Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. PDA Coatings
2.1.1. PDA–O2
2.1.2. PDA–IO4−
2.1.3. PDA–PEI
2.2. PDA Film Chlorination
2.3. Polarization Modulation Reflection Absorptions InfraRed Spectroscopy (PM-RAIRS)
2.4. X-ray Photoelectron Spectroscopy (XPS)
2.5. Water Contact Angle (WCA)
2.6. Chemical 5-Thio-2-nitrobenzoic Acid (TNB) Titration
2.7. Microbiological Tests
2.7.1. Bacteria Growth Capacity (Cultivability of Adhered Bacteria)
2.7.2. Epifluorescence Optical Microscopy Observations
3. Results
3.1. PDA Coating Elaboration
3.2. PDA Coating Chlorination
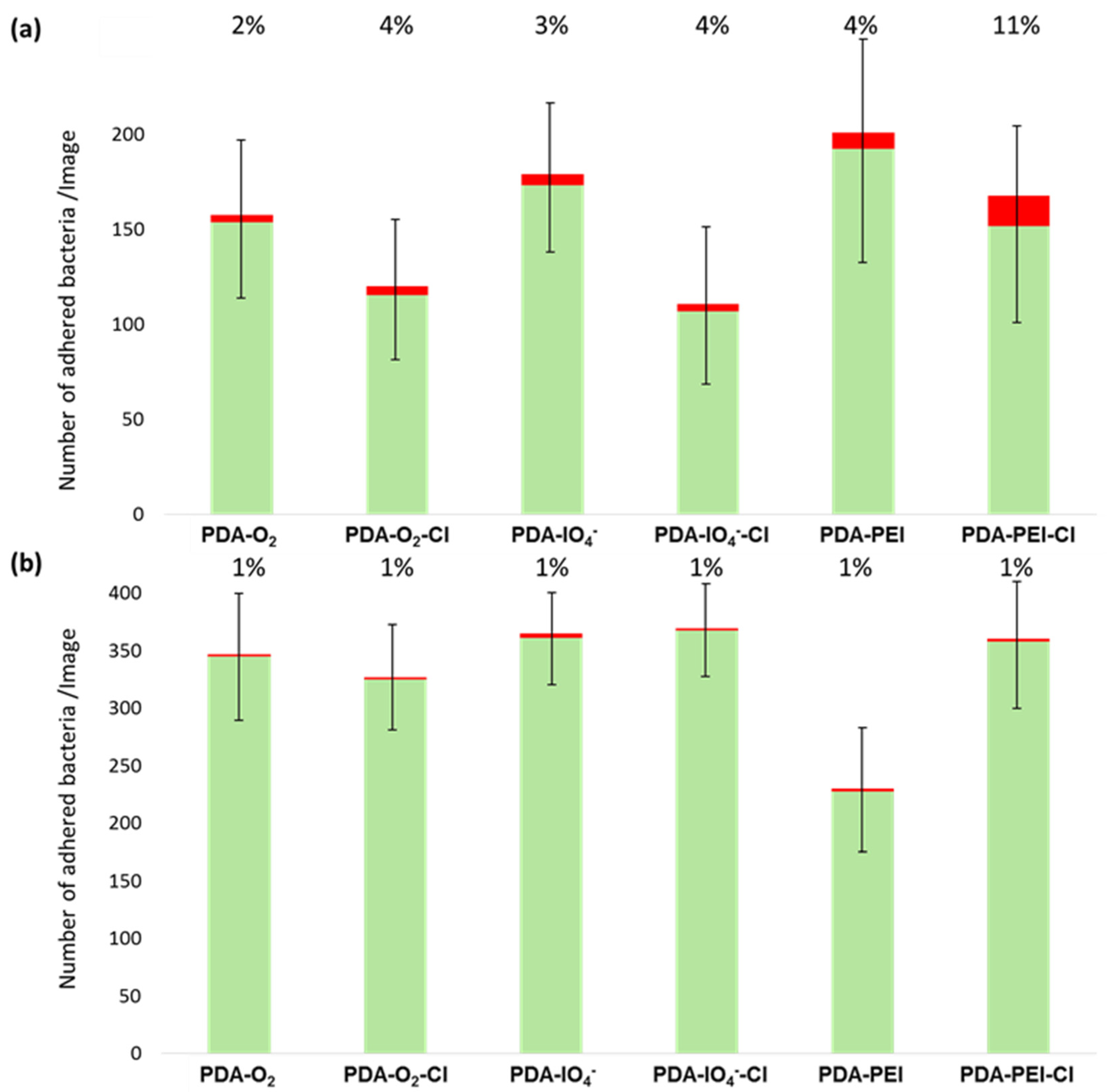
3.3. Antibacterial Properties of Chlorinated PDA Surfaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Carvalho, C.C.C.R. Marine Biofilms: A Successful Microbial Strategy with Economic Implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin Resistance of Pseudomonas Aeruginosa Cells Growing as a Biofilm on Urinary Catheter Material. Antimicrob. Agents Chemother. 1985, 27, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial Biofilm: Structure, Function, and Antimicrobial Resistance. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Alves, D.; Pereira, M.O. Mini-Review: Antimicrobial Peptides and Enzymes as Promising Candidates to Functionalize Biomaterial Surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Xiong, M.; Hu, G.; Zhan, J.; Li, T.; Wang, L.; Wang, Y. Antimicrobial Titanium Surface via Click-Immobilization of Peptide and Its in Vitro/Vivo Activity. ACS Biomater. Sci. Eng. 2019, 5, 1034–1044. [Google Scholar] [CrossRef]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and Eco-Friendly High Efficacy Cinnamaldehyde Antibacterial Surfaces. J. Mater. Chem. B 2021, 9, 2918–2930. [Google Scholar] [CrossRef]
- Nicolas, M.; Beito, B.; Oliveira, M.; Tudela Martins, M.; Gallas, B.; Salmain, M.; Boujday, S.; Humblot, V. Strategies for Antimicrobial Peptides Immobilization on Surfaces to Prevent Biofilm Growth on Biomedical Devices. Antibiotics 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz Khan, M.; Abdelhamid, H.N.; Wu, H.-F. Near Infrared (NIR) Laser Mediated Surface Activation of Graphene Oxide Nanoflakes for Efficient Antibacterial, Antifungal and Wound Healing Treatment. Colloids Surf. B Biointerfaces 2015, 127, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Ren, X.; Huang, T.-S.; Sun, Y. Cytocompatible Antibacterial Fibrous Membranes Based on Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) and Quaternarized N-Halamine Polymer. RSC Adv. 2016, 6, 42600–42610. [Google Scholar] [CrossRef]
