Electrohydrodynamic Instabilities in Free Emulsion Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, S.E. Investigations into the electrical and coalescence behaviour of water-in-crude oil emulsions in high voltage gradients. Colloids Surf. 1988, 29, 29–51. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. Chem. Eng. J. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- Narve, A.; Kallevik, H.; Sjöblom, J. Water-in-crude oil emulsion stability studied by critical electric field measurements. Correlation to physico-chemical parameters and near-infrared spectroscopy. J. Pet. Sci. Eng. 2002, 36, 1–17. [Google Scholar]
- Sameer, M.; Simon, S.; Sjöblom, J.; Xu, Z. Demulsifier assisted film thinning and coalescence in crude oil emulsions under DC electric fields. Chem. Eng. Res. Des. 2018, 134, 117–129. [Google Scholar]
- Abidor, I.G.; Arakelyan, V.B.; Chernomordik, L.V.; Chizmadzhev, Y.A.; Pastushenko, V.F.; Tarasevich, M.P. Electric breakdown of bilayer lipid membranes: I. The main experimental facts and their qualitative discussion. J. Electroanal. Chem. Interf. Electrochem. 1979, 104, 37–52. [Google Scholar] [CrossRef]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Czarnecki, J.; Khristov, K.; Masliyah, J.; Panchev, N.; Taylor, S.D.; Tchoukov, P. Application of Scheludko–Exerowa thin liquid film technique to studies of petroleum W/O emulsions. Colloids Surf. A 2017, 519, 2–10. [Google Scholar] [CrossRef]
- Khristov, K.; Taylor, S.D.; Czarnecki, J.; Masliyah, J. Thin liquid film technique—Application to water-oil-water bitumen emulsion films. Colloids Surf. A 2000, 174, 183–196. [Google Scholar] [CrossRef]
- Tchoukov, P.; Czarnecki, J.; Dabros, T. Study of water-in-oil thin liquid films: Implications for the stability of petroleum emulsions. Colloids Surf. 2010, 372, 15–21. [Google Scholar] [CrossRef]
- Huang, C.; Thompson, T.E. Properties of lipid bilayer membranes separating two aqueous phases: Determination of membrane thickness. J. Mol. Biol. 1965, 13, 183–193. [Google Scholar] [CrossRef]
