An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution
Abstract
:| Contents | |
| Abstract………………………………………………………………………….... | 1 |
| 1. Perspective……………………………………………………………………... | 2 |
| 2. Theories of coacervation ……………………………………………………... | 4 |
| 3. Methods used for the determination of coacervates properties…………. | 8 |
| 4. Factors influencing coacervation…………………………………………….. | 8 |
| 4.1. Ionic strength…………………………………………………………..…….. | 8 |
| 4.2. pH……………………………………………………………….…………….. | 8 |
| 4.3. Molecular weight……………………………………………….…………... | 10 |
| 4.4. Chirality of the polyelectrolytes ………………………………………….. | 10 |
| 4.5. Charge density………………………………………………………………. | 11 |
| 4.6. Temperature………………………………………………………………..... | 12 |
| 4.7. Solvent………………………………………………………………………... | 13 |
| 5. Coacervate Types, Preparation and Properties………………………….… | 14 |
| 5.1. Simple coacervates………………………………………………………….. | 14 |
| 5.2. Complex coacervates……………………………………………………….. | 17 |
| 5.2.1. Polyelectrolyte-polyelectrolyte………………………………………….. | 17 |
| 5.2.2. Polyelectrolyte-surfactant…………………………………………….….. | 20 |
| 5.2.3. Surfactant-surfactant………………………………………………….….. | 24 |
| 5.2.4. Peptide/protein…………………………………………………………..... | 24 |
