al. Drone-based water sampling and characterization of three freshwater harmful algal blooms in the United States. Front. Remote Sens. 2022, 3, 949052. [Google Scholar] [CrossRef]
- Bakirci, M. Evaluating the impact of unmanned aerial vehicles (UAVs) on air quality management in smart cities: A comprehensive analysis of transportation-related pollution. Comput. Electr. Eng. 2024, 119, 109556. [Google Scholar] [CrossRef]
- Bieber, P.; Seifried, T.M.; Burkart, J.; Gratzl, J.; Kasper-Giebl, A.; Schmale, D.G., III.; Grothe, H. A Drone-Based Bioaerosol Sampling System to Monitor Ice Nucleation Particles in the Lower Atmosphere. Remote Sens. 2020, 12, 552. [Google Scholar] [CrossRef]
- Gottwald, T. A Spore and Pollen Trap for Use on Aerial Remotely Piloted Vehicles. Phytopathology 1985, 75, 801–807. [Google Scholar] [CrossRef]
- Ingold, C.T. Fungal Spores: Their Liberation and Dispersal; Clarendon: Oxford, UK, 1971. [Google Scholar]
- Niu, M.; Hu, W.; Cheng, B.; Wu, L.; Ren, L.; Deng, J.; Shen, F.; Fu, P. Influence of rainfall on fungal aerobiota in the urban atmosphere over Tianjin, China: A case study. Atmos. Environ. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Gregory, P.H. The operation of the puff-ball mechanism of lycoperdon perlatum by raindrops shown by ultra-high-speed schlieren cinematography. Trans. Br. Mycol. Soc. 1949, 32, 11–15. [Google Scholar] [CrossRef]
- Pace, L.; Boccacci, L.; Casilli, M.; Fattorini, S. Temporal variations in the diversity of airborne fungal spores in a Mediterranean high altitude site. Atmos. Environ. 2019, 210, 166–170. [Google Scholar] [CrossRef]
- Galante, T.E.; Horton, T.R.; Swaney, D.P. 95% of basidiospores fall within 1m of the cap: A field-and modeling-based study. Mycologia 2011, 103, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Penet, L.; Guyader, S.; Pétro, D.; Salles, M.; Bussière, F. Direct Splash Dispersal Prevails over Indirect and Subsequent Spread during Rains in Colletotrichum gloeosporioides Infecting Yams. PLoS ONE 2014, 9, e115757. [Google Scholar] [CrossRef]
