Tracking Fin Whale Morphology with Drone Photogrammetry: Growth Tendencies, Developmental Changes, and Sexual Dimorphism
Abstract
:1. Introduction
2. Materials and Methods
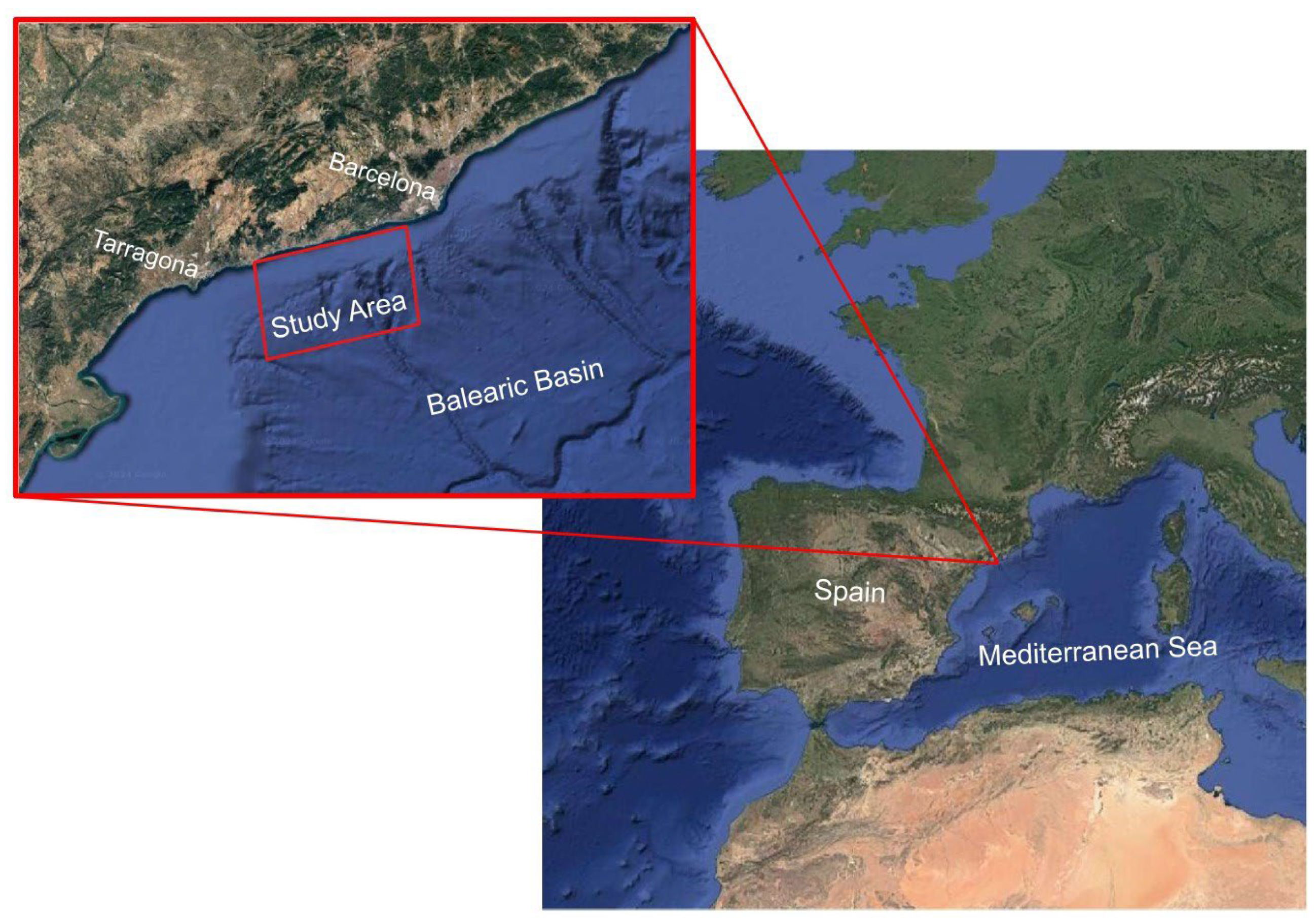
2.1. Study Area
2.2. Data Collection
2.3. Morphometric Measurements and Scaling
2.4. Body Area Index
2.5. Body Development
2.6. Photo-Identification and Life-History Traits
2.7. Mitigation of Uncertainties
2.8. Statistical Analysis
2.8.1. Body Size
2.8.2. Allometric Growth
2.8.3. Developmental Changes
2.8.4. Sexual Dimorphism
3. Results
3.1. Body Size
3.2. Allometric Growth
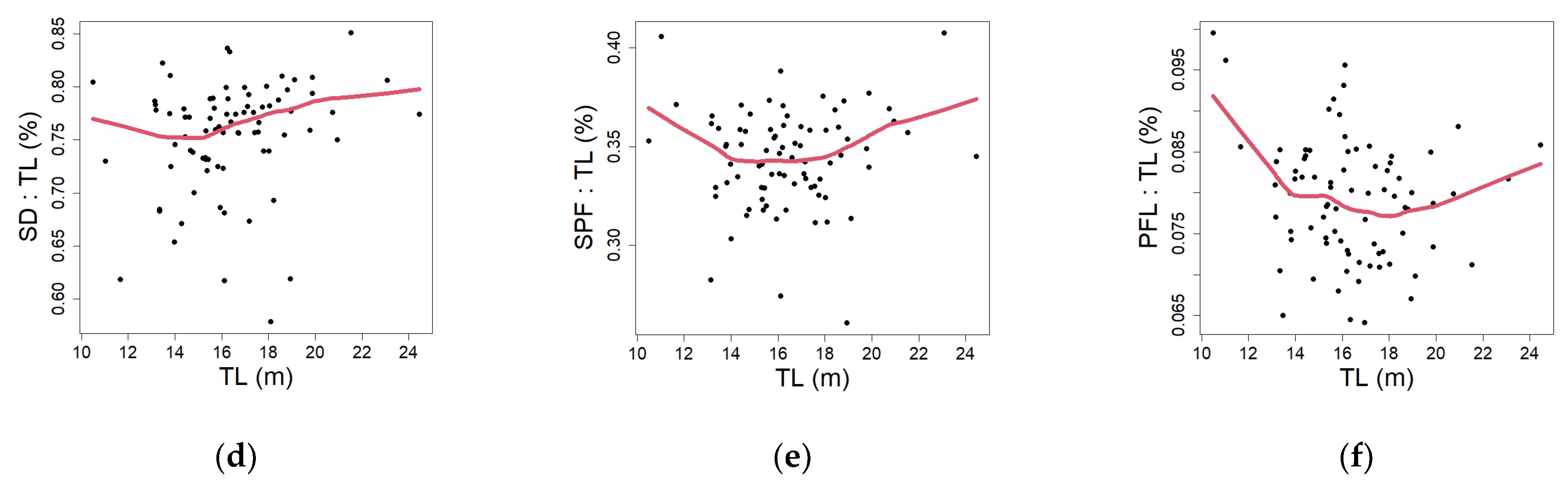
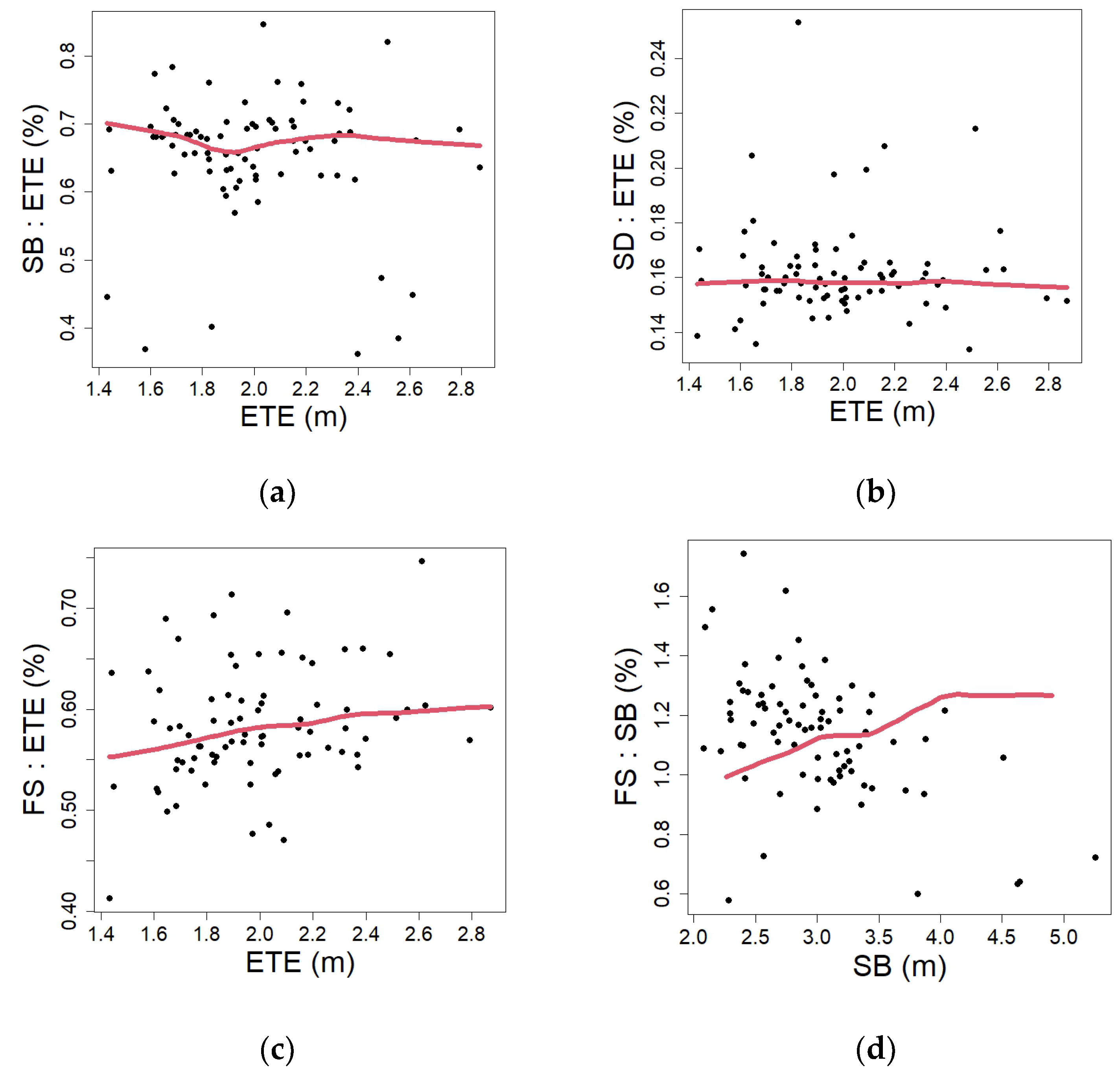
3.3. Developmental Changes
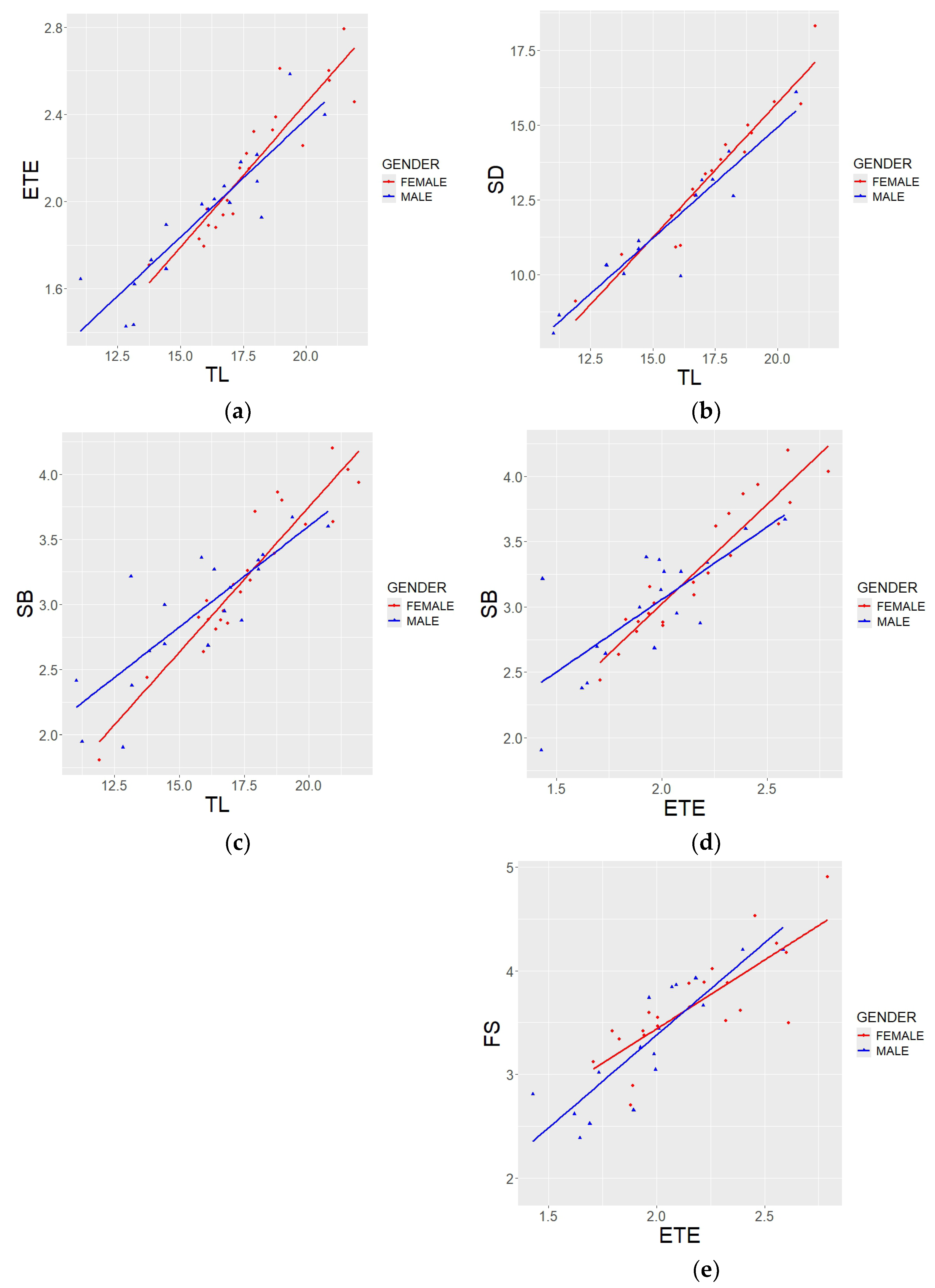
3.4. Sexual Dimorphism
3.5. Uncertanties
4. Discussion
4.1. Difficulties
4.2. Growth Rates and Allometry
4.3. Developmental Changes (Proportions)
4.4. Sexual Dimorphism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFL | Anterior flipper length |
| BAI | Body area index |
| FS | Fluke spread |
| ETE | Eye to eye |
| PFL | Posterior flipper length |
| SAF | Snout to anterior flipper |
| SB | Snout to blowhole |
| SD | Snout to dorsal fin |
| SPF | Snout to posterior flipper |
| TL | Total length |
| UAV | Unmanned aerial vehicle |
References
- Aguilar’, A.; Lockyer, C.H. Growth, physical maturity, and mortality of fin whales (Balaenoptera physalus) inhabiting the temperate waters of the northeast Atlantic. Can. J. Zool. 1987, 65, 253–264. [Google Scholar] [CrossRef]
- Aguilar, A.; García-Vernet, R. Fin Whale: Balaenoptera physalus. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 368–371. [Google Scholar] [CrossRef]
- Jefferson, T.A.; Webber, M.A.; Pitman, R.L.; Jarrett, B. Marine Mammals of the World: A Comprehensive Guide to Their Identification; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar] [CrossRef]