- Mostowfi, F.; Khristov, K.; Czarnecki, J.; Masliyah, J.; Bhattacharjee, S. Electric field mediated breakdown of thin liquid films separating microscopic emulsion droplets. Appl. Phys. Lett. 2007, 90, 184102. [Google Scholar] [CrossRef]
- Mostowfi, F.; Czarnecki, J.; Masliyah, J.; Bhattacharjee, S. A microfluidic electrochemical detection technique for assessing stability of thin films and emulsions. J. Colloid. Intref. Sci. 2008, 317, 593–603. [Google Scholar] [CrossRef]
- Priest, C.; Herminghaus, S.; Seemann, R. Controlled electrocoalescence in microfluidics: Targeting a single lamella. Appl. Phys. Lett. 2006, 89, 134101. [Google Scholar] [CrossRef]
- Schäffer, E.; Thurn-Albrecht, T.; Russell, T.P.; Steiner, U. Electrically induced structure formation and pattern transfer. Nature 2000, 403, 874. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, E.; Thurn-Albrecht, T.; Russell, T.P.; Steiner, U. Electrohydrodynamic instabilities in polymer films. Eur. Phys. Lett. 2001, 53, 518. [Google Scholar] [CrossRef][Green Version]
- Deshpande, P.; Pease, L.F.; Chen, L.; Chou, S.Y.; Russel, W.B. Cylindrically symmetric electrohydrodynamic patterning. Phys. Rev. E 2004, 70, 041601. [Google Scholar] [CrossRef]
- Oron, A.; Davis, S.H.; Bankoff, S.G. Long-scale evolution of thin liquid films. Rev. Mod. Phys. 1997, 69, 931. [Google Scholar] [CrossRef]
- O’Sullivan, M.F. Microcapsules with Liquid Cores and Solid Shells for Pressure Release Applications. Ph.D. Thesis, University of Bristol, Bristol, UK, 2007. [Google Scholar]
- Panchev, N.; Khristov, K.; Czarnecki, J.; Exerowa, D.; Bhattacharjee, S.; Masliyah, J. A new method for water-in-oil emulsion film studies. Colloids Surf. A 2008, 315, 74. [Google Scholar] [CrossRef]
- Scheludko, A.; Platikanov, D. Utersuchung dünner Flüssinger Schichten auf Quecksiber. Kolloid Z. 1961, 175, 150–158. [Google Scholar]
- Sheludko, A. Thins Liquid Films. Adv. Colloid Interface Sci. 1967, 1, 335. [Google Scholar] [CrossRef]
