Development and Characterization of Electrospun Nanostructures Using Polyethylene Oxide: Potential Means for Incorporation of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions Preparation for Electrospinning
2.2. Electrospinning Process
2.3. Experimental Design
2.4. Characterization of Solutions and Nanostructures
2.4.1. Determination of Solution Properties
2.4.2. Characterization of Electrospun Samples
2.5. Incorporation of Jussara Pulp as a Source of Anthocyanins
2.5.1. Solutions Preparation for Electrospinning
2.5.2. Electrospinning Process
2.5.3. Characterization of Electrospun Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. Electrospinning Process
3.2. Experimental Design
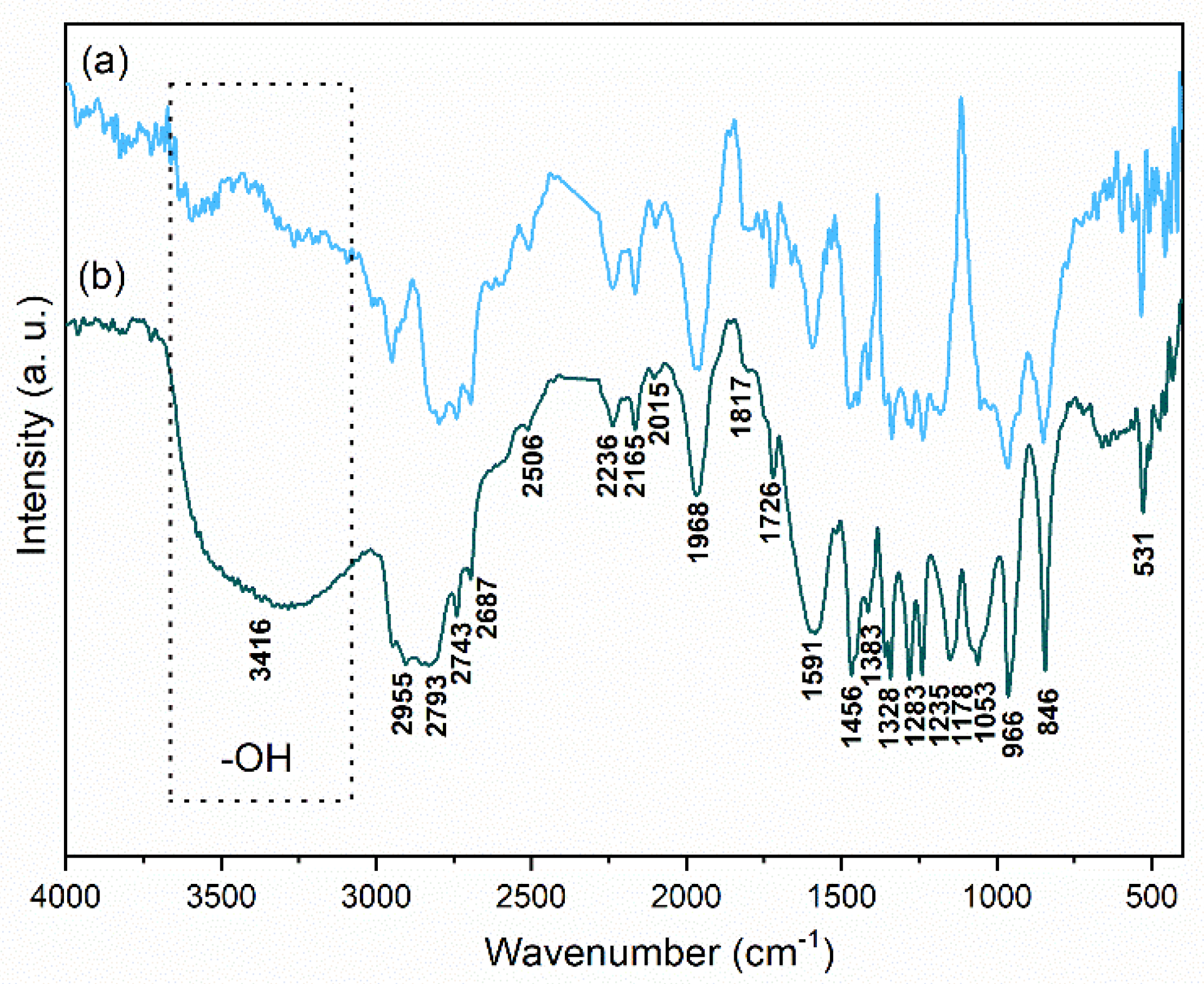
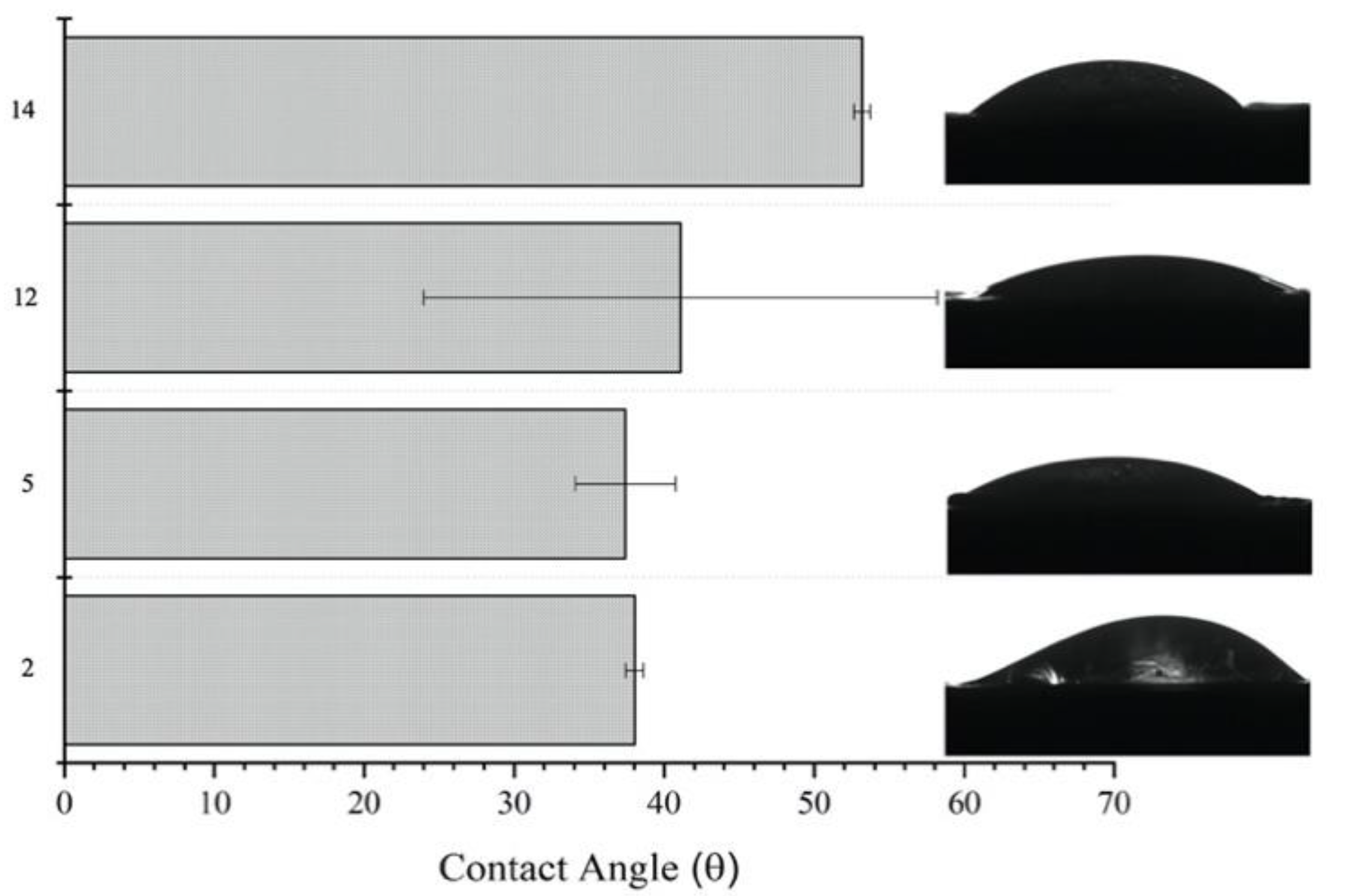
3.3. Characterization of Solutions and Nanostructures
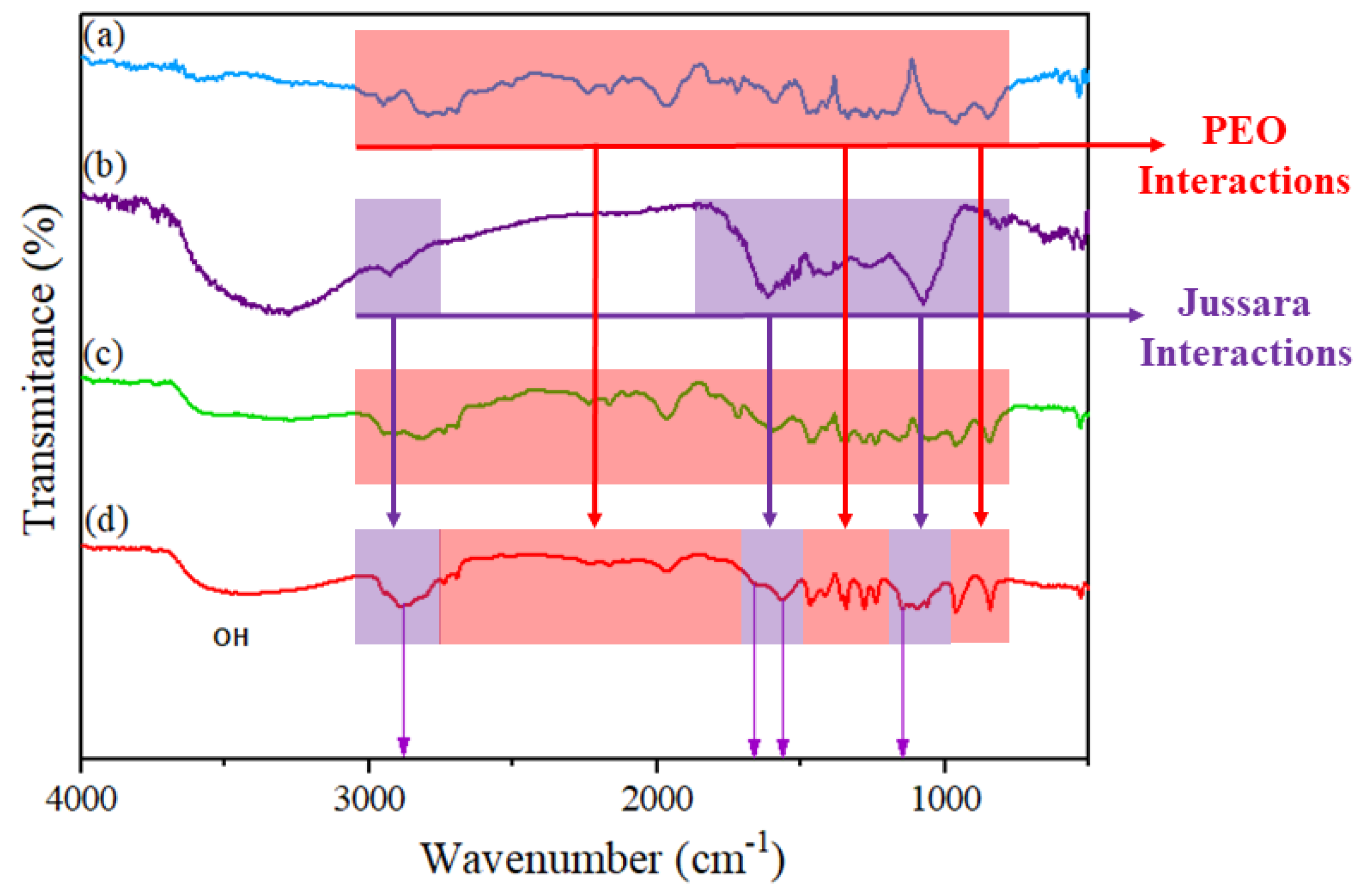
3.4. Incorporation of Jussara Pulp as a Source of Anthocyanins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyselica, R.; Enikov, E.T.; Polyvas, P.; Anton, R. Electrostatic focusing of electrospun Polymer(PEO) nanofibers. J. Electrostat. 2018, 94, 21–29. [Google Scholar] [CrossRef]
- Alehosseini, A.; Ghorani, B.; Sarabi-Jamab, M.; Tucker, N. Principles of electrospraying: A new approach in protection of bioactive compounds in foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2346–2363. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; ISBN 978-981-256-415-3. [Google Scholar]
- Khajavi, R.; Abbasipour, M. Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci. Iran. 2012, 19, 2029–2034. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.G.; Stillings, C.; Dersch, R.; Rudisile, M.; Pranke, P.; Costa, J.A.V.; Wendorff, J. Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, C.; Li, X.Y.; Zhu, J.Y.; Yu, D.G. Medicated Nanofibers Fabricated Using NaCl Solutions as Shell Fluids in Modified Coaxial Electrospinning. J. Nanomater. 2016. [Google Scholar] [CrossRef]