- Tolo-di-sciar, G.A.N.; Zanardelli, M.; Jahoda, M.; Panigada, S.; Airoldi, S. The fin whale Balaenoptera physalus (L. 1758) in the Mediterranean Sea. Mamm. Rev. 2003, 33, 105–150. [Google Scholar] [CrossRef]
- Castellote, M.; Clark, C.W.; Lammers, M.O. Fin whale (Balaenoptera physalus) population identity in the western Mediterranean Sea. Mar. Mamm. Sci. 2012, 28, 325–344. [Google Scholar] [CrossRef]
- Giralt Paradell, O.; Juncà, S.; Marcos, R.; Gimenez, A.; Giménez, J. Encounter rate and relative abundance of eight cetaceans off the central Catalan coast (Northwestern Mediterranean Sea). Mar. Environ. Res. 2023, 191, 106166. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G.; Castellote, M.; Druon, J.N.; Panigada, S. Fin Whales, Balaenoptera physalus: At Home in a Changing Mediterranean Sea? Adv. Mar. Biol. 2016, 75, 75–101. [Google Scholar] [CrossRef]
- Tort Castro, B.; Prieto González, R.; O’Callaghan, S.A.; Dominguez Rein-Loring, P.; Degollada Bastos, E. Ship Strike Risk for Fin Whales (Balaenoptera physalus) off the Garraf coast, Northwest Mediterranean Sea. Front. Mar. Sci. 2022, 9, 867287. [Google Scholar] [CrossRef]
- Gauffier, P.; Verborgh, P.; Giménez, J.; Esteban, R.; Sierra, J.M.S.; De Stephanis, R. Contemporary migration of fin whales through the Strait of Gibraltar. Mar. Ecol. Prog. Ser. 2018, 588, 215–228. [Google Scholar] [CrossRef]
- Geijer, C.K.A.; Notarbartolo di Sciara, G.; Panigada, S. Mysticete migration revisited: Are Mediterranean fin whales an anomaly? Mamm. Rev. 2016, 46, 284–296. [Google Scholar] [CrossRef]
- Degollada, E.; Amigó, N.; O’Callaghan, S.A.; Varola, M.; Ruggero, K.; Tort, B. A Novel Technique for Photo-Identification of the Fin Whale, Balaenoptera physalus, as Determined by Drone Aerial Images. Drones 2023, 7, 220. [Google Scholar] [CrossRef]
