Hydrodynamic Parameters of Strelitzia Gum
Abstract
:1. Introduction
Intrinsic Viscosity and Hydrodynamic Parameters
2. Materials and Methods
2.1. Strelitzia Gum
2.2. Viscosity and Density
2.3. Dynamic Light Scattering
3. Results & Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Symbol | Name | Units |
| η | viscosity | poise |
| t | time drainage | s |
| t0 | solvent drainage time | s |
| ts | solution drainage time | s |
| A | viscometer constant | cm2/s2 |
| ρ | density | g/cm3 |
| ρ0 | solvent density | g/cm3 |
| ρs | solution density | g/cm3 |
| ηs | solution viscosity | poise |
| T | temperature | K |
| ηr | relative viscosity | dimensionless |
| ηsp | specific viscosity | dimensionless |
| c | solution concentration | g/cm3 |
| [η] | intrinsic viscosity | cm3/g |
| kH | Huggins constant | dimensionless |
| kk | Kraemer’s constant | dimensionless |
| kM | Martin’s constant | dimensionless |
| MWv | viscometer molecular weight | g/mol |
| a | “a” Mark–Houwink parameter | dimensionless |
| k | “k” Mark–Houwink parameter | cm3/g |
| NA | Avogadro’s number | 1/mol |
| RHv | viscometer hydrodynamic radius | cm or nm |
| f | solution friction coefficient | poise |
| f0 | solvent friction coefficient | poise |
| P | Perrin number | dimensionless |
| νa/b | Einstein viscosity increment | dimensionless |
| partial specific volume | cm3/g | |
| Vs | volume specific | cm3/g |
| δ | hydration value | g/g |
| D | diffusion coefficient | cm2/g |
| RHDLS | DLS hydrodynamic radius | cm or nm |
| MWDLS | DLS molecular weight | g/mol |
References and Notes
- Odriozola Azurmendi, J.M.; García, J.A. El Cultivo de la Strelitzia; Hojas Divulgadoras del Ministerio de Agricultura: Madrid, España, 1972. [Google Scholar]
- Kronestedt, E.; Walles, B. Anatomy of the Sirelirzia reginae flower (Strelitziaceae). Nord. J. Bot. 1986, 6, 307–320. [Google Scholar] [CrossRef]
- Bayogan, E.R.; Teeranuch Jaroenkit, V.; Paull, R.E. Postharvest life of Bird-of-Paradise inflorescences. Posth. Biol. Technol. 2008, 48, 259–263. [Google Scholar] [CrossRef]
- Ribeiro da Silva Vieira, M.; Cleuma de Medeiros, D.; Nepomuceno Costa, P.; Guimarães Santos, C.M.; de Alencar Paes, R.; de Sousa Fernandez, L.M.; de Oliveira, N.G.; Flavio Silva, A.A. Effect of refrigeration on post-harvest flowers. Afr. J. Biotechnol. 2012, 11, 13065–13068. [Google Scholar] [CrossRef]
- Barbosa, J.G.; Mantovani Alvarenga, E.; Cunha Fernandes Dos Santos Dias, D.; Nunes Vieira, A. Effect of Acid Scarification and Different Temperatures on Physiological Quality of Strelitzia reginae Seeds. Parte da Dissertação de Mestrado do último autor, apresentada à Universidade Federal de Viçosa (UFV). Rev. Bras. Sementes 2005, 27, 71–77. [Google Scholar] [CrossRef]
- Mele, E.; Girardo, S.; Pisignano, D. Strelitzia reginae Leaf as a Natural Template for Anisotropic Wetting and Superhydrophobicity. Langmuir 2012, 28, 5312–5317. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.W. The stigmatic exúdate of Strelitzia. Phyton. Buenos Aires 1970, 27, 47–53. [Google Scholar]
- Kronestedt-Robards, E.C.; Greger, M.; Robards, A.W. The nectar of the Strelitzia reginae flower. Physiol. Plant. 1989, 77, 341–346. [Google Scholar] [CrossRef]
- Kronestedt, E.C.; Robards, A.W. Sugar Secretion from the Nectary of Strelitzia: An Ultrastructural and Physiological Study. Protoplasma 1987, 137, 168–182. [Google Scholar] [CrossRef]
- Jaroenkit, T.; Chen, N.J.; Paull, R.E. Nectar secretion, mucilage production and mold growth on bird-of-paradise inflorescences. Posth. Biol. Technol. 2008, 49, 431–435. [Google Scholar] [CrossRef]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Martin, A.F. Abstr. 103rd Am. Chem. Soc. Meeting, Memphis, TN, USA, April 20–24, 1942; p. 1-c ACS.
