Static and Dynamic Performance of Wet Foam and Polymer-Enhanced Foam in the Presence of Heavy Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Foam Bulk (Static) Experiments
2.3. Foam Dynamic Experiments
3. Results and Discussion
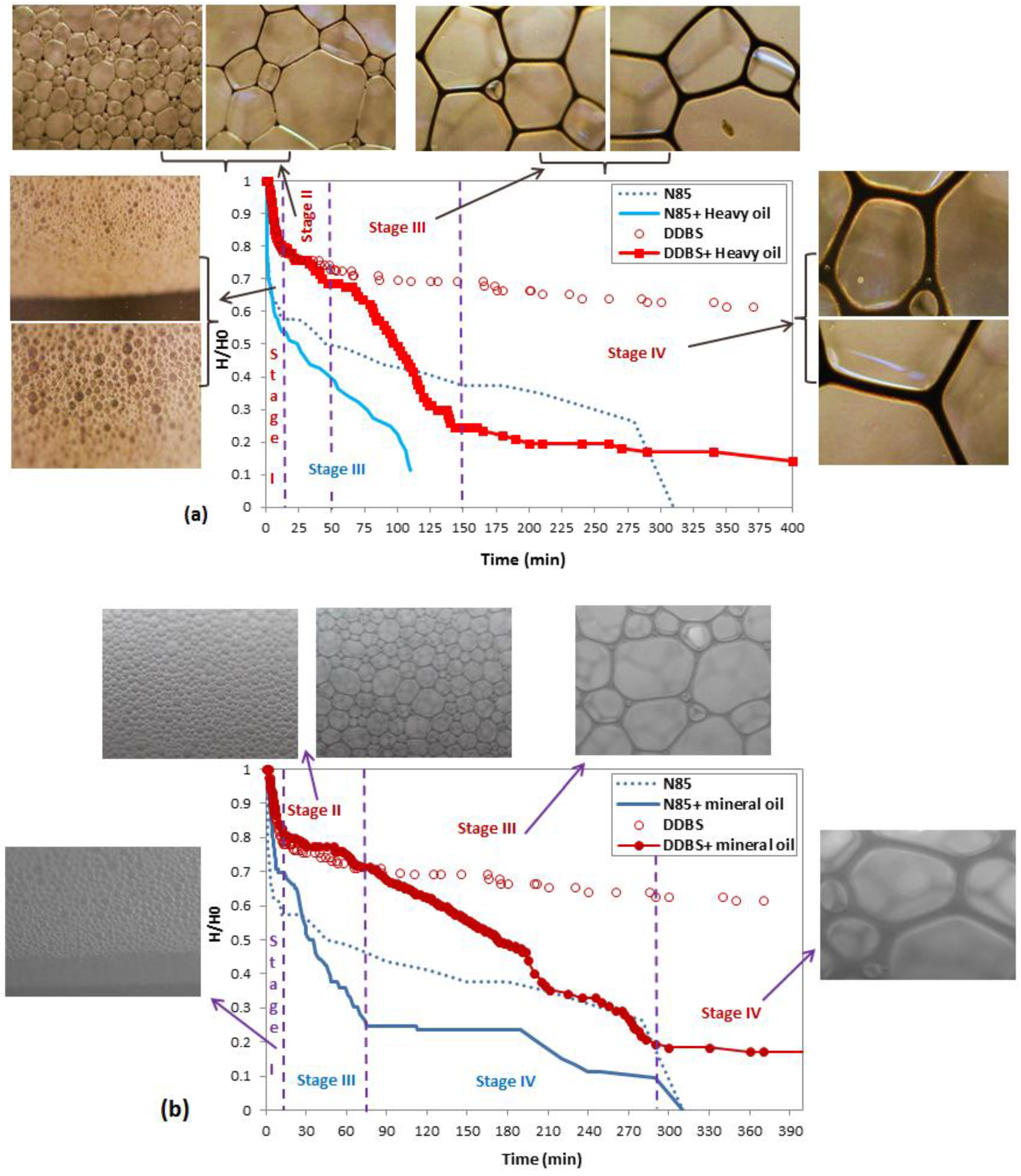
3.1. Static Performance of Foam and PEF in the Absence of Heavy Oil
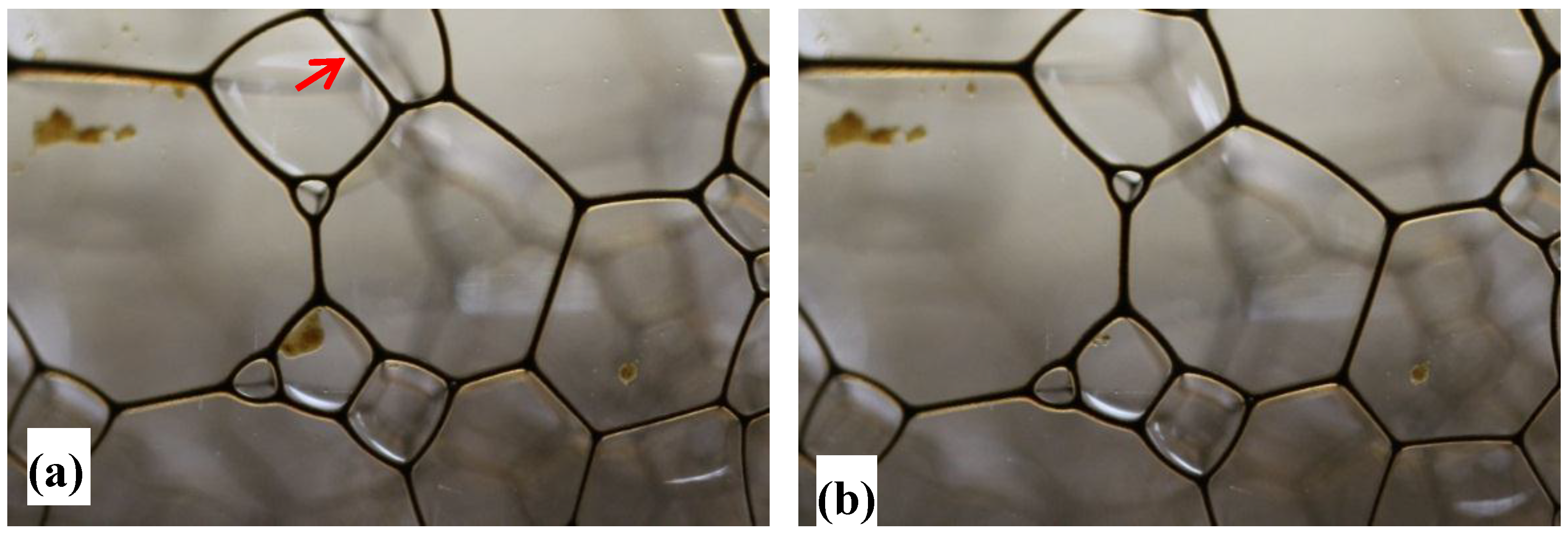
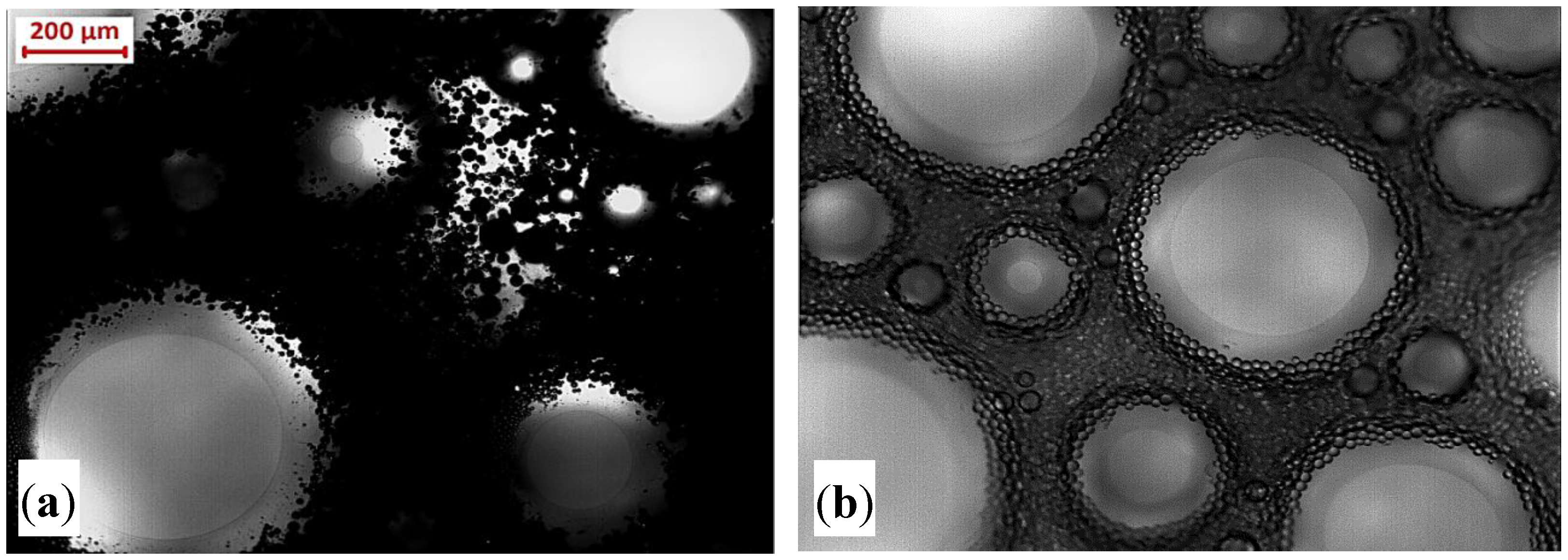
3.2. Static Performance of Foam and PEF in the Presence of Heavy Oil and Mineral Oil
More Insight into Impact of Oil on the Foam Stability
3.3. Dynamic Performance of Foam and PEF in the Absence of Heavy Oil
3.4. Dynamic Performance of Foam and PEF in the Presence of Heavy Oil
4. Conclusions
- Entering and spreading coefficient values are required for discussion of oil–foam interaction, but these may not be enough. Pseudoemulsion film stability should also be considered to support the results of foam stability. Some oils (e.g., mineral oil in this study) may increase the stability of a foam system, which is not expected from the entering and spreading coefficients.
