Electrochemical Perspective on Hematite–Malonate Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Potentiometric Acid–Base Titration
2.2.2. Electrokinetic Measurements
2.2.3. Surface Potential Measurements
3. Results and Discussion
3.1. Surface Charge of Hematite
3.2. Zeta and Surface Potential
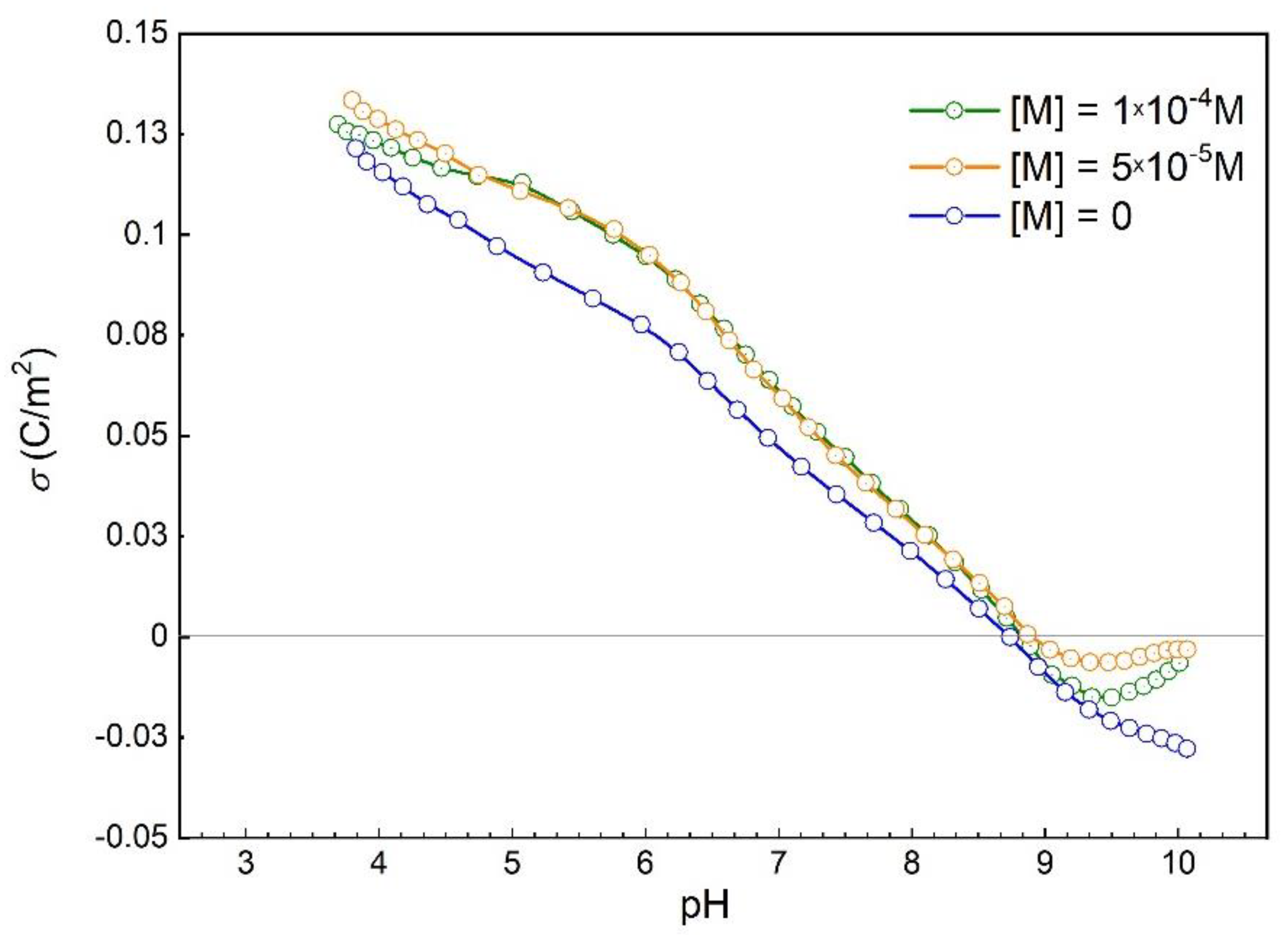
3.3. Malonates Effect on Hematite Surface Charge Density
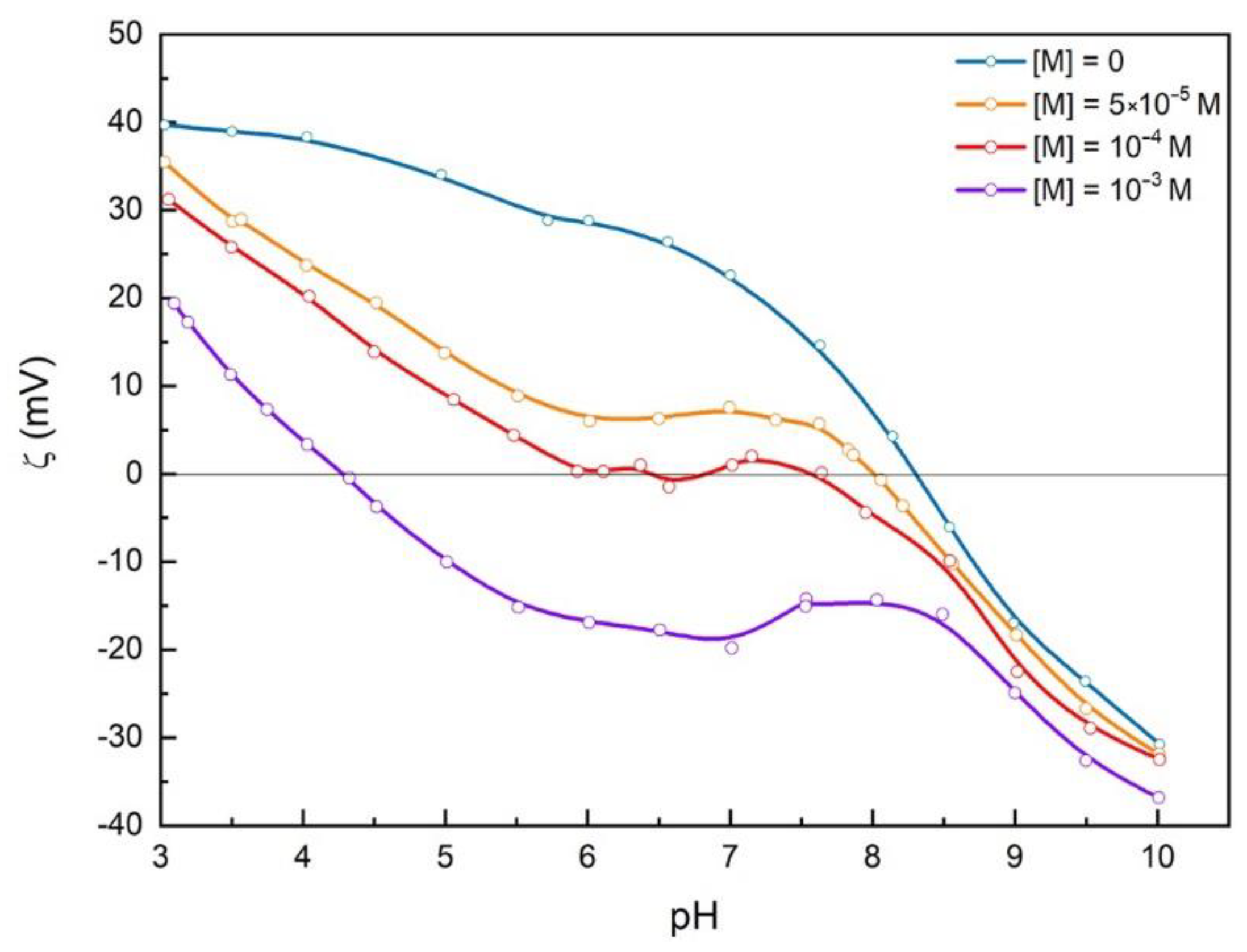
3.4. Malonates Effect on Zeta Potential
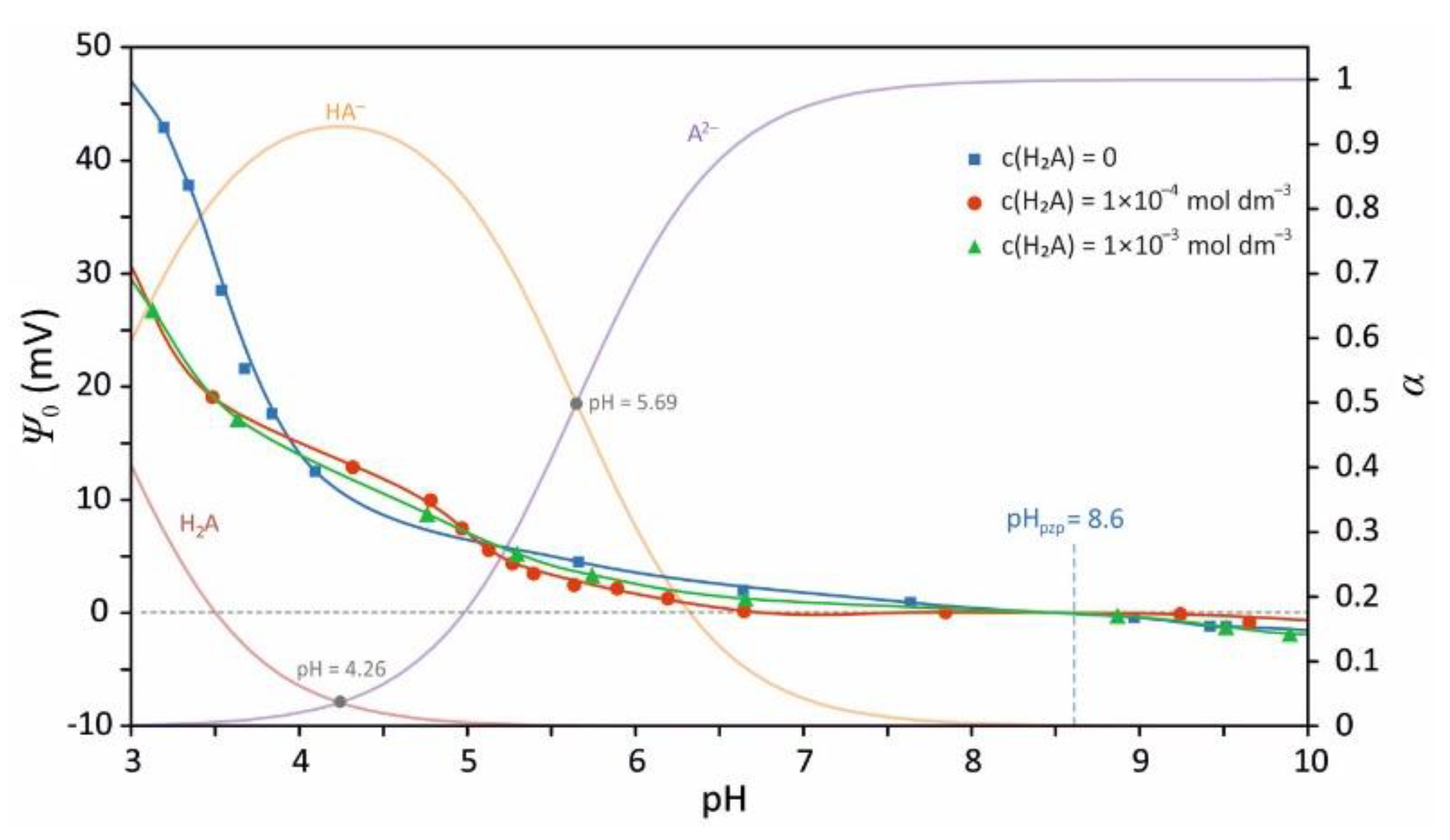
3.5. Insight into Malonates Sorption Modes
3.6. Coexistence of Various Coordination Modes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Scalenghe, R. Soils: Basic Concepts and Future Challenges; Certini, G., Scalenghe, R., Eds.; Cambridge University Press: Cambridge, UK, 2006; Volume 9780521851, ISBN 9780511535802. [Google Scholar]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Chen, C.; Hall, S.J.; Coward, E.; Thompson, A. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 2020, 11, 2255. [Google Scholar] [CrossRef]
- Wagai, R.; Kajiura, M.; Asano, M. Iron and aluminum association with microbially processed organic matter via meso-density aggregate formation across soils: Organo-metallic glue hypothesis. Soil 2020, 6, 597–627. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Kleber, M.; Sollins, P.; Sutton, R. A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Chang. 2018, 8, 1104–1108. [Google Scholar] [CrossRef]
- Torn, M.S.; Trumbore, S.E.; Chadwick, O.A.; Vitousek, P.M.; Hendricks, D.M. Mineral control of soil organic carbon storage and turnover. Nature 1997, 389, 170–173. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Kawamura, K.; Ono, K.; Tachibana, E.; Charriére, B.; Sempéré, R. Distributions of low molecular weight dicarboxylic acids, ketoacids and α-dicarbonyls in the marine aerosols collected over the Arctic Ocean during late summer. Biogeosciences 2012, 9, 4725–4737. [Google Scholar] [CrossRef] [Green Version]