- Panigada, V.; Bodey, T.W.; Friedlaender, A.; Druon, J.N.; Huckstädt, L.A.; Pierantonio, N.; Degollada, E.; Tort, B.; Panigada, S. Targeting fin whale conservation in the North-Western Mediterranean Sea: Insights on movements and behaviour from biologging and habitat modelling. R. Soc. Open Sci. 2024, 11, 231783. [Google Scholar] [CrossRef]
- Espada, R.; Camacho-Sánches, A.; Olaya-Ponzone, L.; Estefanía-Moreno, E.; Patón, D.; García-Gómez, J.C. Fin Whale Balaenoptera physalus Historical Sightings and Strandings, Ship Strikes, Breeding Areas and Other Threats in the Mediterranean Sea: A Review. Environments 2024, 11, 1624–2023. [Google Scholar] [CrossRef]
- Gauffier, P.; Borrell, A.; Silva, M.A.; Víkingsson, G.A.; López, A.; Giménez, J.; Colaço, A.; Halldórsson, S.D.; Vighi, M.; Prieto, R.; et al. Wait your turn, North Atlantic fin whales share a common feeding ground sequentially. Mar. Environ. Res. 2020, 155, 104884. [Google Scholar] [CrossRef]
- Durban, J.W.; Moore, M.J.; Chiang, G.; Hickmott, L.S.; Bocconcelli, A.; Howes, G.; Bahamonde, P.A.; Perryman, W.L.; LeRoi, D.J. Photogrammetry of blue whales with an unmanned hexacopter. Mar. Mamm. Sci. 2016, 32, 1510–1515. [Google Scholar] [CrossRef]
- Gray, P.C.; Bierlich, K.C.; Mantell, S.A.; Friedlaender, A.S.; Goldbogen, J.A.; Johnston, D.W. Drones and convolutional neural networks facilitate automated and accurate cetacean species identification and photogrammetry. Methods Ecol. Evol. 2019, 10, 1490–1500. [Google Scholar] [CrossRef]
- Fortune, S.M.E.; Moore, M.J.; Perryman, W.L.; Trites, A.W. Body growth of North Atlantic right whales (Eubalaena glacialis) revisited. Mar. Mamm. Sci. 2021, 37, 433–447. [Google Scholar] [CrossRef]
- Bando, T.; Nakamura, G.; Fujise, Y.; Kato, H. Developmental Changes in the Morphology of Western North Pacific Bryde’s Whales (Balaenoptera edeni). Open J. Anim. Sci. 2017, 7, 344–355. [Google Scholar] [CrossRef]
- Christiansen, F.; Víkingsson, G.A.; Rasmussen, M.H.; Lusseau, D. Minke whales maximise energy storage on their feeding grounds. J. Exp. Biol. 2013, 216, 427–436. [Google Scholar] [CrossRef]