- Kügler, R.; Bouloussa, O.; Rondelez, F. Evidence of a Charge-Density Threshold for Optimum Efficiency of Biocidal Cationic Surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm Bacteria: Formation and Comparative Susceptibility to Antibiotics. Can. J. Vet. Res. 2002, 66, 86–92. [Google Scholar]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial Polymers: Mechanism of Action, Factors of Activity, and Applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Baltzer, S.A.; Brown, M.H. Antimicrobial Peptides: Promising Alternatives to Conventional Antibiotics. J. Mol. Microbiol. Biotechnol. 2011, 20, 228–235. [Google Scholar] [CrossRef]
- Bang, S.H.; Sekhon, S.S.; Ahn, J.-Y.; Kim, Y.-H.; Min, J. Advances in Antimicrobial Agents Based Lysosomes. Mol. Cell Toxicol. 2014, 10, 229–235. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic Antimicrobial Peptides from Eukaryotic Organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial Action of Epsilon-Poly-L-Lysine. J. Antibiot. 1984, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, A.; Wang, Y.-J.; Gao, Y.; Gao, T.; Gao, G. Chemical Insights into Antibacterial N-Halamines. Chem. Rev. 2017, 117, 4806–4862. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef]
- Nazi, N.; Humblot, V.; Debiemme-Chouvy, C. A New Antibacterial N-Halamine Coating Based on Polydopamine. Langmuir 2020, 36, 11005–11014. [Google Scholar] [CrossRef]