- The porous media experiments have shown better performance of PEF in the presence of heavy oil compared to that of foam. The addition of polymer to the N85 foaming solution accelerates the foam generation and increases its stability in heavy-oil-saturated porous media. In our study, N85 PEF produced 98% of residual oil saturation, while this value was only about 57% for N85 foam.
- Overall, both static and dynamic performances of foam and PEF have shown their potential as displacing fluid for enhanced heavy oil recovery.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirasaki, G.J. The Steam-Foam Process. J. Pet. Technol. 1989, 41, 449–456. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Rossen, W.R. Applying Fractional-Flow Theory to Foam Processes at the Limiting Capillary Pressure. SPE Adv. Technol. 1995, 3, 154–162. [Google Scholar] [CrossRef]
- Smith, D.H. (Ed.) Surfactant-Based Mobility Control: Progress in Miscible-Flood Enhanced Oil Recovery; American Chemical Society Symposium Seri Number 373; American Chemical Society: Washington, DC, USA, 1988; p. 449. [Google Scholar]
- Lake, L.W. Enhanced Oil Recovery; Prentice Hall: Upper Saddle River, NJ, USA, 1989; p. 550. [Google Scholar]
- Zhang, Y.; Yue, X.; Dong, J.; Yu, L. New and Effective Foam Flooding To Recover Oil in Heterogeneous Reservoir. Presented at the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
- Ransohoff, T.C.; Radke, C.J. Mechanisms of Foam Generation in Glass-Bead Packs. SPE Reserv. Eng. 1988, 3, 573–585. [Google Scholar] [CrossRef]
- Rossen, W.R.; Gauglitz, P.A. Percolation Theory of Creation and Mobilization of Foam in Porous Media. AIChE J. 1990, 36, 1176–1188. [Google Scholar] [CrossRef]
- Tanzil, D.; Hirasaki, G.J.; Miller, C.A. Conditions for Foam Generation in Homogeneous Porous Media. Presented at the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar]
- Gauglitz, P.A.; Friedmann, F.; Kam, S.I.; Rossen, W.R. Foam generation in homogeneous porous media. J. Chem. Eng. Sci. 2002, 57, 4037–4052. [Google Scholar] [CrossRef]
- Mattews, C.S. Carbon Dioxide Flooding, in Enhanced Oil Recovery II: Processes and Operations; Donaldson, E.C., Chilingarian, G.V., Yen, T.F., Eds.; Elsevier Scientific Publishing Company: New York, NY, USA, 1989; p. 603. [Google Scholar]
- Heller, J.P. CO2 Foams in Enhanced Oil Recovery, in Foams: Fundamentals and Applications in the Petroleum Industry; Schramm, L.L., Ed.; ACS Advances in Chemistry Series, 3, No. 242; American Chemical Society: Washington, DC, USA, 1994; p. 201. [Google Scholar]
- Vikingstad, A.K.; Skauge, A.; Hoiland, H.; Aarra, M.G. Foam-oil interactions analyzed by static foam tests. Colloids Surf. A Physiochem. Eng. Asp. 2005, 260, 189–198. [Google Scholar] [CrossRef]
- Andrianov, A.; Farajzadeh, R.; Mahmoodi Nick, M.; Talanana, M.; Zitha, P.L.J. Immiscible foam for enhancing oil recovery: Bulk and porous media experiments. Ind. Eng. Chem. Res. 2012, 51, 2214–2226. [Google Scholar] [CrossRef]
- Schramm, L.L.; Novosad, J.J. The destabilization of foams for improved oil recovery by crude oils: Effect of the nature of the oil. J. Pet. Sci. Eng. 1992, 7, 77–90. [Google Scholar] [CrossRef]