- Christiansen, F.; Vivier, F.; Charlton, C.; Ward, R.; Amerson, A.; Burnell, S.; Bejder, L. Maternal body size and condition determine calf growth rates in southern right whales. Mar. Ecol. Prog. Ser. 2018, 592, 267–282. [Google Scholar] [CrossRef]
- Hunt, K.E.; Moore, M.J.; Rolland, R.M.; Kellar, N.M.; Hall, A.J.; Kershaw, J.; Raverty, S.A.; Davis, C.E.; Yeates, L.C.; Fauquier, D.A.; et al. Overcoming the challenges of studying conservation physiology in large whales: A review of available methods. Conserv. Physiol. 2013, 1, cot006. [Google Scholar] [CrossRef]
- Arrigoni, M.; Manfredi, P.; Panigada, S.; Bramanti, L.; Santangelo, G. Life-history tables of the Mediterranean fin whale from stranding data. Mar. Ecol. 2011, 32, 1–9. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Shadwick, R.E.; Lillie, M.A.; Piscitelli, M.A.; Potvin, J.; Pyenson, N.D.; Vogl, A.W. Using morphology to infer physiology: Case studies on rorqual whales (Balaenopteridae). Can. J. Zool. 2014, 93, 687–700. [Google Scholar] [CrossRef]
- Castellini, M. History of polar whaling: Insights into the physiology of the great whales. Comp. Biochem. Physiol. Part A 2000, 126, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.D.; Lemos, L.; Barlow, D.; Wing, M.G.; Chandler, T.; Torres, L.G. Estimating morphometric attributes of baleen whales with photogrammetry from small UASs: A case study with blue and gray whales. Mar. Mamm. Sci. 2019, 35, 108–139. [Google Scholar] [CrossRef]
- de Oliveira, L.; Andriolo, A.; Cremer, M.; Zerbini, A. Aerial photogrammetry techniques using drones to estimate morphometric measurements and body condition in South American small cetaceans. Mar. Mamm. Sci. 2023, 39, 811–829. [Google Scholar] [CrossRef]
- Dawson, S.M.; Bowman, M.H.; Leunissen, E.; Sirguey, P. Inexpensive aerial photogrammetry for studies of whales and large marine animals. Front. Mar. Sci. 2017, 4, 366. [Google Scholar] [CrossRef]