- Chayad, F.; Jabur, A.; Jalal, N. Effect of NaCl Solution Addition on Improving Some of the Physical Properties of Nylon 6 Solutions used for Electro Spinning Purpose. Eng. Technol. J. 2016, 34, 1265–1274. [Google Scholar]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials (Basel) 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2014, 45, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordillo, B.; Sigurdson, G.T.; Lao, F.; González-Miret, M.L.; Heredia, F.J.; Giusti, M.M. Assessment of the color modulation and stability of naturally copigmented anthocyanin-grape colorants with different levels of purification. Food Res. Int. 2018, 106, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Domínguez-Hernández, C.R.; García-Alvarado, M.A.; García-Galindo, H.S.; Salgado-Cervantes, M.A.; Beristáin, C.I. Stability, antioxidant activity and bioavailability of nano-emulsified astaxanthin. Rev. Mex. Ing. Quim. 2016, 15, 457–468. [Google Scholar]
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Biluca, F.C.; Gonzaga, L.V.; da Borges, G.S.C.; Vitali, L.; Micke, G.A.; de Gois, J.S.; de Almeida, T.S.; Borges, D.L.G.; Miller, P.R.M.; et al. Bioaccessibility of bioactive compounds and antioxidant potential of juçara fruits (Euterpe edulis Martius) subjected to in vitro gastrointestinal digestion. Food Chem. 2017, 228, 447–454. [Google Scholar] [CrossRef]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V.V. Bioavailability and biological effects of bioactive compounds extracted with natural deep eutectic solvents and ionic liquids: Advantages over conventional organic solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Braga, A.R.C.; da Figueira, F.S.; da Silveira, J.T.; de Morais, M.G.; Costa, J.A.V.; Kalil, S.J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Da Figueira, F.S.; Gettens, J.G.; Costa, J.A.V.; de Morais, M.G.; Moraes, C.C.; Kalil, S.J. Production of nanofibers containing the bioactive compound C-phycocyanin. J. Nanosci. Nanotechnol. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; de Almeida, A.C.A.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate and phenolic compounds microalgae electrospun nanofibers: A novel nanomaterial with antibacterial activity. Int. J. Biol. Macromol. 