- Bakirci, M. Smart City Air Quality Management through Leveraging Drones for Precision Monitoring. Sustain. Cities Soc. 2024, 106, 105390. [Google Scholar] [CrossRef]

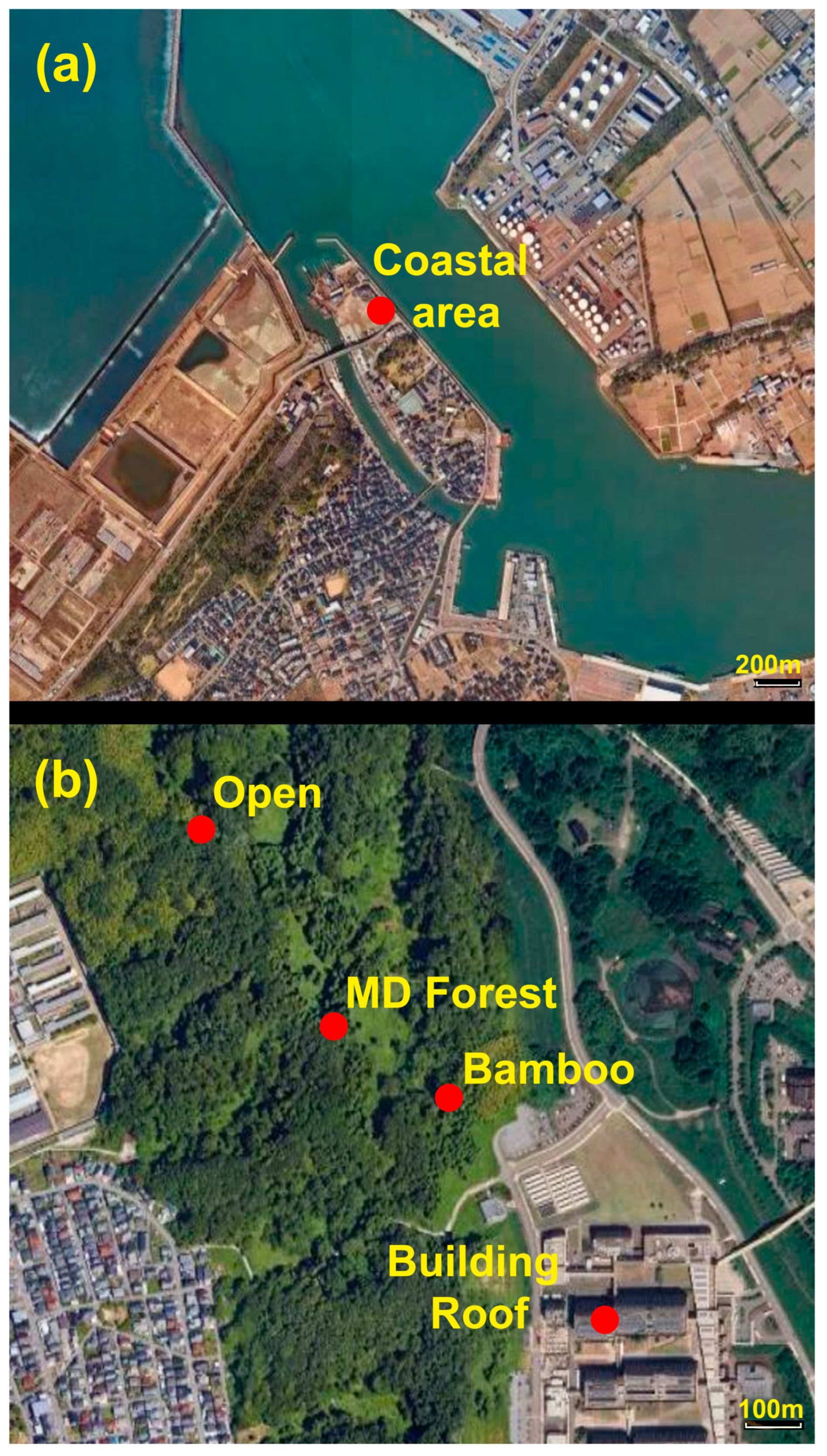

| Site | Coordinates | Type | Sampling Date | Heights Sampled (m) | Elevation (Meters Above Sea Level) |
|---|---|---|---|---|---|
| KU Kakuma Campus Site 1 (Bamboo) | N36° 32.822′ E136° 42.173′ | Forest | April 2024–December 2024 | Ground, ~10 m, 50 m | 127 |
| KU KC Site 2 (MD Forest) | N36° 32.858′ E136° 42.050′ | Forest | April 2024–November 2024 | Ground, ~8 m, 50 m | 144 |
| KU KC Site 3 (Open) | N36° 33.013′ E136° 41.903′ | Forest | May 2024– November 2024 | Ground, ~11 m, 50 m | 132 |
| KU KC Building 1 | N36° 32.678′ E136° 42.277′ | Urban | July 2024–November 2024 | Roof | 148 |
| Kanazawa, Coastal area | N 36° 37.106′ E136° 36.316′ | Coastal, urban | June 2024 | Ground 20 m, 50 m | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangay, R.; Matsuki, A.; Tuno, N. A Novel Drone Sampling Method for Lower Atmospheric Fungal Spores. Drones 2025, 9, 91. https://doi.org/10.3390/drones9020091
Bangay R, Matsuki A, Tuno N. A Novel Drone Sampling Method for Lower Atmospheric Fungal Spores. Drones. 2025; 9(2):91. https://doi.org/10.3390/drones9020091
Chicago/Turabian StyleBangay, Rohit, Atsushi Matsuki, and Nobuko Tuno. 2025. "A Novel Drone Sampling Method for Lower Atmospheric Fungal Spores" Drones 9, no. 2: 91. https://doi.org/10.3390/drones9020091
APA StyleBangay, R., Matsuki, A., & Tuno, N. (2025). A Novel Drone Sampling Method for Lower Atmospheric Fungal Spores. Drones, 9(2), 91. https://doi.org/10.3390/drones9020091