- Torres, W.; Bierlich, K. MorphoMetriX: A photogrammetric measurement GUI for morphometric analysis of megafauna. J. Open Source Softw. 2020, 5, 1825. [Google Scholar] [CrossRef]
- Castrillon, J.; Bengtson Nash, S. Evaluating cetacean body condition; a review of traditional approaches and new developments. Ecol. Evol. 2020, 10, 6144–6162. [Google Scholar] [CrossRef]
- Ratnaswamy, M.J.; Winn, H.E. Photogrammetric Estimates of Allometry and Calf Production in Fin Whales, Balaenoptera physalus. J. Mammal. 1993, 74, 323–330. [Google Scholar] [CrossRef]
- Murphy, S.; Rogan, E. External morphology of the short-beaked common dolphin, Delphinus delphis: Growth, allometric relationships and sexual dimorphism. Acta Zool. 2006, 87, 315–329. [Google Scholar] [CrossRef]
- Lloret, J.; Palomera, I.; Salat, J.; Sole, I. Impact of freshwater input and wind on landings of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) in shelf waters surrounding the Ebre (Ebro) River delta (north-western Mediterranean). Fish. Oceanogr. 2004, 13, 102–110. [Google Scholar] [CrossRef]
- Rigual-Hernández, A.S.; Bárcena, M.A.; Sierro, F.J.; Flores, J.A.; Hernández-Almeida, I.; Sanchez-Vidal, A.; Palanques, A.; Heussner, S. Seasonal to interannual variability and geographic distribution of the silicoflagellate fluxes in the Western Mediterranean. Mar. Micropaleontol. 2010, 77, 46–57. [Google Scholar] [CrossRef]
- Puig, P.; Palanques, A.; Guilleh, J.; Garcı, E. Deep slope currents and suspended particle fuxes in and around the Foix submarine canyon (NW Mediterranean). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2000, 47, 343–366. [Google Scholar] [CrossRef]
- Real Decreto 1727/2007, de 21 de Diciembre, Por El Que Se Establecen Medidas de Protección de los Cetáceos. Referencia:BOE-A-2008-516. Available online: https://www.boe.es/eli/es/rd/2007/12/21/1727 (accessed on 5 March 2024).