- Tanglertpaibul, T.; Rao, M.A. Intrinsic viscosity of tomato serum as affected by methods of determination and methods of processing concentrates. J. Food Sci. 1987, 52, 1642–1688. [Google Scholar] [CrossRef]
- Higiro, J.; Herald, T.J.; Alavi, S. Rheological study of xanthan and locust bean gum interaction in dilute solution. Food Res. Int. 2006, 39, 165–175. [Google Scholar] [CrossRef]
- Higiro, J.; Herald, T.J.; Alavi, S.; Bean, S. Rheological study of xanthan and locust bean gum interaction in dilute solution: Effect of salt. Food Res. Int. 2007, 40, 435–447. [Google Scholar] [CrossRef]
- Mark, H. Der Feste Körper; Sänger, R., Ed.; Hirzel, Switzerland; Leipzig, Germany, 1938; pp. 65–104. [Google Scholar]
- Houwink, R. Zusammenhang zwischen viscosimetrisch und osmotisch bestimm-ten polymerisationsgraden bei hochpolymeren. J. Prakt. Chem. 1940, 157, 15. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Takara, A.; Acosta, A. Hydrodynamic properties of tragacanthin. Study of temperature influence. J. Argent. Chem. Soc. 2013, 100, 25–34. [Google Scholar]
- Masuelli, M.A. Mark-Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. J. Pol. Biopol. Phys. Chem. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Masuelli, M.A. Viscometric study of pectin. Effect of temperature on the hydrodynamic properties. Int. J. Biol. Macromol. 2011, 48, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, M.A.; Sansone, M.G. Hydrodynamic properties of Gelatine. Studies from intrinsic viscosity measurements. In Products and Applications of Biopolymers; Verbeek, C.J.R., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 5; ISBN 978-953-51-0226-7. [Google Scholar]
- Masuelli, M.A. Hydrodynamic Properties of Whole Arabic Gum. Am. J. Food Sci. Technol. 2013, 1, 60–66. [Google Scholar]
- Harding, S.E. The Viscosity Intrinsic of Biological Macromolecules. Progress in Measurement, Interpretation and Application to Structure in Dilute Solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef]
- Curvale, R.; Masuelli, M.; Perez Padilla, A. Intrinsic viscosity of bovine serum albumin conformers. Int. J. Biol. Macromol. 2008, 42, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, M.A. Study of Bovine Serum Albumin Solubility in Aqueous Solutions by Intrinsic Viscosity Measurements. Adv. Phys. Chem. 2013, 2013, 360239. [Google Scholar] [CrossRef] [Green Version]
- Sartor, M. Dynamic Light Scattering; University of California: San Diego, CA, USA, 2003; pp. 2–21. [Google Scholar]
- Rojas, R.; Giacomelli, C. Effect of structure and bonding on the interfacial properties and the reactivity of layered double hydroxides and Zn hydroxide salts. Eng. Asp. 2013, 419, 166–173. [Google Scholar] [CrossRef]
- Zanon, M.; Masuelli, M. Purification and Characterization of Alcayota Gum. Exp. Rev. Biopolym. Res. 2018, 2, 105. [Google Scholar]
- Kuhn, W.; Kuhn, H. Die abhängigkeit der viskosität vom strömungsgefälle bei hochverdünnten suspensionen und lösungen. Helv. Chim. Acta 1945, 28, 97–127. [Google Scholar] [CrossRef]
- Sakurada, I. Kasen Koenshu 1940, 5, 33–44.
- Sakurada, I. Kasen Koenshu 1941, 6, 177–184.