- Koczo, K.; Lobo, L.; Wasan, D.T. Effect of oil on foam stability: Aqueous film stabilized by emulsions. J. Colloid Interface Sci. 1992, 150, 492–506. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Zitha, P.L.J. Investigation of Immiscible and Miscible Foam for Enhancing Oil Recovery. Ind. Eng. Chem. Res. 2010, 49, 1910–1919. [Google Scholar] [CrossRef]
- Simjoo, M.; Rezaei, T.; Andrianov, A.; Zitha, P.L.J. Foam stability in the presence of oil: Effect of surfactant concentration and oil type. Colloids Surf. A Physiochem. Eng. Asp. 2013, 438, 148–158. [Google Scholar] [CrossRef]
- Nikolov, A.D.; Wasan, D.T.; Huang, D.W.; Edwards, D.A. The Effect of Oil on Foam Stability: Mechanisms and Implications for Oil Displacement by Foam in Porous Media. Presented at the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 5–8 October 1986. [Google Scholar]
- Yan, W.; Miller, C.A.; Hirasaki, G.J. Foam sweep in fractures for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 348–359. [Google Scholar] [CrossRef]
- Flick, E.W. Industrial Surfactants, 2nd ed.; Noyes Publications: Park Ridge, NJ, USA, 1993; p. 547. [Google Scholar]
- Urban, D.G. How to Formulate and Compound Industrial Detergents; Book Surge Publishing: Charleston, CA, USA, 2003; p. 234. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 616. [Google Scholar]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids; Elsvier: Amsterdam, The Netherlands, 1948; p. 216. [Google Scholar]
- Marinova, K.G.; Dimitrova, L.M.; Marinov, R.Y.; Denkov, N.D.; Kingma, A. Impact of the Surfactant Structure on the Foaming/Defoaming Performance of Nonionic Block Copolymers in Na Caseinate Solutions. Bulg. J. Phys. 2012, 39, 53–64. [Google Scholar]
- Israelachvili, J.N. Intermolecular & Surface Forces, 3rd ed.; Academic Press: San Diego, CA, USA, 2010; p. 710. [Google Scholar]
- Schramm, L.L.; Wassmuth, F. Foams: Basic Principles in Foams: Fundamentals and Application in the Petroleum Industry; Schramm, L.L., Ed.; American Chemical Society: Washington, DC, USA, 1994; p. 201. [Google Scholar]
- Denkov, N.D. Mechanisms of Foam Destruction by Oil-Based Antifoams. Langmuir 2004, 20, 9463–9505. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lhermerout, R.; Rio, E.; Langevin, D.; Saint-Jalmes, A. Dual gas and oil dispersions in water: Production and stability of foamulsion. Soft Matter 2012, 8, 699–706. [Google Scholar] [CrossRef]
- Manlowe, D.J.; Radke, C.J. A Pore-Level Investigation of Foam/Oil Interactions in Porous Media. SPE Reserv. Eng. 1990, 5, 495–502. [Google Scholar] [CrossRef]
- Hadjiiski, A.; Denkov, N.D.; Tcholakova, S.; Ivanov, I.B. Role of entry barriers in the foam destruction by oil drops. In Adsorption and Aggregation of Surfactants in Solution; Mittal, K.L., Shah, D.O., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 465–498. [Google Scholar]
- Ross, S.; Suzin, Y. Measurement of Dynamic Foam Stability. Langmuir 1985, 1, 145–149. [Google Scholar] [CrossRef]
- Martinez, A.C.; Rio, E.; Delon, G.; Saint-Jalmes, A.; Langevin, D.; Binks, B.P. On the origin of the remarkable stability of aqueous foams stabilised by nanoparticles: Link with microscopic surface properties. Soft Matter 2008, 4, 1531–1535. [Google Scholar] [CrossRef]
- Li, R.F.; Yan, W.; Liu, S.; Hirasaki, G.; Miller, C.A. Foam Mobility Control for Surfactant Enhanced Oil Recovery. SPE J. 2010, 15, 934–948. [Google Scholar] [CrossRef]