- Bierlich, K.C.; Schick, R.S.; Hewitt, J.; Dale, J.; Goldbogen, J.A.; Friedlaender, A.S.; Johnston, D.W. Bayesian approach for predicting photogrammetric uncertainty in morphometric measurements derived from drones. Mar. Ecol. Prog. Ser. 2021, 673, 193–210. [Google Scholar] [CrossRef]
- Soledade Lemos, L.; Burnett, J.D.; Chandler, T.E.; Sumich, J.L.; Torres, L.G. Intra- and inter-annual variation in gray whale body condition on a foraging ground. Ecosphere 2020, 11, e03094. [Google Scholar] [CrossRef]
- Durban, J.W.; Fearnbach, H.; Paredes, A.; Hickmott, L.S.; LeRoi, D.J. Size and body condition of sympatric killer whale ecotypes around the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2021, 677, 209–217. [Google Scholar] [CrossRef]
- Christiansen, F.; Sironi, M.; Moore, M.J.; Di Martino, M.; Ricciardi, M.; Warick, H.A.; Irschick, D.J.; Gutierrez, R.; Uhart, M.M. Estimating body mass of free-living whales using aerial photogrammetry and 3D volumetrics. Methods Ecol. Evol. 2019, 10, 2034–2044. [Google Scholar] [CrossRef]
- Cheney, B.J.; Dale, J.; Thompson, P.M.; Quick, N.J. Spy in the sky: A method to identify pregnant small cetaceans. Remote Sens. Ecol. Conserv. 2022, 8, 492–505. [Google Scholar] [CrossRef]
- Glarou, M.; Gero, S.; Frantzis, A.; Brotons, J.M.; Vivier, F.; Alexiadou, P.; Cerdà, M.; Pirotta, E.; Christiansen, F. Estimating body mass of sperm whales from aerial photographs. Mar. Mamm. Sci. 2023, 39, 251–273. [Google Scholar] [CrossRef]
- Best, P.B.; Ruther, D.H. Aerial photogrammetry of southern right whales, Eubalaena australis. Zool. Soc. Lond. 1992, 228, 595–614. [Google Scholar] [CrossRef]
- Fox, J.; Bouchet-Valat, M.; Munoz Marquez, M.; Andronic, L.; Ash, M.; Boye, T.; Calza, S.; Chang, A.; Gegzna, G.; Grosjean, P.; et al. R Commander. R Package Version 2.9.5. 2024. Available online: https://github.com/RCmdr-Project/rcmdr (accessed on 5 March 2024).
- Reinhar, A. Fetch Economic and Financial Time Series Data from Public Sources. R Package Version 0.3.3. 2024. Available online: https://github.com/abielr/pdfetch (accessed on 5 March 2024).
- Jeffrey, A.R.; Joshua, M.U. xts: eXtensible Time Series. R Package Version 0.12.1. 2020. Available online: https://CRAN.R-project.org/package=xts (accessed on 5 March 2024).
- Golding, N. versions: Query and Install Specific Versions of Packages on CRAN. R Package Version 0.3. 2016. Available online: https://CRAN.R-project.org/package=versions (accessed on 5 March 2024).
- Wickham, H.; Henry, L.; Vaughan, D. vctrs: Vector Helpers. R Package Version 0.6.5. 2023. Available online: https://CRAN.R-project.org/package=vctrs (accessed on 5 March 2024).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R Package Version 1.3.1. 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 5 March 2024).
- Müller, K.; Wickham, H. tibble: Simple Data Frames. R Package Version 3.1.8. 2022. Available online: https://CRAN.R-project.org/package=tibble (accessed on 5 March 2024).
- Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. R Package Version 2.8.17. 2024. Available online: https://CRAN.R-project.org/package=sjPlot (accessed on 5 March 2024).
- Lüdecke, D. sjmisc: Data and Variable Transformation Functions. J. Open Source Softw. 2018, 3, 754. [Google Scholar] [CrossRef]
- Lüdecke, D. sjlabelled: Labelled Data Utility Functions (Version 1.2.0). 2022. Available online: https://CRAN.R-project.org/package=sjlabelled (accessed on 5 March 2024).
- Csárdi, G.; Hester, J.; Wickham, H.; Chang, W.; Morgan, M.; Tenenbaum, D. remotes: R Package Installation from Remote Repositories, Including “GitHub”. R Package Version 2.4.2. 2021. Available online: https://CRAN.R-project.org/package=remotes (accessed on 5 March 2024).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.4.0. 2022. Available online: https://CRAN.R-project.org/package=readxl (accessed on 5 March 2024).
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Rudis, B. hrbrthemes: Additional Themes, Theme Components and Utilities for “ggplot2”. 2024. Available online: https://CRAN.R-project.org/package=hrbrthemes (accessed on 5 March 2024).
- Patil, I. Visualizations with statistical details: The ’ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Lboukadel, K. ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 March 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. 2016. Available online: https://ggplot2.tidyverse.org (accessed on 5 March 2024).
- Pedersen, T. ggforce: Accelerating “ggplot2”. R Package Version 0.4.1. 2022. Available online: https://CRAN.R-project.org/package=ggforce (accessed on 5 March 2024).
- Cameron, A.; van den Brand, T. geomtextpath: Curved Text in “ggplot2”. R Package Version 0.1.3. 2024. Available online: https://CRAN.R-project.org/package=geomtextpath (accessed on 5 March 2024).
- Bryan, J. gapminder: Data from Gapminder. R Package Version 1.0.0. 2023. Available online: https://CRAN.R-project.org/package=gapminder (accessed on 5 March 2024).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. R Package Version 0.9.4. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 5 March 2024).
- Gohel, D.; Skintzos, P. flextable: Functions for Tabular Reporting. R Package Version 0.9.6. 2024. Available online: https://CRAN.R-project.org/package=flextable (accessed on 5 March 2024).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 5 March 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.9. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 5 March 2024).