- Chien, H.-W.; Chiu, T.-H. Stable N-Halamine on Polydopamine Coating for High Antimicrobial Efficiency. Eur. Polym. J. 2020, 130, 109654. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Ball, V. Physicochemical Perspective on “Polydopamine” and “Poly(Catecholamine)” Films for Their Applications in Biomaterial Coatings. Biointerphases 2014, 9, 030801. [Google Scholar] [CrossRef]
- Ponzio, F.; Ball, V. Polydopamine Deposition at Fluid Interfaces. Polym. Int. 2016, 65, 1251–1257. [Google Scholar] [CrossRef]
- Ponzio, F.; Barthès, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant Control of Polydopamine Surface Chemistry in Acids: A Mechanism-Based Entry to Superhydrophilic-Superoleophobic Coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, S.-J.; Du, Y.; Yang, H.-C.; Xu, Z.-K. Co-Deposition Kinetics of Polydopamine/Polyethyleneimine Coatings: Effects of Solution Composition and Substrate Surface. Langmuir 2018, 34, 13123–13131. [Google Scholar] [CrossRef]
- Chien, H.W.; Chiu, T.H.; Lee, Y.L. Rapid Biocidal Activity of N-Halamine-Functionalized Polydopamine and Polyethylene Imine Coatings. Langmuir 2021, 37, 8037–8044. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Hequet, A.; Berjeaud, J.-M.; Pradier, C.