- Fee, M.; Errington, N.; Jumel, K.; Illum, L.; Smith, A.; Harding, S.E. Correlation of SEC/MALLS with ultracentrifuge and viscometric data for chitosans. Eur. Biophys. J. 2003, 32, 457–464. [Google Scholar] [CrossRef] [PubMed]


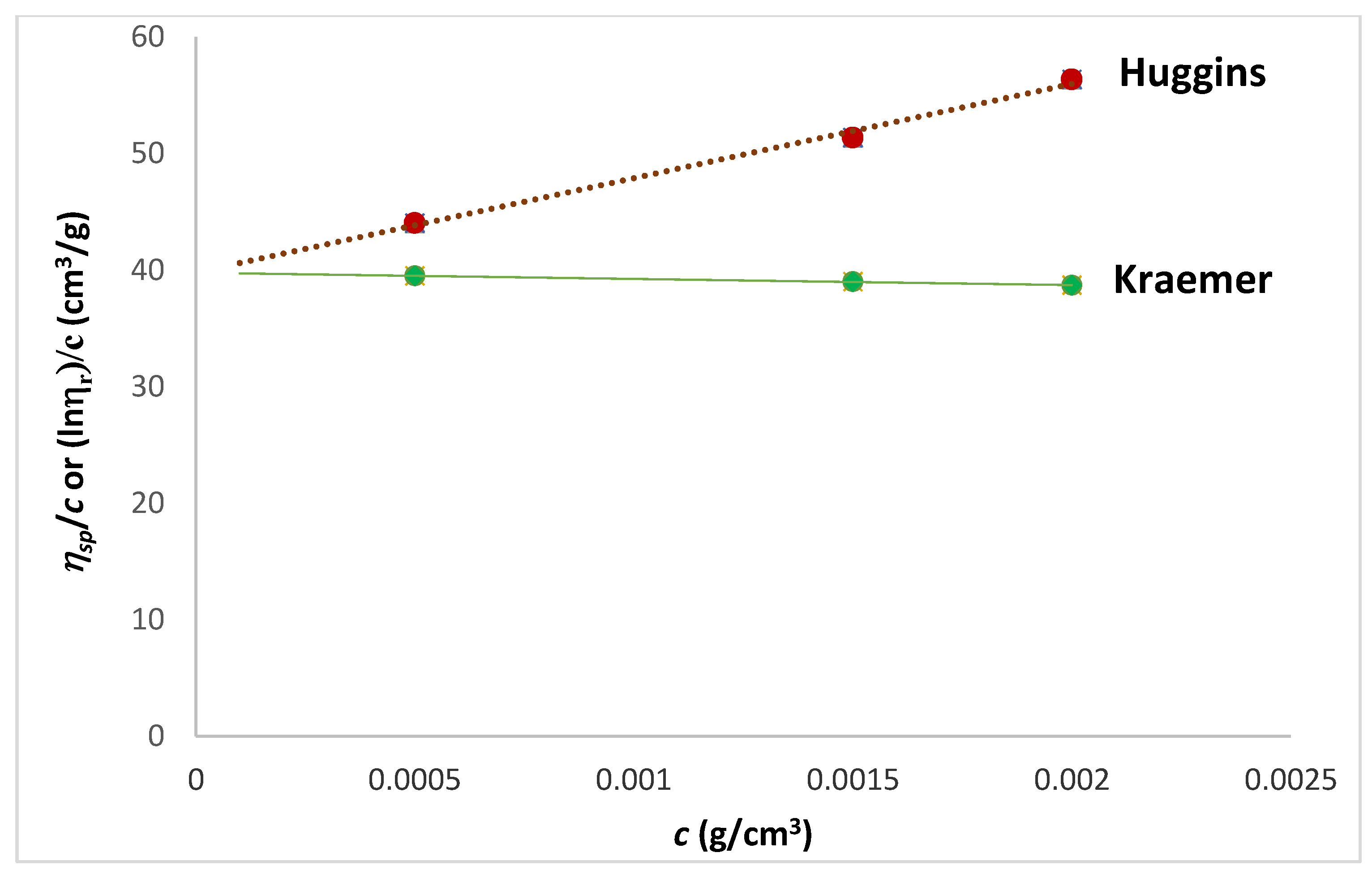

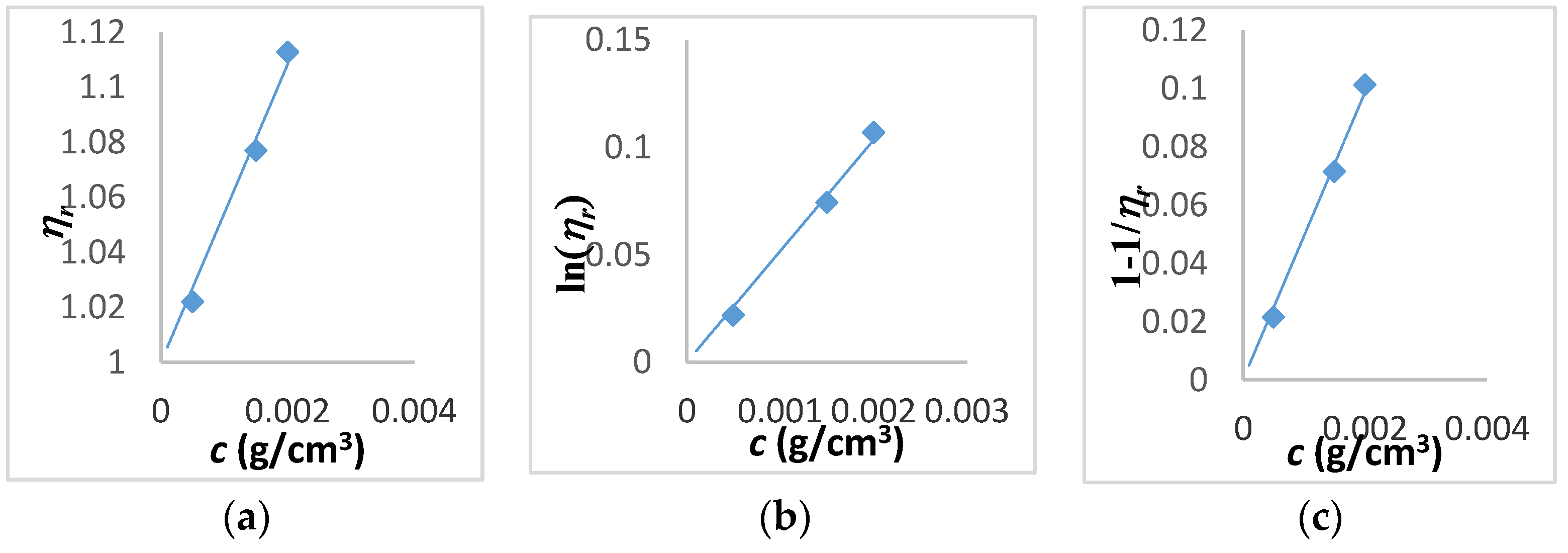


| Tanglertpeibul & Rao | |||||||
|---|---|---|---|---|---|---|---|
| Huggins | Kraemer | H-K Media * | Martin | a | b | c | |
| [η] (cm3/g) | 38 ± 0.3 | 39 ± 1 | 39 ± 0.5 | 40 ± 1.8 | 54 ± 21 | 51 ± 17 | 49 ± 13 |
| R2 | 0.9934 | 0.9978 | - | 0.9977 | 0.9850 | 0.9893 | 0.9930 |
| ER% | - | 2.57 | 1.28 | 4.44 | 39.66 | 33.24 | 27.23 |
