Two Down Syndrome Patients with Bilateral Profound Hearing Loss: Case Report and Literature Review
Abstract
:1. Case Introduction
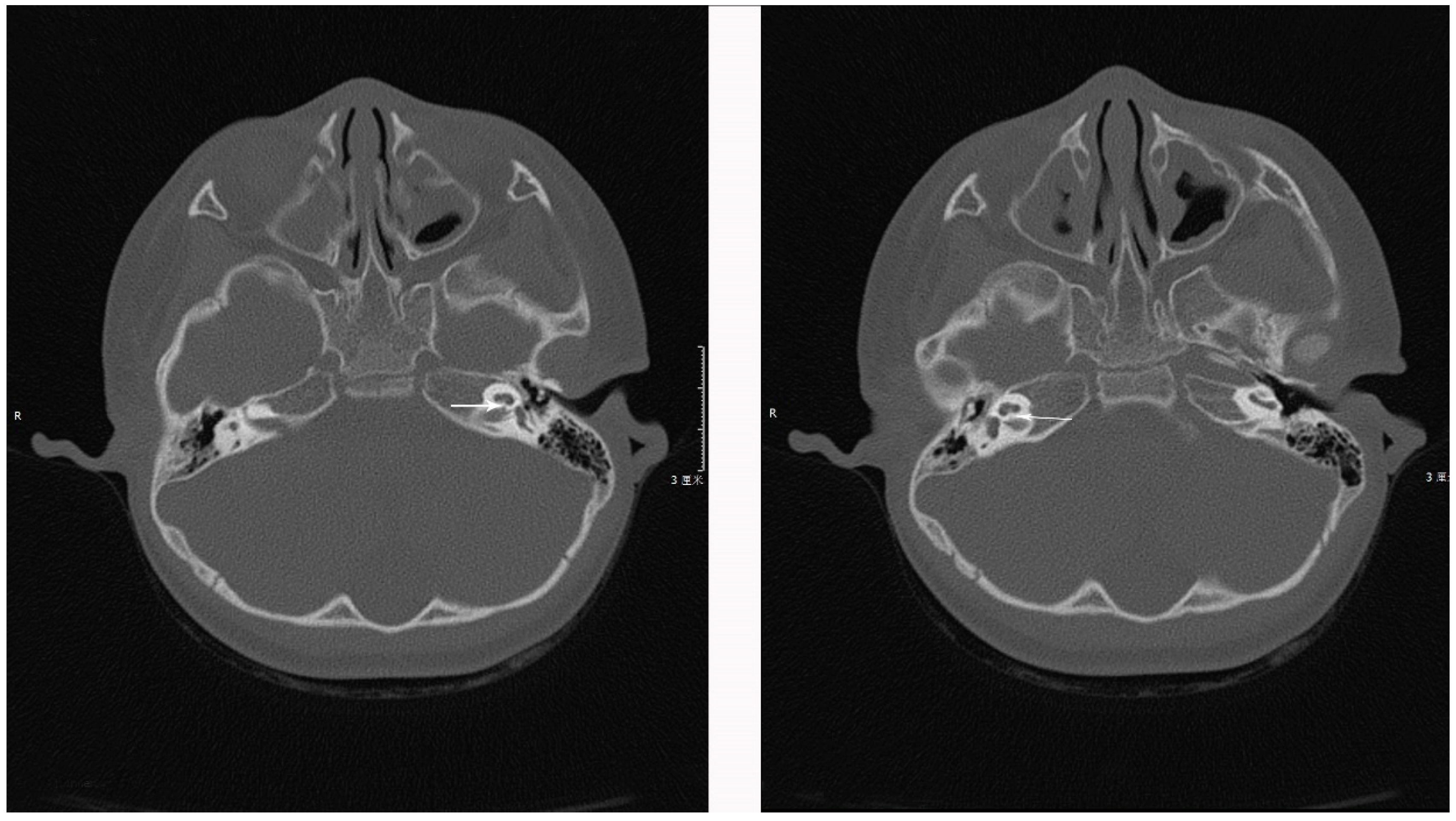
1.1. Case 1
1.2. Case 2
2. Discussion
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Informed Consent and Ethic Relative
References
- Blaser, S.; Propst, E.J.; Martin, D.; Feigenbaum, A.; James, A.L.; Shannon, P.; Papsin, B.C. Inner ear dysplasia is common in children with Down syndrome (trisomy 21). Laryngoscope 2006, 116, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Roizen, N.J.; Wolters, C.; Nicol, T.; Blondis, T.A. Hearing loss in children with Down syndrome. J. Pediatr. 1993, 123, S9–S12. [Google Scholar] [CrossRef]
- Shott, S.R. Down syndrome: Common otolaryngologic manifestations. Am. J. Med. Genet. Part C Semin. Med. Genet. 2006, 142, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Intrapiromkul, J.; Aygun, N.; Tunkel, D.E.; Carone, M.; Yousem, D.M. Inner ear anomalies seen on CT images in people with Down syndrome. Pediatr. Radiol. 2012, 42, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Saliba, I.; Sbeity, S.; El-Zir, E.; Yammine, F.G.; Noun, C.T.; Haddad, A. Down syndrome: An electrophysiological and radiological profile. Laryngoscope 2014, 124, E141–E147. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Ko, C.H.; Cheng, W.W. Prevalence and parental awareness of hearing loss in children with Down syndrome. Chin. Med. J. 2015, 128, 1091–1095. [Google Scholar] [PubMed]
- Austeng, M.E.; Akre, H.; Falkenberg, E.; Øverland, B.; Abdelnoor, M.; Kværner, K.J. Hearing level in children with Down syndrome at the age of eight. Res. Dev. Disabil. 