-M. The Antibacterial Activity of Magainin I Immobilized onto Mixed Thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Del Frari, D.; Bour, J.; Ball, V.; Toniazzo, V.; Ruch, D. Degradation of Polydopamine Coatings by Sodium Hypochlorite: A Process Depending on the Substrate and the Film Synthesis Method. Polym. Degrad. Stab. 2012, 97, 1844–1849. [Google Scholar] [CrossRef]
- Ma, K.; Jiang, Z.; Li, L.; Liu, Y.; Ren, X.; Huang, T.-S. N-Halamine Modified Polyester Fabrics: Preparation and Biocidal Functions. Fibers Polym. 2014, 15, 2340–2344. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Riddles, P.W.; Blakeley, R.L.; Zerner, B. Ellman’s Reagent: 5,5′-Dithiobis(2-Nitrobenzoic Acid) a Reexamination. Anal. Biochem. 1979, 94, 75–81. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Haskouri, S.; Folcher, G.; Cachet, H. An Original Route to Immobilize an Organic Biocide onto a Transparent Tin Dioxide Electrode. Langmuir 2007, 23, 3873–3879. [Google Scholar] [CrossRef]
- Segut, O.; Herlem, G.; Lakard, B.; Blondeau-Patissier, V.; Nardin, M.; Gree, S.; Rauch, J.-Y. Electrochemically Deposited Polyethyleneimine Films and Their Characterization. Synth. Met. 2010, 160, 1359–1364. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Chen, T.-P.; Liu, T.; Su, T.-L.; Liang, J. Self-Polymerization of Dopamine in Acidic Environments without Oxygen. Langmuir 2017, 33, 5863–5871. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hydrophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Zheng, W.; Fan, H.; Wang, L.; Jin, Z. Oxidative Self-Polymerization of Dopamine in an Acidic Environment. Langmuir 2015, 31, 11671–11677. [Google Scholar] [CrossRef]
- Weidman, S.W.; Kaiser, E.T. The Mechanism of the Periodate Oxidation of Aromatic Systems. III. A Kinetic Study of the Periodate Oxidation of Catechol. J. Am. Chem. Soc. 1966, 88, 5820–5827. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculation of Electron Inelastic Mean Free Paths (IMFPs) VII. Reliability of the TPP-2M IMFP Predictive Equation. Surf. Interface Anal. 2003, 35, 268–275. [Google Scholar] [CrossRef]
- Xu, L.; Zuo, Y.Y. Reversible Phase Transitions in the Phospholipid Monolayer. Langmuir 2018, 34, 8694–8700. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Chen, Y. Stability of Polydopamine Coatings on Gold Substrates Inspected by Surface Plasmon Resonance Imaging. Langmuir 2018, 34, 3565–3571. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual Salt and pH Induced Changes in Polyethylenimine Solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Chen, Z.; Sun, Y. Controlling Biofilm Formation with an N-Halamine-Based Polymeric Additive. J. Biomed. Mater. Res. 2006, 77A, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Biedroń, R.; Białecka, A.; Kasprowicz, A.; Mak, M.; Targosz, M. Susceptibility of Propionibacterium Acnes and Staphylococcus Epidermidis to Killing by MPO-Halide System Products. Implication for Taurine Bromamine as a New Candidate for Topical Therapy in Treating Acne Vulgaris. Arch. Immunol. Ther. Exp. 2006, 54, 61–68. [Google Scholar] [CrossRef]
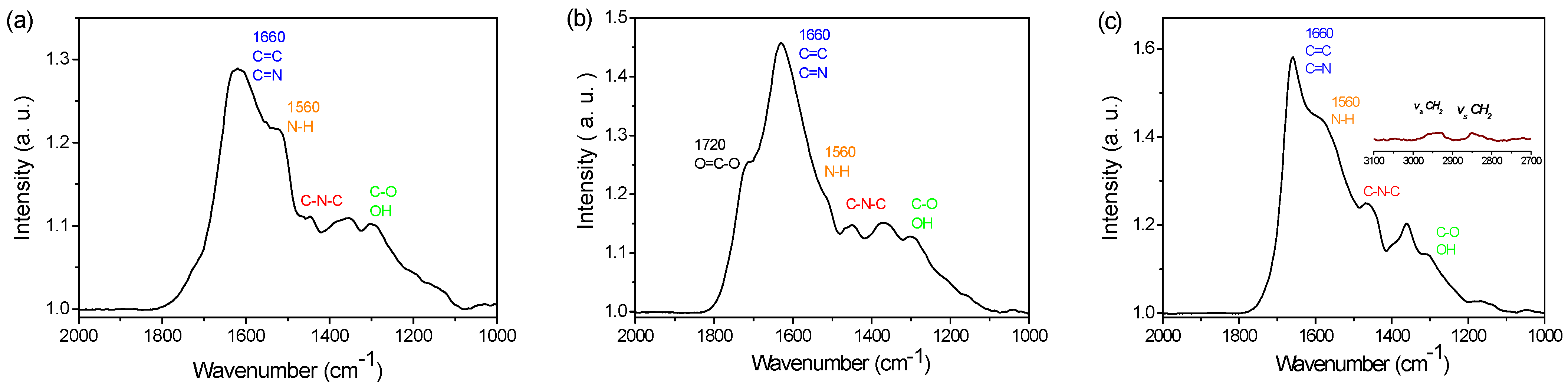
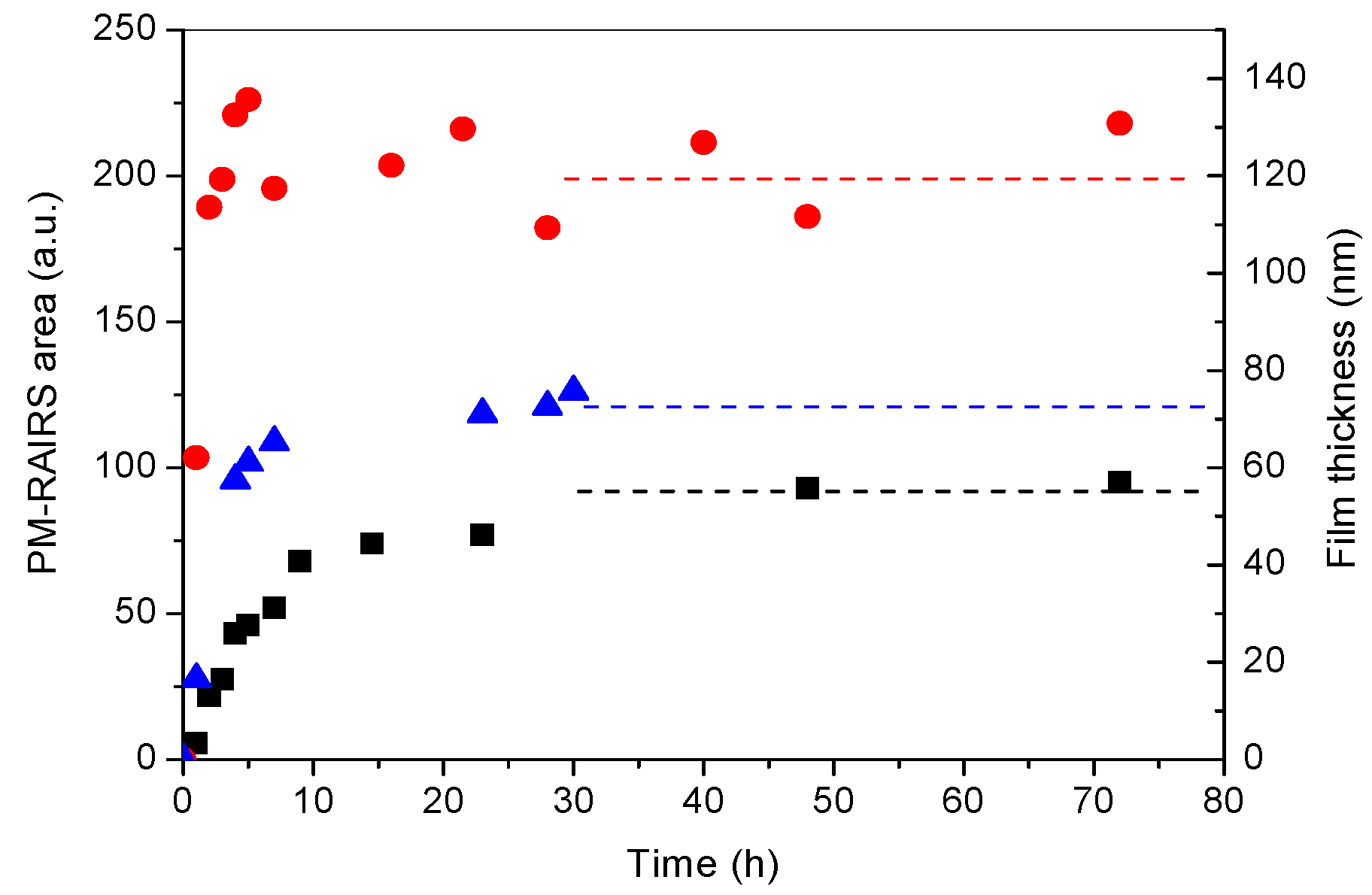
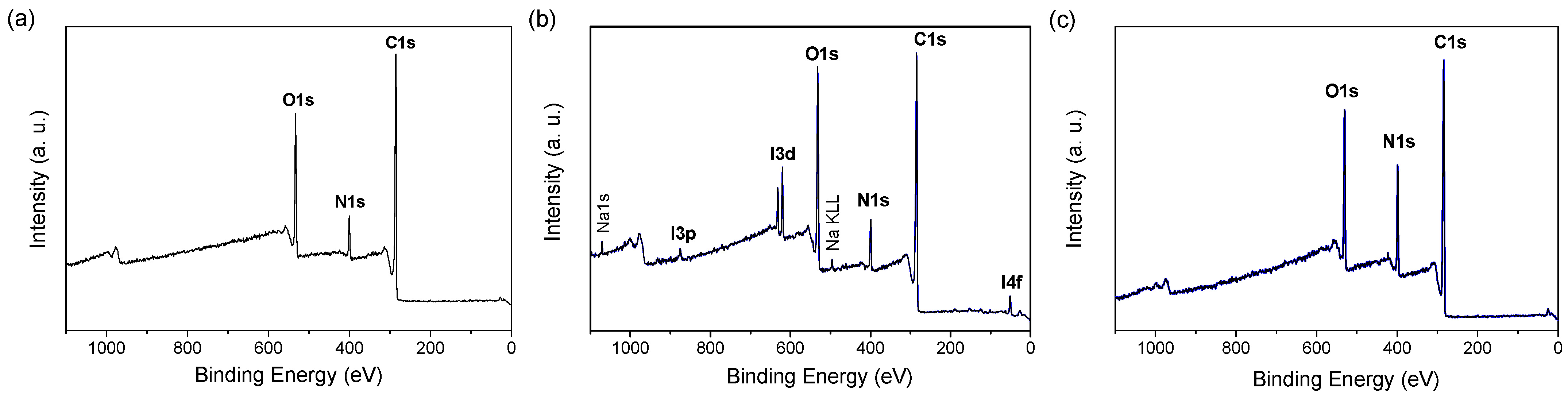
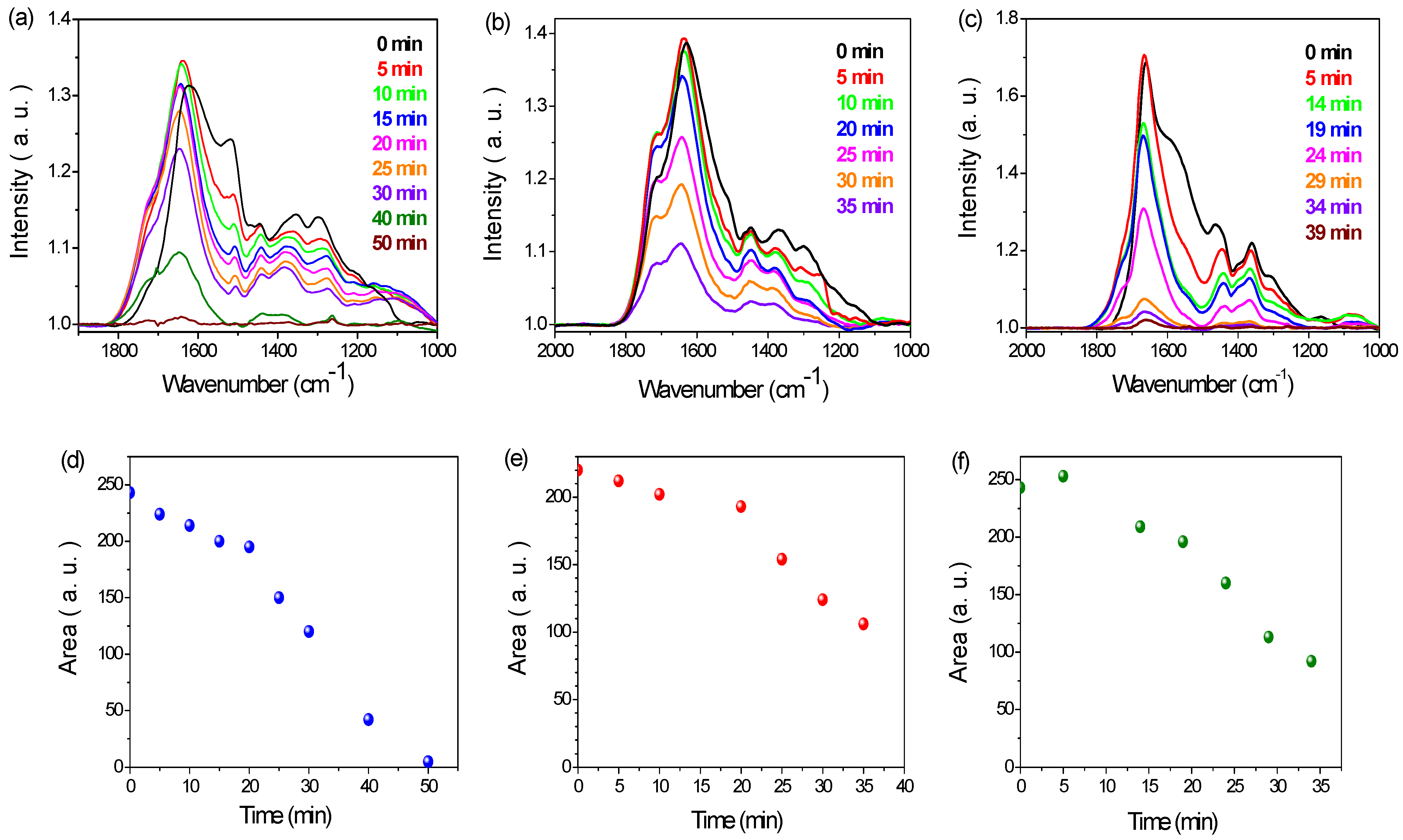
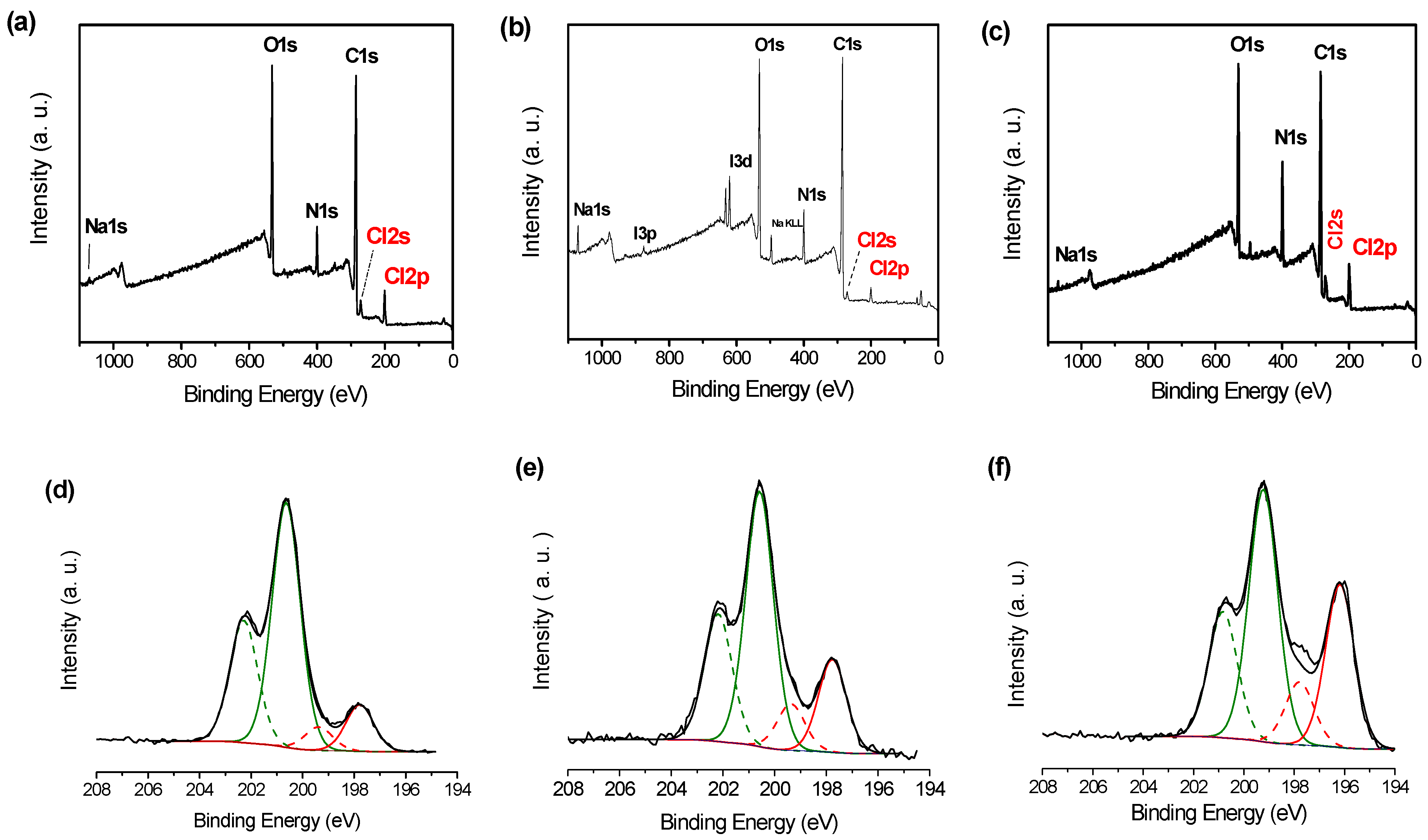


| C | N | O | Au | I | Na | N/C | |
|---|---|---|---|---|---|---|---|
| PDA–O2 | 75.5 | 7.3 | 17.2 | ND | - | - | 0.1 |
| PDA–IO4− | 72.2 | 7.1 | 19.3 | ND | 0.9 | 0.5 | 0.1 |
| PDA–PEI | 69.4 | 15.4 | 15.2 | ND | - | - | 0.2 |
| C | N | O | I | Au | Na | N/C | Cltotal | Cl200 | Cl200/N | |
|---|---|---|---|---|---|---|---|---|---|---|
| PDA–O2–Cl | 69.85 | 6.45 | 18.9 | - | - | - | 0.1 | 4.8 | 4.0 | 0.6 |
| PDA–IO4−–Cl | 69.2 | 6.5 | 20.0 | 0.7 | - | 1.3 | 0.1 | 2.3 | 1.7 | 0.3 |
| PDA–PEI–Cl | 61.4 | 13.7 | 16.4 | - | - | 0.8 | 0.2 | 7.7 | 4.5 | 0.3 |
| A at 412 nm | Cl (at.) | dCl (at cm−3) | Cl200/N | |
|---|---|---|---|---|
| PD–-O2–Cl | 0.30 | 4.0 × 1016 | 2.5 × 1021 | 0.47 |