- Mitlin, V.S.; Petviashvili, N.V. Nonlinear dynamics of dewetting: Kinetically stable structures. Phys. Lett. A 1994, 192, 323. [Google Scholar] [CrossRef]
- Herminghaus, S. Dynamical Instability of Thin Liquid Films Between Conducting Media. Phys. Rev. Lett. 1999, 83, 2359. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Sharma, A. Electric field induced instabilities in thin confined bilayers. J. Colloid Interface Sci. 2007, 311, 595. [Google Scholar] [CrossRef] [PubMed]
- Ruckenstein, E.; Jain, R.K. Spontaneous rupture of thin liquid films. J. Chem. Soc. Faraday Trans. 1974, 70, 132. [Google Scholar] [CrossRef]
- Mileva, E.; Radoev, B. Hydrodynamic interactions and stability of emulsion films. In Emulsions: Structure Stability and Interactions; Elsevier: New York, NY, USA, 2004; pp. 215–258. [Google Scholar]

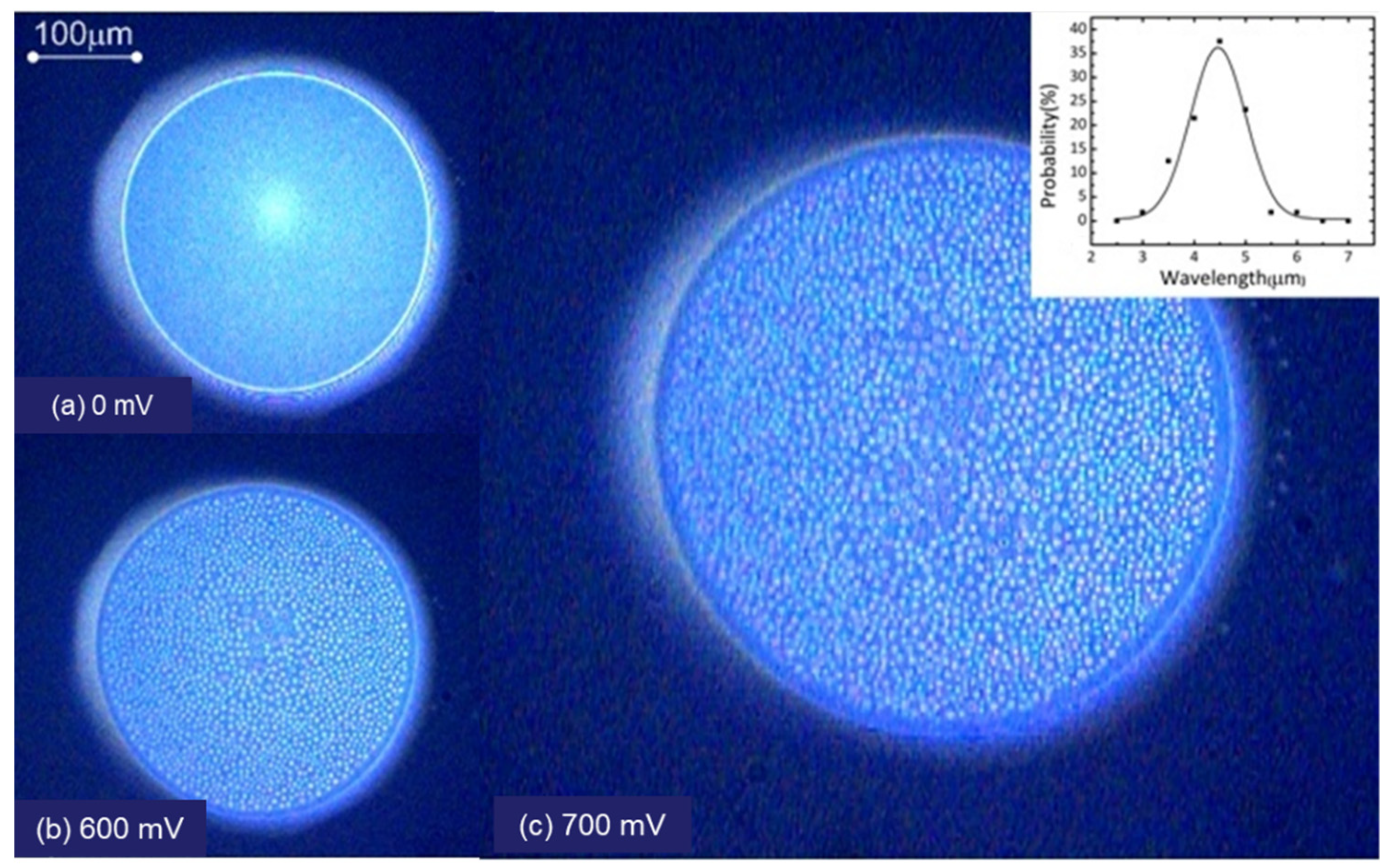

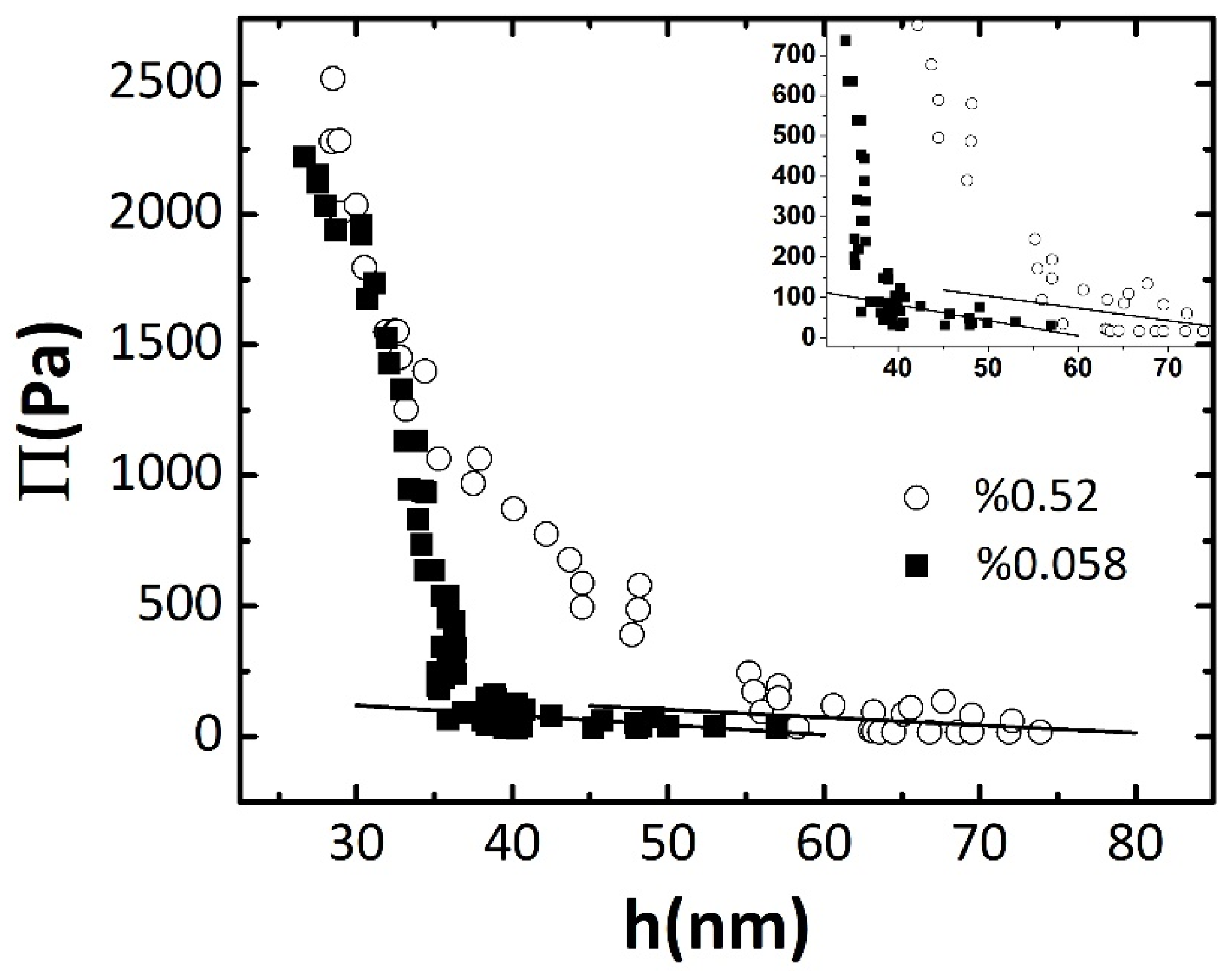
| Conc. (wt.%) | Equilibrium Thickness (nm) | Equilibrium Interfacial Tension (mJ/m2) | (Pa/nm) |
|---|---|---|---|
| 0.058 | 57 ± 1 | 5.8 ± 0.5 | −3.8 |
| 0.52 | 72 ± 1 | 3.2 ± 0.5 | −3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostowfi, F.; Tchoukov, P.; Panchev, N.; Dabros, T.; Czarnecki, J. Electrohydrodynamic Instabilities in Free Emulsion Films. Colloids Interfaces 2021, 5, 36. https://doi.org/10.3390/colloids5030036
Mostowfi F, Tchoukov P, Panchev N, Dabros T, Czarnecki J. Electrohydrodynamic Instabilities in Free Emulsion Films. Colloids and Interfaces. 2021; 5(3):36. https://doi.org/10.3390/colloids5030036
Chicago/Turabian StyleMostowfi, Farshid, Plamen Tchoukov, Nikolay Panchev, Tadeusz Dabros, and Jan Czarnecki. 2021. "Electrohydrodynamic Instabilities in Free Emulsion Films" Colloids and Interfaces 5, no. 3: 36. https://doi.org/10.3390/colloids5030036
APA StyleMostowfi, F., Tchoukov, P., Panchev, N., Dabros, T., & Czarnecki, J. (2021). Electrohydrodynamic Instabilities in Free Emulsion Films. Colloids and Interfaces, 5(3), 36. https://doi.org/10.3390/colloids5030036