- Kovscek, A.R. Reservoir Simulation of Foam Displacement Processes. Presented at the 7th UNITAR International Conference on Heavy Crude and Tar Sands, Beijing, China, 27–31 October 1998. [Google Scholar]
- Telmadarreie, A.; Trivedi, J.J. Post-Surfactant CO2 Foam/Polymer-Enhanced Foam Flooding for Heavy Oil Recovery: Pore-Scale Visualization in Fractured Micromodel. Transp. Porous Media 2016, 113, 717–733. [Google Scholar] [CrossRef]
- Telmadarreie, A.; Trivedi, J.J. New Insight on Carbonate-Heavy-Oil Recovery: Pore-Scale Mechanisms of Post-Solvent Carbon Dioxide Foam/Polymer-Enhanced-Foam Flooding. SPE J. 2016, 21, 1655–1668. [Google Scholar] [CrossRef]
- Heins, R.; Simjoo, M.; Zitha, P.L.J.; Rossen, W.R. Oil Relative Permeability during Enhanced Oil Recovery by Foam Flooding. Presented at the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014. SPE 170810. [Google Scholar]
- Farshbaf Zinati, F.; Farajzadeh, R.; Zitha, P.L.J. Modeling and CT scan Study of the Effect of Core Heterogeneity on Foam Flow for Acid Diversion. Presented at the European Formation Damage Conference, Scheveningen, The Netherlands, 30 May–1 June 2007. SPE 107790. [Google Scholar]
- Ettinger, R.A.; Radke, C.J. Influence of Texture on Steady Foam Flow in Berea Sandstone. SPE Reserv. Eng. 1992, 7, 83–90. [Google Scholar] [CrossRef]
- Zanganeh, M.N.; Kam, S.I.; LaForce, T.; Rossen, W.R. The Method of Characteristics Applied to Oil Displacement by Foam. SPE J. 2011, 16, 8–23. [Google Scholar] [CrossRef]
- Kovscek, A.R.; Bertin, H.J. Foam Mobility in Heterogeneous Porous Media. Transp. Porous Media 2003, 52, 17–35. [Google Scholar] [CrossRef]













| Experiment | Porous Media Length (cm) | Ø (%) | K (D) | Soi (%) | WF-RF (%) | Total RF (%) |
|---|---|---|---|---|---|---|
| AOS Foam | 24.5 | 36.28 | 37.75 | NA | NA | NA |
| 24.3 | 36.85 | 37.42 | 92.5 | 33 | 91.6 | |
| N85 Foam | 24.5 | 36.67 | 37.88 | NA | NA | NA |
| 24.4 | 37.22 | 37.72 | 93.4 | 33.1 | 57 | |
| N85 PEF | 24.4 | 37.22 | 38.19 | NA | NA | NA |
| 24.4 | 36.82 | 37.91 | 92.3 | 33 | 98 |
| Surfactant Solution (0.29 wt%) | Mineral Oil | Heavy Oil | ||||
|---|---|---|---|---|---|---|
| IFT (mN/m) | E | S | IFT (mN/m) | E | S | |
| N85 | 0.51 | 7.21 | 6.19 | 0.58 | 10.08 | 8.92 |
| DDBS | 0.33 | 3.03 | 2.37 | 0.54 | 6.04 | 4.96 |
| AOS | 0.50 | 4.7 | 3.7 | 1.10 | 7.7 | 5.7 |
| CTAB | 0.16 | 9.56 | 9.24 | 0.90 | 12.9 | 11.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telmadarreie, A.; Trivedi, J.J. Static and Dynamic Performance of Wet Foam and Polymer-Enhanced Foam in the Presence of Heavy Oil. Colloids Interfaces 2018, 2, 38. https://doi.org/10.3390/colloids2030038
Telmadarreie A, Trivedi JJ. Static and Dynamic Performance of Wet Foam and Polymer-Enhanced Foam in the Presence of Heavy Oil. Colloids and Interfaces. 2018; 2(3):38. https://doi.org/10.3390/colloids2030038
Chicago/Turabian StyleTelmadarreie, Ali, and Japan J. Trivedi. 2018. "Static and Dynamic Performance of Wet Foam and Polymer-Enhanced Foam in the Presence of Heavy Oil" Colloids and Interfaces 2, no. 3: 38. https://doi.org/10.3390/colloids2030038
APA StyleTelmadarreie, A., & Trivedi, J. J. (2018). Static and Dynamic Performance of Wet Foam and Polymer-Enhanced Foam in the Presence of Heavy Oil. Colloids and Interfaces, 2(3), 38. https://doi.org/10.3390/colloids2030038