2018, 113, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- de Farias, B.S.; Vidal, É.M.; Ribeiro, N.T.; da Silveira, N.; da Silva Vaz, B.; Kuntzler, S.G.; de Morais, M.G.; Cadaval, T.R.S.A.; de Almeida Pinto, L.A.; de Farias, B.S.; et al. Electrospun chitosan/poly(ethylene oxide) nanofibers applied for the removal of glycerol impurities from biodiesel production by biosorption. J. Mol. Liq. 2018, 268, 365–370. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Braga, A.R.C.; de Mesquita, L.M.S.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Alberti, A.; Zielinski, A.A.F.; Couto, M.; Judacewski, P.; Mafra, L.I.; Nogueira, A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J. Food Sci. Technol. 2017, 54, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: A meta-analytic investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef]
- Jamar, G.; Santamarina, A.B.; Mennitti, L.V.; de Cássia Cesar, H.; Oyama, L.M.; de Rosso, V.V.; Pisani, L.P. Bifidobacterium spp. reshaping in the gut microbiota by low dose of juçara supplementation and hypothalamic insulin resistance in Wistar rats. J. Funct. Foods 2018, 46, 212–219. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; Ribeiro, D.A.; Cardoso, C.M.; De Rosso, V.V.; Oyama, L.M.; Pisani, L.P. Polyphenols-Rich Fruit (Euterpe edulis Mart.) Prevents Peripheral Inflammatory Pathway Activation by the Short-Term High-Fat Diet. Molecules 2019, 24, 1655. [Google Scholar] [CrossRef] [Green Version]
- Oyama, L.M.; Silva, F.P.; Carnier, J.; de Miranda, D.A.; Santamarina, A.B.; Ribeiro, E.B.; Oller do Nascimento, C.M.; De Rosso, V.V. Juçara pulp supplementation improves glucose tolerance in mice. Diabetol. Metab. Syndr. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, C.A.; Oyama, L.M.; de Oliveira, J.L.; Garcia, M.C.; De Rosso, V.V.; Amigo, L.S.M.; do Nascimento, C.M.O.; Pisani, L.P. Jussara (Euterpe edulis Mart.) Supplementation during Pregnancy and Lactation Modulates the Gene and Protein Expression of Inflammation Biomarkers Induced by trans-Fatty Acids in the Colon of Offspring. Mediators Inflamm. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; De Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian Atlantic forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; dos Ramos, S.P.; Pereira, C.F.; Lemes, A.C.; De Rosso, V.V.; Braga, A.R. Cavalcante Overcoming restrictions of bioactive compounds biological effects in food using nanometer-sized structures. Food Hydrocoll. 2020. In press. [Google Scholar] [CrossRef]