- Kallay, N.; Dojnović, Z.; Čop, A. Surface potential at the hematite-water interface. J. Colloid Interface Sci. 2005, 286, 610–614. [Google Scholar] [CrossRef]
- Preočanin, T.; Čop, A.; Kallay, N. Surface potential of hematite in aqueous electrolyte solution: Hysteresis and equilibration at the interface. J. Colloid Interface Sci. 2006, 299, 772–776. [Google Scholar] [CrossRef]
- Zarzycki, P.; Chatman, S.; Preočanin, T.; Rosso, K.M. Electrostatic potential of specific mineral faces. Langmuir 2011, 27, 7986–7990. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P.; Rosso, K.M.; Chatman, S.; Preočanin, T.; Kallay, N.; Piasecki, W. Theory, experiment and computer simulation of the electrostatic potential at crystal/electrolyte interfaces. Croat. Chem. Acta 2010, 83, 457–474. [Google Scholar]
- Preočanin, T.; Kallay, N. Effect of electrolyte on surface potential of haematite in aqueous electrolyte solutions. Surf. Eng. 2008, 24, 253–258. [Google Scholar] [CrossRef]
- Yanina, S.V.; Rosso, K.M. Linked Reactivity at Mineral-Water Interfaces Through Bulk Crystal Conduction. Science 2008, 320, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Chatman, S.; Zarzycki, P.; Rosso, K.M. Surface potentials of (001), (012), (113) hematite (α-Fe2O3) crystal faces in aqueous solution. Phys. Chem. Chem. Phys. 2013, 15, 13911–13921. [Google Scholar] [CrossRef]
- Shimizu, K.; Boily, J.-F. Electrochemical Properties and Relaxation Times of the Hematite/Water Interface. Langmuir 2014, 30, 9591–9598. [Google Scholar] [CrossRef]
