Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria, Culture Conditions and Preparation of Cell Concentrate
2.3. Preparation of Antibubbles
2.4. Survival of L. casei during the Encapsulation Process
2.5. Triggered Release along the Digestive Tract
2.6. Survival of Unencapsulated and Encapsulated L. casei at Low pH
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antibubble Production
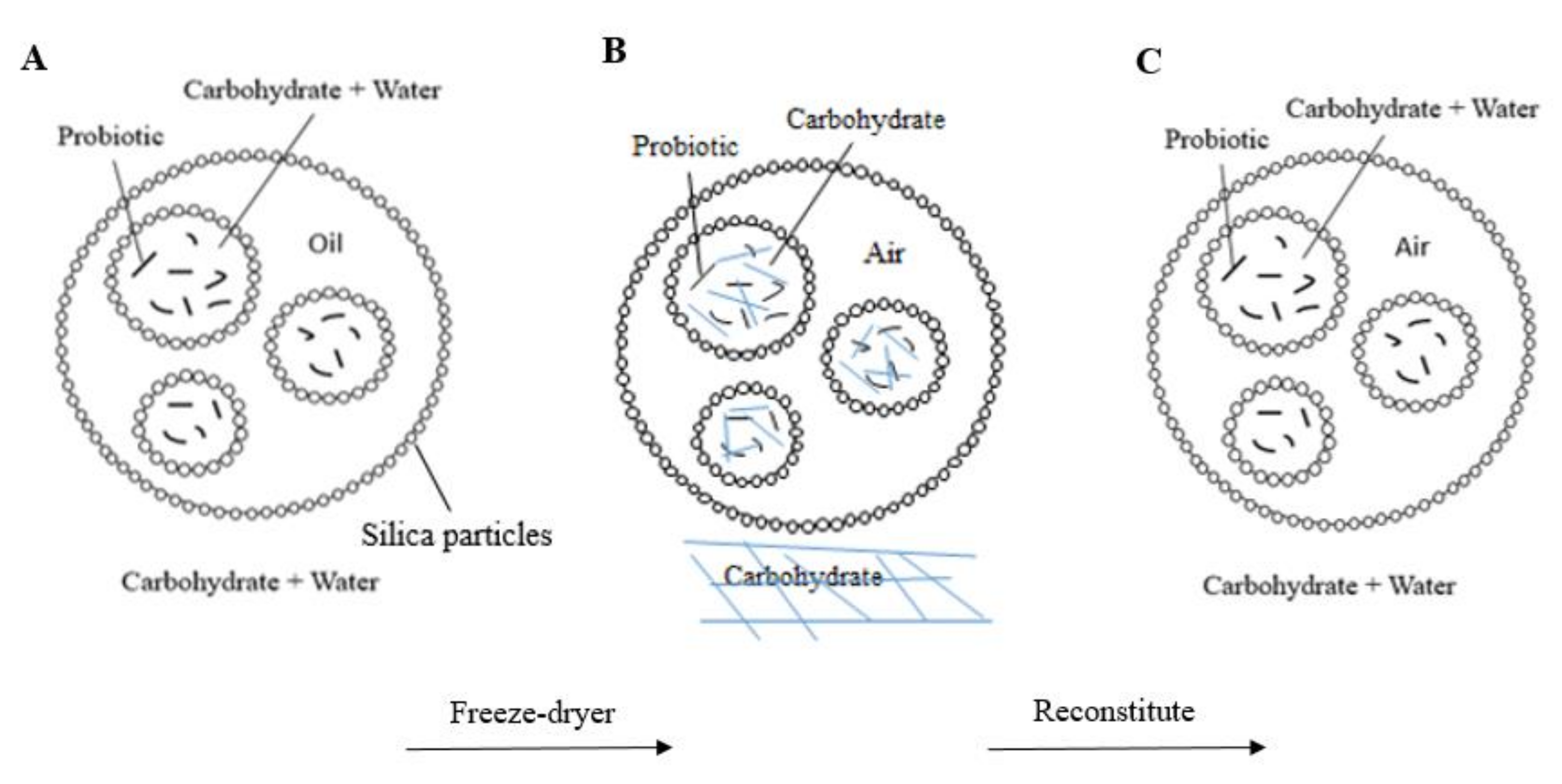
3.2. Survival of L. casei after Freeze-Drying and after Encapsulation inside Antibubbles
3.3. Survival of Unencapsulated Versus Encapsulated L. casei at Low pH
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.M.P.; Malcata, F.X. Bifidobacterium spp. and Lactobacillus acidophilus: Biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 1991, 10, 139–157. [Google Scholar] [CrossRef]
- Yao, M.; Li, B.; Ye, H.; Huang, W.; Luo, Q.; Xiao, H.; Mcclements, D.J.; Li, L. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocoll. 2018, 83, 246–252. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and methods of microencapsulation of probiotic microorganisms. Iran. J. Biotechnoly 2007, 5, 1–18. [Google Scholar]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living bacteria: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic bacteria for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Petrovic, T.; Dimitrijevicbrankovic, S.; Levic, S.; Nedovic, V.; Kourkoutas, Y. Survival of spray dried microencapsulated Lactobacillus casei ATCC 393 in simulated gastrointestinal conditions and fermented milk. LWT Food Sci. Technol. 2016, 71, 169–174. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Silpe, J.E.; Nunes, J.K.; Poortinga, A.T.; Stone, H.A. Generation of Antibubbles from Core–Shell Double Emulsion Templates Produced by Microfluidics. Langmuir 2013, 29, 8782–8787. [Google Scholar] [CrossRef]
- Vitry, Y.; Dorbolo, S.; Vermant, J.; Scheid, B. Controlling the lifetime of antibubbles. Adv. Colloid Interface Sci. 2019, 270, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Poortinga, A.T. Micron-sized antibubbles with tunable stability. Colloids Surf. A 2013, 419, 15–20. [Google Scholar] [CrossRef]
- Poortinga, A.T. Long-Lived Antibubbles: Stable Antibubbles through Pickering Stabilization. Langmuir 2011, 27, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Marefati, A.; Bertranda, M.; Sjööa, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Villota, R.; Hawkes, J.G.; Cockrane, H. Food applications and the toxicological and nutritional implications of amorphous silicon dioxide. Crit. Rev. Food Sci. 1986, 23, 289–321. [Google Scholar] [CrossRef]
- Binks, B.P.; Clint, J.H. Solid wettability from surface energy components: Relevance to Pickering emulsions. Langmuir 2002, 18, 1270–1273. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanisms behind resistance of whole bacteria to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of homogenization on bead size and survival of encapsulated probiotic bacteria. Food Res. Int. 2007, 40, 1261–1269. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Effect of cooling rate, freeze-drying, and storage on survival of free and immobilized Lactobacillus casei ATCC 393. LWT Food Sci. Technol. 2016, 69, 468–473. [Google Scholar] [CrossRef]