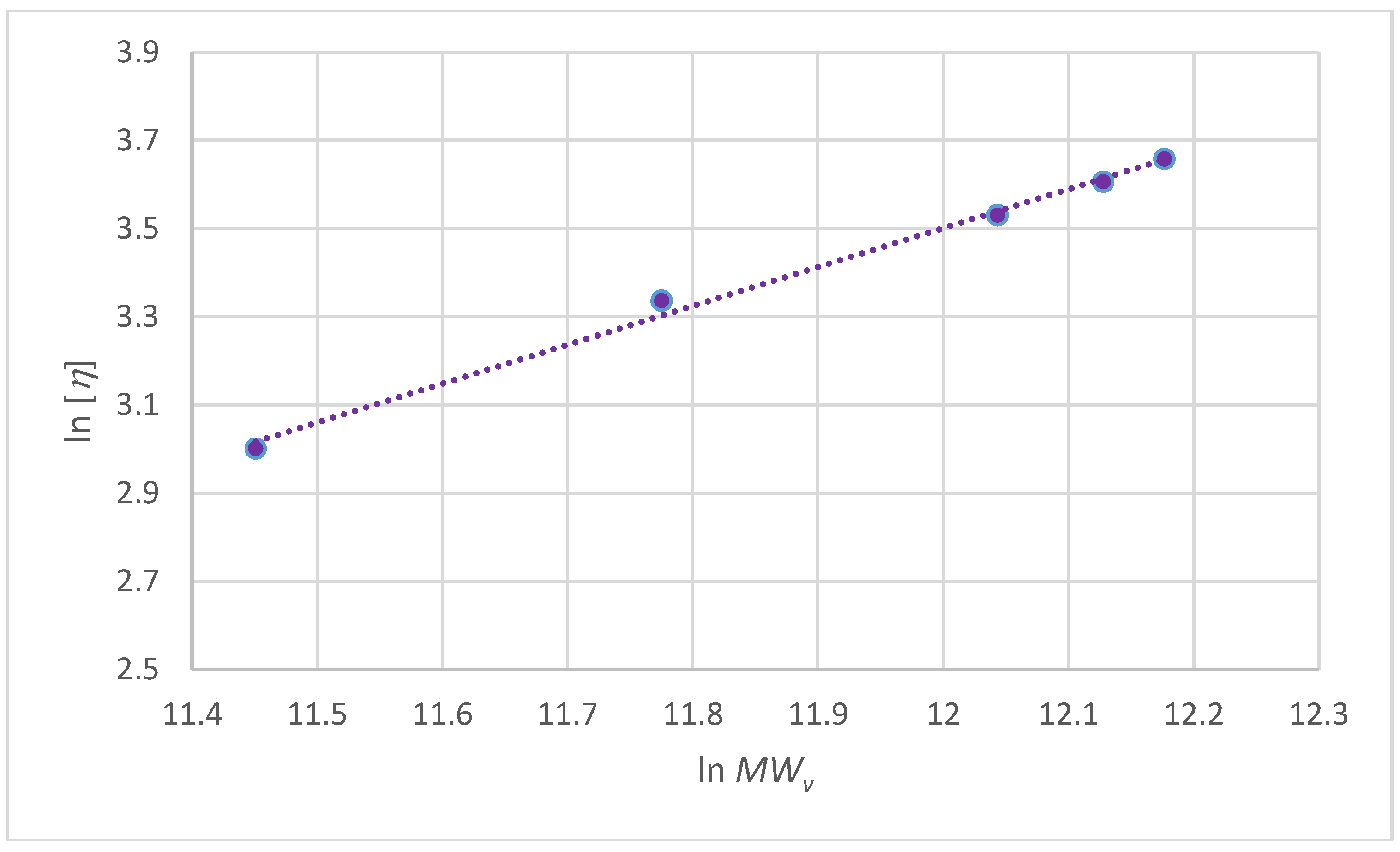
| kH | (cm3/g) | δ (gH2O/g) | RHv (nm) | MWv (g/mol) | νa/b | P | k (cm3/g) | a |
|---|---|---|---|---|---|---|---|---|
| 5.4 ± 0.25 | 0.14 ± 0.03 | 445 ± 34 | 20 ± 1 | 200,000 | 11.4 ± 0.15 | 1.6 ± 0.24 | 0.00124 | 0.8500 |
| c (M) | D (cm2/s) × 107 | MWDLS (g/mol) | [η] (cm3/g) | MWv (g/mol) |
|---|---|---|---|---|
| 0 | 1.26 ± 0.13 | 230,000 | 38 ± 0.3 | 200,000 |
| 0.005 | 1.41 ± 0.27 | 205,000 | 36 ± 0.8 | 185,000 |
| 0.01 | 1.61 ± 0.24 | 180,000 | 34 ± 0.2 | 170,000 |
| 0.025 | 1.94 ± 0.07 | 130,000 | 28 ± 0.1 | 130,000 |
| 0.05 | 2.55 ± 0.16 | 90,000 | 20 ± 0.2 | 94,000 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuelli, M.A. Hydrodynamic Parameters of Strelitzia Gum. Colloids Interfaces 2018, 2, 45. https://doi.org/10.3390/colloids2040045
Masuelli MA. Hydrodynamic Parameters of Strelitzia Gum. Colloids and Interfaces. 2018; 2(4):45. https://doi.org/10.3390/colloids2040045
Chicago/Turabian StyleMasuelli, Martin A. 2018. "Hydrodynamic Parameters of Strelitzia Gum" Colloids and Interfaces 2, no. 4: 45. https://doi.org/10.3390/colloids2040045
APA StyleMasuelli, M. A. (2018). Hydrodynamic Parameters of Strelitzia Gum. Colloids and Interfaces, 2(4), 45. https://doi.org/10.3390/colloids2040045