- Shimizu, K.; Boily, J.-F. Electrochemical Signatures of Crystallographic Orientation and Counterion Binding at the Hematite/Water Interface. J. Phys. Chem. C 2015, 119, 5988–5994. [Google Scholar] [CrossRef]
- Shimizu, K.; Nyström, J.; Geladi, P.; Lindholm-Sethson, B.; Boily, J.-F. Electrolyte ion adsorption and charge blocking effect at the hematite/aqueous solution interface: An electrochemical impedance study using multivariate data analysis. Phys. Chem. Chem. Phys. 2015, 17, 11560–11568. [Google Scholar] [CrossRef] [Green Version]
- Preočanin, T.; Janusz, W.; Kallay, N. Evaluation of equilibrium parameters of the anatase/aqueous electrolyte solution interface by introducing surface potential data. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 30–37. [Google Scholar] [CrossRef]
- Kallay, N.; Preočanin, T.; Šupljika, F. Measurement of surface potential at silver chloride aqueous interface with single-crystal AgCl electrode. J. Colloid Interface Sci. 2008, 327, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Lützenkirchen, J.; Preočanin, T.; Kovačević, D.; Tomišić, V.; Lövgren, L.; Kallay, N. Potentiometric Titrations as a Tool for Surface Charge Determination. Croat. Chem. Acta 2012, 85, 391–417. [Google Scholar] [CrossRef]
- Preočanin, T.; Namjesnik, D.; Klačić, T.; Šutalo, P. The Effects on the Response of Metal Oxide and Fluorite Single Crystal Electrodes and the Equilibration Process in the Interfacial Region. Croat. Chem. Acta 2017, 90, 333–344. [Google Scholar] [CrossRef]
- Determination of Organic Structures by Physical Methods, 1st ed.; Braude, E.A.; Nachod, F.C. (Eds.) Academic Press Inc.: New York, NY, USA, 1955; ISBN 9781483275727. [Google Scholar]