| PDA–IO4−–Cl | 0.50 | 5.8 × 1015 | 5.0 × 1020 | 0.10 |
| PDA–PEI–Cl | 0.20 | 9.25 × 1016 | 9.25 × 1021 | 0.29 |
| PDA–Cl | PDA–IO4−-Cl | PDA–PEI–Cl | ||||
|---|---|---|---|---|---|---|
| Reference | Inoculum | PDA | Inoculum | PDA–IO4− | Inoculum | PDA–PEI |
| E. coli ATCC 25922 | 99.0 | 34.0 | 97.7 | 91.4 | 97.4 | 86.2 |
| S. epidermidis CIP 6821 | 70.0 | 59.4 | 83.0 | 76.6 | 66.6 | 58.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazi, N.; Marguier, A.; Debiemme-Chouvy, C.; Humblot, V. Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine. Colloids Interfaces 2022, 6, 9. https://doi.org/10.3390/colloids6010009
Nazi N, Marguier A, Debiemme-Chouvy C, Humblot V. Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine. Colloids and Interfaces. 2022; 6(1):9. https://doi.org/10.3390/colloids6010009
Chicago/Turabian StyleNazi, Nadia, Adeline Marguier, Catherine Debiemme-Chouvy, and Vincent Humblot. 2022. "Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine" Colloids and Interfaces 6, no. 1: 9. https://doi.org/10.3390/colloids6010009
APA StyleNazi, N., Marguier, A., Debiemme-Chouvy, C., & Humblot, V. (2022). Optimization and Antibacterial Response of N-Halamine Coatings Based on Polydopamine. Colloids and Interfaces, 6(1), 9. https://doi.org/10.3390/colloids6010009