2013, 34, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Park, A.H.; Wilson, M.A.; Stevens, P.T.; Harward, R.; Hohler, N. Identification of Hearing Loss in Pediatric Patients with Down Syndrome. Otolaryngol. Head Neck Surg. 2011, 146, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hans, P.S.; England, R.; Prowse, S.; Young, E.; Sheehan, P.Z. UK and Ireland experience of cochlear implants in children with Down Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Phelan, E.; Pal, R.; Henderson, L.; Green, K.M.J.; Bruce, I.A. The management of children with Down syndrome and profound hearing loss. Cochlear Implant. Int. 2015, 17, 52–57. [Google Scholar] [CrossRef] [PubMed]

| Score | Level | |
|---|---|---|
| Gross Motor | 29 | severe defects |
| Adaptability | 27 | severe defects |
| Sociability | 27 | severe defects |
| Fine motor | 40 | moderate defect |
| Language | 14 | extremely severe defect |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Yang, J.-M.; Zhao, M.; Qian, X.-Q.; Chi, F.-L. Two Down Syndrome Patients with Bilateral Profound Hearing Loss: Case Report and Literature Review. J. Otorhinolaryngol. Hear. Balance Med. 2018, 1, 8. https://doi.org/10.3390/ohbm1020008
Zheng Y, Yang J-M, Zhao M, Qian X-Q, Chi F-L. Two Down Syndrome Patients with Bilateral Profound Hearing Loss: Case Report and Literature Review. Journal of Otorhinolaryngology, Hearing and Balance Medicine. 2018; 1(2):8. https://doi.org/10.3390/ohbm1020008
Chicago/Turabian StyleZheng, Yu, Juan-Mei Yang, Meng Zhao, Xiao-Qing Qian, and Fang-Lu Chi. 2018. "Two Down Syndrome Patients with Bilateral Profound Hearing Loss: Case Report and Literature Review" Journal of Otorhinolaryngology, Hearing and Balance Medicine 1, no. 2: 8. https://doi.org/10.3390/ohbm1020008
APA StyleZheng, Y., Yang, J.-M., Zhao, M., Qian, X.-Q., & Chi, F.-L. (2018). Two Down Syndrome Patients with Bilateral Profound Hearing Loss: Case Report and Literature Review. Journal of Otorhinolaryngology, Hearing and Balance Medicine, 1(2), 8. https://doi.org/10.3390/ohbm1020008