| 6. Applications of Coacervates…………………………………………..……... | 26 |
| 6.1. Wastewater treatment …………………………………………………….... | 26 |
| 6.2. Protein purification………………………………………………………..... | 28 |
| 6.3. Food formulation…………………………………………………………..... | 28 |
| 6.4. Cellular mimics…………………………………………………………........ | 29 |
| 6.5. Nanoparticle synthesis………………………………………………...…..... | 29 |
| 6.6. Delivery carrier…………………………………………………………......... | 30 |
| 7. Summary……………………………………………………………………....... | 32 |
| References…………………………………………………………………………. | 33 |
1. Perspective
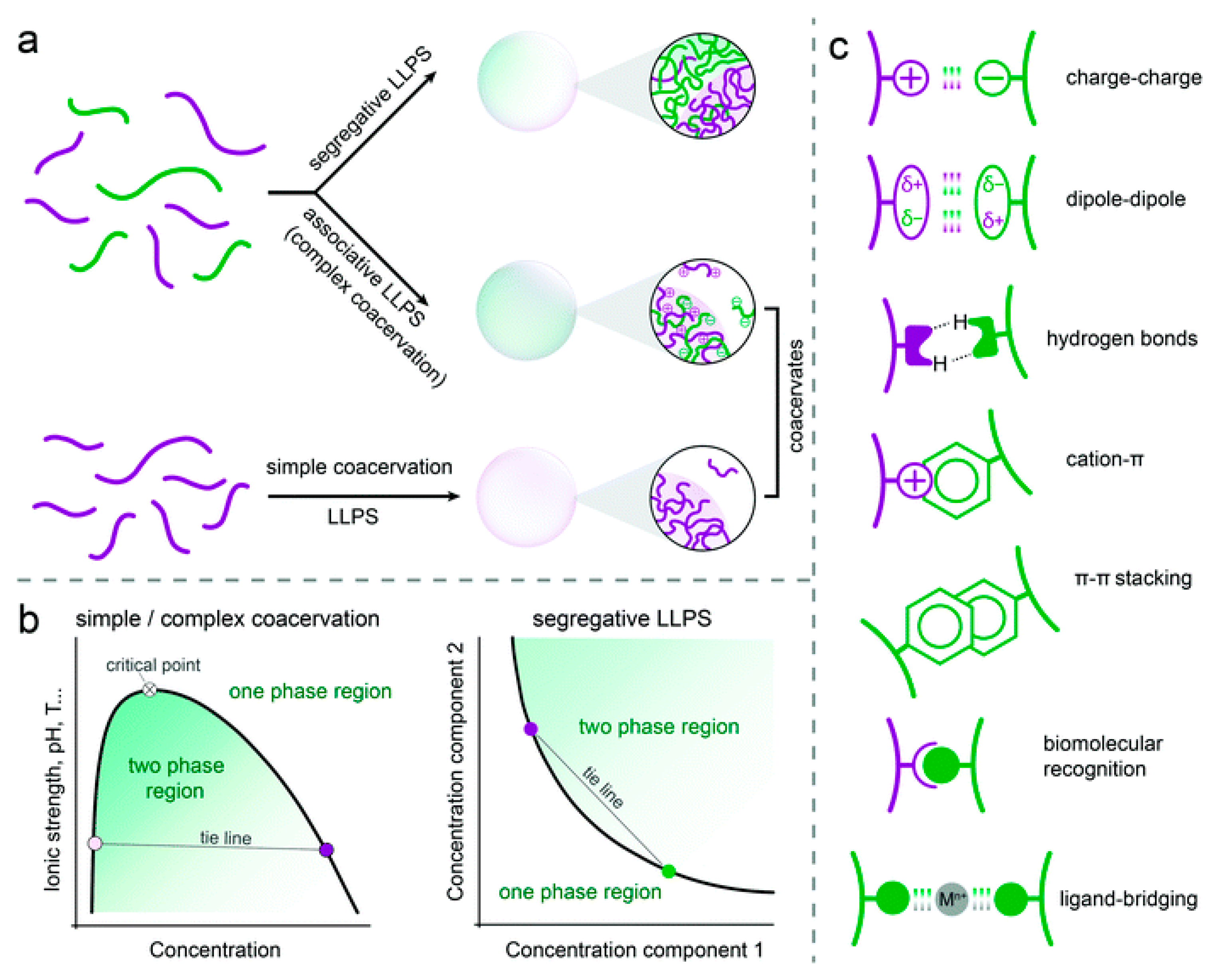
2. Theories of Coacervation
3. Methods Used for the Determination of Coacervates Properties
4. Factors Influencing Coacervation
4.1. Ionic Strength
4.2. pH
4.3. Molecular Weight
4.4. Chirality
4.5. Charge Density
4.6. Temperature
4.7. Solvent
5. Coacervate Types, Preparation, and Properties
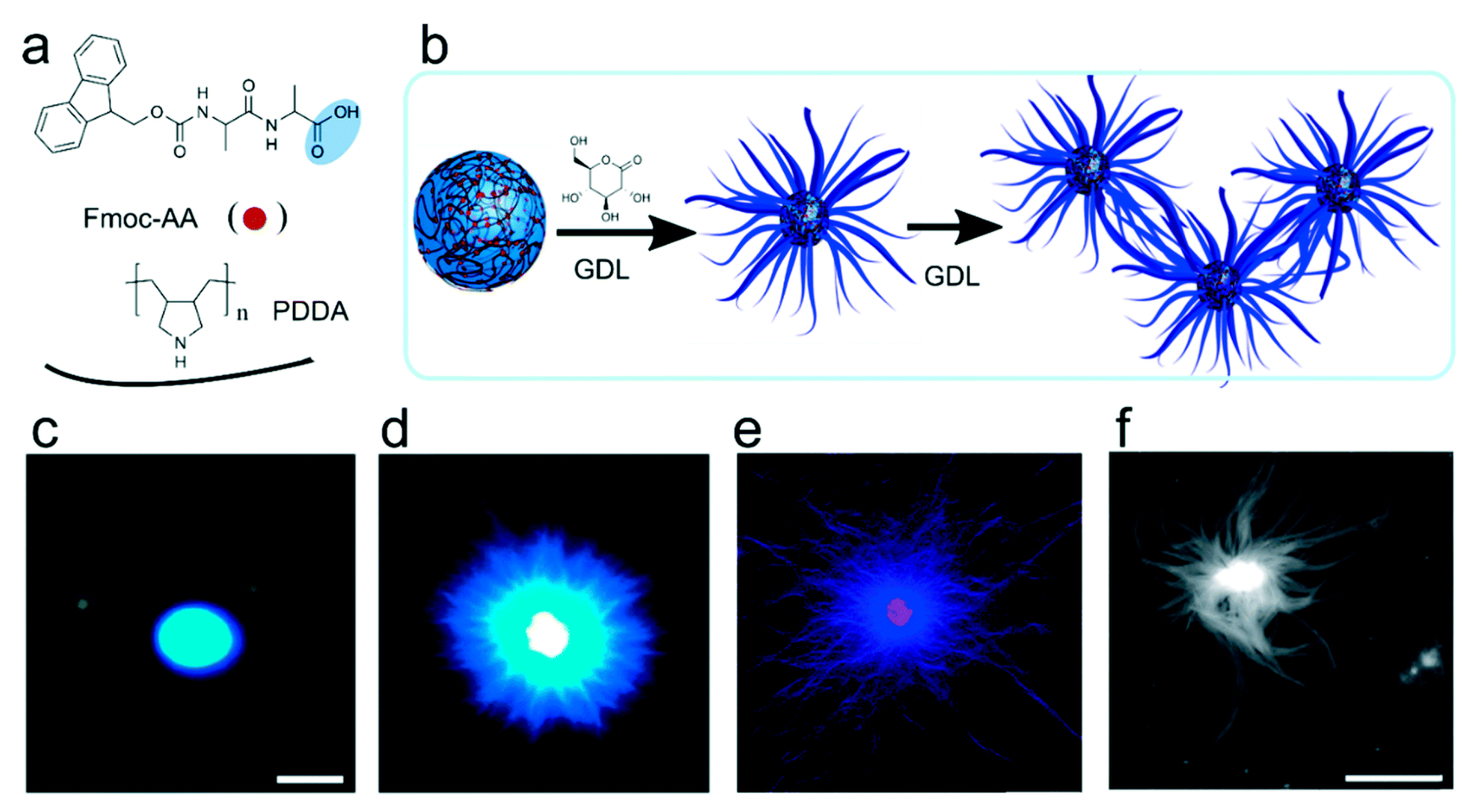
5.1. Simple Coacervates
5.2. Complex Coacervates
5.2.1. Polyelectrolyte–Polyelectrolyte Types
5.2.2. Polyelectrolyte–Surfactant Types
5.2.3. Surfactant–Surfactant Types
5.2.4. Peptide/Protein Types
6. Applications of Coacervates
6.1. Wastewater Treatment
6.2. Protein Purification
6.3. Food Formulation