- Szymanek, K.; Charmas, R.; Piasecki, W. A study on the mechanism of Ca2+ adsorption on TiO2 and Fe2O3 with the usage of calcium ion-selective electrode. Chemosphere 2020, 242, 125162. [Google Scholar] [CrossRef]
- Piasecki, W.; Szymanek, K.; Charmas, R. Fe2+ adsorption on iron oxide: The importance of the redox potential of the adsorption system. Adsorption 2019, 25, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, C.M.; Jonsson, C.L.; Sverjensky, D.A.; Cleaves, H.J.; Hazen, R.M. Attachment of L-glutamate to rutile (α-TiO2): A Potentiometric, adsorption, and surface complexation study. Langmuir 2009, 25, 12127–12135. [Google Scholar] [CrossRef]
- Aquatic Chemical Kinetics—Reaction Rates of Processes in Natural Waters; Stumm, W. (Ed.) John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1991; Volume 152, ISBN 0471510297. [Google Scholar]
- Sposito, G. On Points of Zero Charge. Environ. Sci. Technol. 1998, 32, 2815–2819. [Google Scholar] [CrossRef]
- Preočanin, T.; Kallay, N. Evaluation of surface potential from single crystal electrode potential. Adsorption 2013, 19, 259–267. [Google Scholar] [CrossRef]
- Kedra-Królik, K.; Rosso, K.M.; Zarzycki, P. Probing size-dependent electrokinetics of hematite aggregates. J. Colloid Interface Sci. 2017, 488, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Toczydłowska, D.; Kedra-Królik, K.; Nejbert, K.; Preočanin, T.; Rosso, K.M.; Zarzycki, P. Potentiometric and electrokinetic signatures of iron(ii) interactions with (α,γ)-Fe2O3. Phys. Chem. Chem. Phys. 2015, 17, 26264–26269. [Google Scholar] [CrossRef]