- Moyaho, A.; Beristain-Castillo, E. Experimental Design: Basic Concepts. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: Oxford, UK, 2019; pp. 471–479. ISBN 978-0-12-813252-4. [Google Scholar]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G., Jr. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wang, L.; Sun, D. Pattern deposition of electrosprayed polymer nanoparticles. In Proceedings of the 2007 7th IEEE Conference on Nanotechnology (IEEE NANO), Hong Kong, China, 2–5 August 2007; pp. 271–274. [Google Scholar]
- Morota, K.; Matsumoto, H.; Mizukoshi, T.; Konosu, Y.; Minagawa, M.; Tanioka, A.; Yamagata, Y.; Inoue, K. Poly(ethylene oxide) thin films produced by electrospray deposition: Morphology control and additive effects of alcohols on nanostructure. J. Colloid Interface Sci. 2004, 279, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, D.A.; Costa, J.A.V.; de Morais, M.G. A novel nanocomposite for food packaging developed by electrospinning and electrospraying. Food Packag. Shelf Life 2019, 20, 100314. [Google Scholar] [CrossRef]
- Seo, H.B.; Kim, S.S.; Lee, H.Y.; Jung, K.H. High-level Production of Ethanol during Fed-batch Ethanol Fermentation with a Controlled Aeration Rate and Non-sterile Glucose Powder Feeding of Saccharomyces cerevisiae. Biotechnol. Bioprocess Eng. 2009, 14, 591–598. [Google Scholar] [CrossRef]
- Da Vaz, B.S.; da Mastrantonio, D.J.S.; Costa, J.A.V.; de Morais, M.G.; de Morais, M.G. Green alga cultivation with nanofibers as physical adsorbents of carbon dioxide: Evaluation of gas biofixation and macromolecule production. Bioresour. Technol. 2019, 287, 121406. [Google Scholar] [CrossRef]