- Wilke, C. cowplot: Streamlined Plot Theme and Plot Annotations for “ggplot2”. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 5 March 2024).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowely, J. GGally: Extension to “ggplot2”. R Package Version 2.1.2. 2021. Available online: https://CRAN.R-project.org/package=GGally (accessed on 5 March 2024).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Ohsumi, S. Relative Growth of the Fin Whale, Balaenoptera physalus (Linn.). Sci. Rep. WHales. Res. Inst. 1960, 15, 17–84. [Google Scholar]
- Cook, R.D.; Weisberc, S. An Introduction to Regression Graphics, 23rd ed.; Branett, V., Bradley, R.A., Fisher, N.I., Hunter, J.S., Kadane, J.B., Kendall, D.G., Smith, A.F.M., Stigler, S.M., Teugles, J.L., Watson, G.S., Eds.; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 1994; pp. 23–34. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Sherrill, M.; Bernier-Graveline, A.; Ewald, J.; Pang, Z.; Moisan, M.; Marzelière, M.; Muzzy, M.; Romano, T.A.; Michaud, R.; Verreault, J. Scaled mass index derived from aerial photogrammetry associated with predicted metabolic pathway disruptions in free-ranging St. Lawrence Estuary belugas. Front. Mar. Sci. 2024, 11, 1360374. [Google Scholar] [CrossRef]
- Castilla, A.M.G. Evaluation of Body Condition of Resident Bottlenose Dolphins (Tursiops truncatus) in the Sado Region Using Unmanned Aircraft Systems and Photogrammetry. Master’s Thesis, Universidade de Evora (Portugal), Évora, Portugal, 2023. [Google Scholar]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.Y.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7, e01468. [Google Scholar] [CrossRef]
- Ohsumi, S. Yearly Change in age and body length at sexual maturity of a fin whale stock in the eastern north pacific. Sci. Rep. Whales Res. Inst. 1986, 1–16. Available online: https://www.icrwhale.org/pdf/SC0371-16.pdf (accessed on 26 April 2023).
- Weber, P.W.; Howle, L.E.; Murray, M.M.; Reidenberg, J.S.; Fish, F.E. Hydrodynamic performance of the flippers of large-bodied cetaceans in relation to locomotor ecology. Mar. Mamm. Sci. 2014, 30, 413–432. [Google Scholar] [CrossRef]
- Woodward, B.L.; Winn, J.P.; Fish, F.E. Morphological specializations of baleen whales associated with hydrodynamic performance and ecological niche. J. Morphol. 2006, 267, 1284–1294. [Google Scholar] [CrossRef]
- Roston, R.A.; Lickorish, D.; Buchholtz, E.A. Anatomy and age estimation of an early blue whale (Balaenoptera musculus) fetus. Anat. Rec. 2013, 296, 709–722. [Google Scholar] [CrossRef]
- Kim, Y.; Nishimura, F.; Bando, T.; Fujise, Y.; Nakamura, G.; Murase, H.; Kato, H. Fetal development in tail flukes of the antarctic minke whale. Cetacean Popul. Stud. (CPOPS) 2021, 3, 231–238. [Google Scholar] [CrossRef]
- Gavazzi, L.; Cooper, L.N.; Usip, S.; Suydam, R.; Stimmelmayr, R.; George, J.C.; O’Corry-Crowe, G.; Hsu, C.W.; Thewissen, J. Comparative embryology of Delphinapterus leucas (beluga whale), Balaena mysticetus (bowhead whale), and Stenella attenuata (pan-tropical spotted dolphin) (Cetacea: Mammalia). J. Morphol. 2023, 284, e21543. [Google Scholar] [CrossRef] [PubMed]
- Shingleton, A.W. Allometry and Relative Growth. Nat. Educ. Knowl. 2010, 3, 1–17. [Google Scholar]
- Brownell, R.L., Jr.; Ralls, K. Potential for sperm competition in baleen whales. Rep. Int. Whal. Comm. 1986, 8, 97–112. [Google Scholar]
- Lockyer, C. Body fat condition in northeast Atlantic fin whales, Balaenoptera physalus, and its relationship with reproduction and food resource. Can. J. Fish. Aquat. Sci. 1986, 43, 142–147. [Google Scholar] [CrossRef]
- Aoki, K.; Isojunno, S.; Bellot, C.; Iwata, T.; Kershaw, J.; Akiyama, Y.; Martín López, L.M.; Ramp, C.; Biuw, M.; Swift, R.; et al. Aerial photogrammetry and tag-derived tissue density reveal patterns of lipid-store body condition of humpback whales on their feeding grounds. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202307. [Google Scholar] [CrossRef]
- Bradford, A.L.; Weller, D.W.; Punt, A.E.; Ivashchenko, Y.V.; Burdin, A.M.; Vanblaricom, G.R.; Brownell, R.L. Leaner leviathans: Body condition variation in a critically endangered whale population. J. Mammal. 2012, 93, 251–266. [Google Scholar] [CrossRef]




| Body Measurement | Description | Abbreviation |
|---|---|---|
| Total length | From the tip of the rostrum until the notch of the fluke | TL |
| Fluke spread | Width of the fluke | FS |
| Snout to blowhole | From the tip of the rostrum until the anterior of the blowhole | SB |