- Zarzycki, P.; Preočanin, T. Point of zero potential of single-crystal electrode/inert electrolyte interface. J. Colloid Interface Sci. 2012, 370, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, P. Comparison of the Monte Carlo estimation of surface electrostatic potential at the hematite (0 0 0 1)/electrolyte interface with the experiment. Appl. Surf. Sci. 2007, 253, 7604–7612. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, O.W.; Martin, S.T. Surface complexation and dissolution of hematite by C1-C6 dicarboxylic acids at pH = 5.0. Geochim. Cosmochim. Acta 2001, 65, 4289–4301. [Google Scholar] [CrossRef]
- Litter, M.I.; Villegas, M.; Blesa, M.A. Photodissolution of iron oxides in malonic acid. Can. J. Chem. 1994, 72, 2037–2043. [Google Scholar] [CrossRef]
- Zarzycki, P.; Charmas, R.; Piasecki, W. Formal mathematical analysis of the existence of the common intersection point in relation to determining the parameters describing ion adsorption at the oxide/electrolyte interface: Comparison of the triple and four-layer models. Adsorption 2004, 10, 139–149. [Google Scholar] [CrossRef]
- Kallay, N.; Preocanin, T.; Ivsić, T. Determination of surface potentials from the electrode potentials of a single-crystal electrode. J. Colloid Interface Sci. 2007, 309, 21–27. [Google Scholar] [CrossRef]
- Zarzycki, P.; Rosso, K.M. Nonlinear response of the surface electrostatic potential formed at metal oxide/electrolyte interfaces. A Monte Carlo simulation study. J. Colloid Interface Sci. 2010, 341, 143–152. [Google Scholar] [CrossRef]
- Persson, P.; Axe, K. Adsorption of oxalate and malonate at the water-goethite interface: Molecular surface speciation from IR spectroscopy. Geochim. Cosmochim. Acta 2005, 69, 541–552. [Google Scholar] [CrossRef]