- Alves, F.G.; Maugeri, F.; Burkert, J.F.M.; Kalil, S.J. Maximization of b-galactosidase production: A simultaneous investigation of agitation and aeration effects. Appl. Biochem. Biotechnol. 2010, 160, 1528–1539. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Gomes, P.A.; Kalil, S.J. Formulation of Culture Medium with Agroindustrial Waste for β-Galactosidase Production from Kluyveromyces marxianus ATCC 16045. Food Bioprocess Technol. 2012, 5, 1653–1663. [Google Scholar] [CrossRef]
- Machado, J.R.; Behling, M.B.; Braga, A.R.C.; Kalil, S.J. β-Galactosidase production using glycerol and byproducts: Whey and residual glycerin. Biocatal. Biotransform. 2015, 33, 208–215. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Silva, M.F.; Vladimir Oliveira, J.; Treichel, H.; Kalil, S.J. Effect of compressed fluids treatment on β-galactosidase activity and stability. Bioprocess Biosyst. Eng. 2012, 35, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Gondaliya, N.; Kanchan, D.K.; Sharma, P.; Joge, P. Structural and conductivity studies of poly (ethylene oxide)–silver triflate polymer electrolyte system. Mater. Sci. Appl. 2011, 2, 1639. [Google Scholar] [CrossRef] [Green Version]
- Isik, B.S.; Altay, F.; Capanoglu, E. The uniaxial and coaxial encapsulations of sour cherry (Prunus cerasus L.) concentrate by electrospinning and their in vitro bioaccessibility. Food Chem. 2018, 265, 260–273. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, C.; Wu, H.; Chang, F. Hydrogen bonding effect on the poly (ethylene oxide), phenolic resin, and lithium perchlorate–based solid-state electrolyte. J. Appl. Polym. Sci. 2004, 91, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.L.; Rabolt, J.F. Self-assembled thin-film blends by polymer codeposition: Poly (ethylene oxide) and poly (methyl methacrylate). Macromolecules 1996, 29, 2543–2547. [Google Scholar] [CrossRef]
- Li, X.; Hsu, S.L. An analysis of the crystallization behavior of poly (ethylene oxide)/poly (methyl methacrylate) blends by spectroscopic and calorimetric techniques. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1331–1342. [Google Scholar] [CrossRef]
- Rajendran, S.; Kannan, R.; Mahendran, O. Ionic conductivity studies in poly (methylmethacrylate)–polyethlene oxide hybrid polymer electrolytes with lithium salts. J. Power Sources 2001, 96, 406–410. [Google Scholar] [CrossRef]