| Snout to dorsal fin | From the tip of the rostrum until the tip of the dorsal fin | SD |
| Snout anterior flipper | From the tip of the rostrum until the anterior insertion of the flipper | SAF |
| Snout posterior flipper | From the tip of the rostrum until the anterior–posterior of the flipper | SPF |
| Anterior flipper length | From the anterior flipper insertion until the tip of the flipper | AFL |
| Posterior flipper length | From the posterior flipper insertion until the tip of the flipper | PFL |
| Eye to eye | Width between the eyes | ETE |
| Body Proportions | Abbreviation |
|---|---|
| Fluke spread compared to total length | FS:TL |
| Snout to blowhole compared to total length | SB:TL |
| Snout to dorsal fin compared to total length | SD:TL |
| Snout to anterior flipper compared to total length | SAF:TL |
| Snout to posterior flipper compared to total length | SPF:TL |
| Eye to eye distance compared to total length | ETE:TL |
| Anterior flipper length compared to total length | AFL:TL |
| Posterior flipper length compared to total length | PFL:TL |
| Eye to eye distance compared to snout to blowhole | ETE:SB |
| Eye to eye distance compared to snout to dorsal fin | EYE:SD |
| Eye to eye distance compared to fluke spread | EYE:FS |
| Anterior flipper length compared to eye to eye distance | AFL:ETE |
| Anterior flipper length compared to snout to blowhole | AFL:SB |
| Anterior flipper length compared to snout to dorsal fin | AFL:SD |
| Anterior flipper length compared to fluke spread | AFL:FS |
| fluke spread compared to snout to blowhole | FS:SB |
| fluke spread compared to snout to dorsal fin | FS:SD |
| Abbreviation | N | R2 | Intercept | Allometric Coefficient | Relative Growth Pattern | SER (%) |
|---|---|---|---|---|---|---|
| ETE | 106 | 0.800 | 0.39 | −0.792 | Negative | 0.6 |
| FS | 107 | 0.698 | 0.38 | −0.527 | Negative | 0.2 |
| SB | 100 | 0.514 | 0.47 | −0.833 | Negative | 0.1 |
| SD | 90 | 0.844 | 0.48 | −0.238 | Negative | 0.07 |
| AFL | 102 | 0.730 | 0.37 | −0.764 | Negative | 0.2 |
| PFL | 100 | 0.677 | 0.38 | −0.949 | Negative | 0.4 |
| SAF | 102 | 0.420 | 0.41 | −0.476 | Negative | 0.8 |
| SPF | 102 | 0.806 | 0.44 | −0.482 | Negative | 0.8 |
| Female | Male | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviation | Mean (m) | Std | Range (m) | p-Value | Mean (m) | Std | Range (m) | p-Value | F-Value | p-Value |
| TL | 17.63 | 1.99 | 13.76–21.51 | 0.07 | 15.71 | 2.26 | 11.50–20.73 | 0.45 | 3.61 | 0.14 |
| ETE | 2.15 | 0.31 | 1.70–2.79 | 0.01 | 1.90 | 0.27 | 1.43–2.39 | 0.10 | 3.09 | 0.05 |
| FS | 3.64 | 0.45 | 2.89–4.91 | 0.03 | 3.25 | 0.60 | 2.38–4.20 | 0.16 | 2.23 | 0.11 |
| SB | 3.3 | 0.56 | 2.44–4.64 | 0.02 | 2.94 | 0.61 | 2.21–4.62 | 0.43 | 3.15 | 0.04 |
| SD | 13.57 | 2.01 | 10.66–18.31 | 0.01 | 11.72 | 2.14 | 10.31–16.09 | 0.10 | 3.63 | 0.03 |
| AFL | 1.95 | 0.26 | 1.64–2.56 | 0.04 | 1.81 | 0.30 | 1.46–2.29 | 0.53 | 2.83 | 0.06 |
| PFL | 1.38 | 0.18 | 1.10–1.84 | 0.04 | 1.26 | 0.22 | 1.01–1.65 | 0.40 | 2.53 | 0.08 |
| SAF | 5.03 | 0.92 | 3.34–6.75 | 0.18 | 4.49 | 1.05 | 1.75–5.56 | 0.20 | 1.00 | 0.37 |
| SPF | 6.05 | 0.94 | 5.52–7.68 | 0.07 | 5.54 | 1.03 | 4.76–7.65 | 0.45 | 1.94 | 0.14 |
| Sex | Female | Male | ||
|---|---|---|---|---|
| Adjusted R2 | Significance | Adjusted R2 | Adjusted R2 | |
| ETE~TL | 0.7942 | <0.001 | 0.8631 | 0.7579 |
| SD~TL | 0.5175 | <0.001 | 0.6107 | 0.4698 |
| SB~TL | 0.8401 | 0 < x < 0.001 | 0.8928 | 0.8525 |
| SD~ETE | 0.689 | <0.001 | 0.6894 | 0.2957 |
| FS~ETE | 0.6234 | <0.001 | 0.5649 | 0.3827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészáros, D.; Tort, B.; Degollada, E. Tracking Fin Whale Morphology with Drone Photogrammetry: Growth Tendencies, Developmental Changes, and Sexual Dimorphism. Drones 2025, 9, 290. https://doi.org/10.3390/drones9040290
Mészáros D, Tort B, Degollada E. Tracking Fin Whale Morphology with Drone Photogrammetry: Growth Tendencies, Developmental Changes, and Sexual Dimorphism. Drones. 2025; 9(4):290. https://doi.org/10.3390/drones9040290
Chicago/Turabian StyleMészáros, Dorottya, Beatriu Tort, and Eduard Degollada. 2025. "Tracking Fin Whale Morphology with Drone Photogrammetry: Growth Tendencies, Developmental Changes, and Sexual Dimorphism" Drones 9, no. 4: 290. https://doi.org/10.3390/drones9040290
APA StyleMészáros, D., Tort, B., & Degollada, E. (2025). Tracking Fin Whale Morphology with Drone Photogrammetry: Growth Tendencies, Developmental Changes, and Sexual Dimorphism. Drones, 9(4), 290. https://doi.org/10.3390/drones9040290