- Lenhart, J.J.; Bargar, J.R.; Davis, J.A. Spectroscopic evidence for ternary surface complexes in the lead(II)-malonic acid-hematite system. J. Colloid Interface Sci. 2001, 234, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltis, J.A.; Schwartzberg, A.M.; Zarzycki, P.; Penn, R.L.; Rosso, K.M.; Gilbert, B. Electron Mobility and Trapping in Ferrihydrite Nanoparticles. ACS Earth Space Chem. 2017, 1, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Katz, J.E.; Zhang, X.; Attenkofer, K.; Chapman, K.W.; Frandsen, C.; Zarzycki, P.; Rosso, K.M.; Falcone, R.W.; Waychunas, G.A.; Gilbert, B. Electron small polarons and their mobility in iron (oxyhydr)oxide nanoparticles. Science 2012, 337, 1200–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangiante, D.M.; Schaller, R.D.; Zarzycki, P.; Banfield, J.F.; Gilbert, B. Mechanism of Ferric Oxalate Photolysis. ACS Earth Space Chem. 2017, 1, 270–276. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędra, K.; Łazarczyk, M.; Begović, T.; Namjesnik, D.; Lament, K.; Piasecki, W.; Zarzycki, P. Electrochemical Perspective on Hematite–Malonate Interactions. Colloids Interfaces 2021, 5, 47. https://doi.org/10.3390/colloids5040047
Kędra K, Łazarczyk M, Begović T, Namjesnik D, Lament K, Piasecki W, Zarzycki P. Electrochemical Perspective on Hematite–Malonate Interactions. Colloids and Interfaces. 2021; 5(4):47. https://doi.org/10.3390/colloids5040047
Chicago/Turabian StyleKędra, Karolina, Marzena Łazarczyk, Tajana Begović, Danijel Namjesnik, Karolina Lament, Wojciech Piasecki, and Piotr Zarzycki. 2021. "Electrochemical Perspective on Hematite–Malonate Interactions" Colloids and Interfaces 5, no. 4: 47. https://doi.org/10.3390/colloids5040047
APA StyleKędra, K., Łazarczyk, M., Begović, T., Namjesnik, D., Lament, K., Piasecki, W., & Zarzycki, P. (2021). Electrochemical Perspective on Hematite–Malonate Interactions. Colloids and Interfaces, 5(4), 47. https://doi.org/10.3390/colloids5040047