- Xu, Y.; Zou, L.; Lu, H.; Kang, T. Effect of different solvent systems on PHBV/PEO electrospun fibers. RSC Adv. 2017, 7, 4000–4010. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, T.; Tadokoro, H.; Murahashi, S. Normal Vibrations of the Polymer Molecules of Helical Conformation. IV. Polyethylene Oxide and Polyethylene-d 4 Oxide. J. Chem. Phys. 1964, 41, 2902–2911. [Google Scholar] [CrossRef]
- Yokoyama, M.; Ishihara, H.; Iwamoto, R.; Tadokoro, H. Structure of poly (ethylene oxide) complexes. III. Poly (ethylene oxide)-mercuric chloride complex. Type II. Macromolecules 1969, 2, 184–192. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Yusof, A.P.M. The use of Fourier transform infrared (FTIR) spectroscopy and artificial neural networks (ANNs) to assess wine quality. Mod. Chem. Appl. 2013. [Google Scholar] [CrossRef] [Green Version]
- Vasincu, A.; Paulsen, B.; Diallo, D.; Vasincu, I.; Aprotosoaie, A.; Bild, V.; Charalambous, C.; Constantinou, A.; Miron, A.; Gavrilescu, C. Vernonia kotschyana roots: Therapeutic potential via antioxidant activity. Molecules 2014, 19, 19114–19136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; Silva, E.C.; Oliveira, J.M., Jr.; Vila, M.M.D.C.; Tubino, M. Physicochemical characterization of a crude anthocyanin extract from the fruits of jussara (Euterpe edulis Martius): Potential for food and pharmaceutical applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.-J.; Chen, T.-L.; Chen, C.-H.; Cho, K.-C.; Lai, X.-R. Characterization of natural dye extracted from wormwood and purple cabbage for dye-sensitized solar cells. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef]
- Muchuweti, M.; Chikwambi, Z. Isolation and identification of anthocyanins in the fruit peels of starkrimson and marx red bartlett common pear cultivars and their bud mutants. Am. J. Food Technol. 2008, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vashisth, P.; Sharma, M.; Nikhil, K.; Singh, H.; Panwar, R.; Pruthi, P.A.; Pruthi, V. Antiproliferative activity of ferulic acid-encapsulated electrospun PLGA/PEO nanofibers against MCF-7 human breast carcinoma cells. 3 Biotech 2015, 5, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Pezeshki, A.; Ghanbarzadeh, B.; Mohammadi, M.; Fathollahi, I.; Hamishehkar, H. Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv. Pharm. Bull. 2014, 4, 563–568. [Google Scholar]