- Xu, M.; Gagné-Bourque, F.; Dumont, M.J.; Jabaji, S. Encapsulation of Lactobacillus casei ATCC 393 bacteria and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J. Food Eng. 2016, 168, 52–59. [Google Scholar] [CrossRef]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R.P. Effect of disaccharides on survival during storage of freeze dried probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, X.; Xu, H.; Aguilar, Z.P.; Wei, H. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT Food Sci. Technol. 2016, 68, 8–13. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of Gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kos, B.; Suskovic, J.; Goreta, J.; Matosic, S. Effect of protectors on the viability of Lactobacillus acidophilus M92 in simulated gastrointestinal conditions. Food Technol. Biotechnol. 2000, 38, 121–127. [Google Scholar]
- Sanchez, M.T.; Ruiz, M.A.; Lasserrot, A.; Hormigo, M.; Morales, M.E. An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study. Food Hydrocoll. 2017, 69, 67–75. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic bacteria. Int. Dairy J. 2009, 19, 77–84. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M.R. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.K.; Singh, K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for tunable drug release: Using displacement of air to control delivery rates. J. Am. Chem. Soc. 2012, 134, 2016–2019. [Google Scholar] [CrossRef] [Green Version]
- Binks, B.P.; Muijlwijk, K.; Koman, H.; Poortinga, A.T. Food-grade Pickering stabilisation of foams by in situ hydrophobisation of calcium carbonate particles. Food Hydrocoll. 2017, 63, 585–592. [Google Scholar] [CrossRef]



| Antibubble | Oil Phase | Inner Water Phase |
|---|---|---|
| CM | Cyclohexane | 10% Maltodextrin |
| CMS | 10% Maltodextrin + 8% Sucrose | |
| DM | Decane | 10% Maltodextrin |
| DMS | 10% Maltodextrin + 8% Sucrose |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardani Ghahfarokhi, V.; Pescarmona, P.P.; Euverink, G.-J.W.; Poortinga, A.T. Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH. Colloids Interfaces 2020, 4, 40. https://doi.org/10.3390/colloids4030040
Mardani Ghahfarokhi V, Pescarmona PP, Euverink G-JW, Poortinga AT. Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH. Colloids and Interfaces. 2020; 4(3):40. https://doi.org/10.3390/colloids4030040
Chicago/Turabian StyleMardani Ghahfarokhi, Vida, Paolo P. Pescarmona, Gert-Jan W. Euverink, and Albert T. Poortinga. 2020. "Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH" Colloids and Interfaces 4, no. 3: 40. https://doi.org/10.3390/colloids4030040
APA StyleMardani Ghahfarokhi, V., Pescarmona, P. P., Euverink, G.-J. W., & Poortinga, A. T. (2020). Encapsulation of Lactobacillus casei (ATCC 393) by Pickering-Stabilized Antibubbles as a New Method to Protect Bacteria against Low pH. Colloids and Interfaces, 4(3), 40. https://doi.org/10.3390/colloids4030040