- Li, Y.; Yang, H.; Cao, F.; Zhao, X.; Wang, J. The stability of C-phycocyanin doped silica biomaterials in UV irradiation. J. Wuhan Univ. Technol. Sci. Ed. 2009, 24, 852. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]



| Samples | PEO (%) (w/v) | NaCl (%) (w/v) | TCD (cm) | Voltage (kV) | Feeding Rate (μ·h−1) | pH | Conductivity (μS·cm−1) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.0 | 0.0 | 10.0 | 10.0 | 3000 | 8.76 | 171.8 | −0.3 |
| 2 | 8.0 | 0.0 | 10.0 | 10.0 | 150 | 8.64 | 128.8 | −0.3 |
| 3 | 6.0 | 2.5 | 10.0 | 10.0 | 150 | 8.26 | 27,500.0 | −0.9 |
| 4 | 8.0 | 2.5 | 10.0 | 10.0 | 3000 | 8.37 | 23,900.0 | −0.9 |
| 5 | 6.0 | 0.0 | 15.0 | 10.0 | 150 | 8.76 | 171.8 | −0.3 |
| 6 | 8.0 | 0.0 | 15.0 | 10.0 | 3000 | 8.64 | 128.8 | −0.3 |
| 7 | 6.0 | 2.5 | 15.0 | 10.0 | 3000 | 8.26 | 27,500.0 | −0.9 |
| 8 | 8.0 | 2.5 | 15.0 | 10.0 | 150 | 8.37 | 23,900.0 | −0.9 |
| 9 | 6.0 | 0.0 | 10.0 | 24.0 | 150 | 8.76 | 171.8 | −0.3 |
| 10 | 8.0 | 0.0 | 10.0 | 24.0 | 3000 | 8.64 | 128.8 | −0.3 |
| 11 | 6.0 | 2.5 | 10.0 | 24.0 | 3000 | 8.26 | 27,500.0 | −0.9 |
| 12 | 8.0 | 2.5 | 10.0 | 24.0 | 150 | 8.37 | 23,900.0 | −0.9 |
| 13 | 6.0 | 0.0 | 15.0 | 24.0 | 3000 | 8.76 | 171.8 | −0.3 |
| 14 | 8.0 | 0.0 | 15.0 | 24.0 | 150 | 8.64 | 128.8 | −0.3 |
| 15 | 6.0 | 2.5 | 15.0 | 24.0 | 150 | 8.26 | 27,500.0 | −0.9 |
| 16 | 8.0 | 2.5 | 15.0 | 24.0 | 3000 | 8.37 | 23,900.0 | −0.9 |
| 17 | 7.0 | 1.25 | 12.5 | 17.0 | 1575 | 8.39 | 16,680.0 | −0.4 |
| 18 | 7.0 | 1.25 | 12.5 | 17.0 | 1575 | 8.39 | 16,680.0 | −0.4 |
| 19 | 7.0 | 1.25 | 12.5 | 17.0 | 1575 | 8.39 | 16,680.0 | −0.4 |
| 20 | 7.0 | 1.25 | 12.5 | 17.0 | 1575 | 8.39 | 16,680.0 | −0.4 |
| Measurements (Samples) | Mean Diameter (nm) | Minimum Diameter (nm) | Maximum Diameter (nm) |
|---|---|---|---|
| 2 | 525.16ab ± 120 | 119.7 | 1333.62 |
| 5 | 126.00a ± 50 | 52.22 | 199.50 |
| 7 | 5589.37d ± 659 | 3108.60 | 9442.59 |
| 8 | 2408.09c ± 980 | 1533.12 | 3871.30 |
| 9 | 2643.67c ± 666 | 1870.25 | 8195.25 |
| 11 | 29,957.27e ± 6032 | 3710.90 | 50,939.74 |
| 12 | 94.02a ± 32 | 51.50 | 177.94 |
| 13 | 906.35b ± 132 | 249.08 | 1618.59 |
| 14 | 484.88ab ± 98 | 72.46 | 1549.17 |
| 15 | 2577.16c ± 765 | 1381.50 | 4918.4 |
| Wavenumber (cm−1) | Assignment |
|---|---|
| 850 | ρ (CH2) + δ (C-O-C) |
| 960 | ρas (CH2) + ν (CH2) |
| 1052 | ν (C-C) + ρ (CH2) |
| 1150 | νs (C-O-C) |
| 1238, 1278 | τs (CH2), τas (CH2) |
| 1338, 1358 | A doublet ω (CH2) |
| 1445, 1471 | δs (CH2), δas (CH2) |
| 1722 | ν (C=O) |
| 2950–2700 | νs (CH2), νas (CH2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, S.d.P.; Giaconia, M.A.; Do Marco, J.T.; Paiva, R.d.S.; De Rosso, V.V.; Lemes, A.C.; Egea, M.B.; Assis, M.; Mazzo, T.M.; Longo, E.; et al. Development and Characterization of Electrospun Nanostructures Using Polyethylene Oxide: Potential Means for Incorporation of Bioactive Compounds. Colloids Interfaces 2020, 4, 14. https://doi.org/10.3390/colloids4020014
Ramos SdP, Giaconia MA, Do Marco JT, Paiva RdS, De Rosso VV, Lemes AC, Egea MB, Assis M, Mazzo TM, Longo E, et al. Development and Characterization of Electrospun Nanostructures Using Polyethylene Oxide: Potential Means for Incorporation of Bioactive Compounds. Colloids and Interfaces. 2020; 4(2):14. https://doi.org/10.3390/colloids4020014
Chicago/Turabian StyleRamos, Sergiana dos P., Michele A. Giaconia, Jonas T. Do Marco, Robert da S. Paiva, Veridiana V. De Rosso, Ailton C. Lemes, Mariana B. Egea, Marcelo Assis, Tatiana M. Mazzo, Elson Longo, and et al. 2020. "Development and Characterization of Electrospun Nanostructures Using Polyethylene Oxide: Potential Means for Incorporation of Bioactive Compounds" Colloids and Interfaces 4, no. 2: 14. https://doi.org/10.3390/colloids4020014
APA StyleRamos, S. d. P., Giaconia, M. A., Do Marco, J. T., Paiva, R. d. S., De Rosso, V. V., Lemes, A. C., Egea, M. B., Assis, M., Mazzo, T. M., Longo, E., & Braga, A. R. C. (2020). Development and Characterization of Electrospun Nanostructures Using Polyethylene Oxide: Potential Means for Incorporation of Bioactive Compounds. Colloids and Interfaces, 4(2), 14. https://doi.org/10.3390/colloids4020014