6.4. Cellular Mimics
6.5. Nanoparticle Synthesis
6.6. Delivery Carrier
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veis, A. A Review of the Early Development of the Thermodynamics of the Complex Coacervation Phase Separation. Adv. Colloid Interface Sci. 2011, 167, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kayitmazer, A.B. Thermodynamics of Complex Coacervation. Adv. Colloid Interface Sci. 2017, 239, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Menger, F.M.; Sykes, B.M. Anatomy of a Coacervate. Langmuir 1998, 14, 4131–4137. [Google Scholar] [CrossRef]
- Bungenberg De Jong, H.G.; Kruyt, H.R. Coacervation (partial miscibility in colloid systems). Proc. K. Ned. Akad. Wet. 1929, 32, 849–856. [Google Scholar]
- Priftis, D.; Tirrell, M. Phase Behaviour and Complex Coacervation of Aqueous Polypeptide Solutions. Soft Matter 2012, 8, 9396–9405. [Google Scholar] [CrossRef]
- Sing, C.E. Development of the Modern Theory of Polymeric Complex Coacervation. Adv. Colloid Interface Sci. 2017, 239, 2–16. [Google Scholar] [CrossRef]
- Tiebackx, F.W. GleichzeitigeAusflockungzweierKolloide. Zeitschr. F. Chem. Und Ind. Der Kolloide 1911, 8, 198–201. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y. Applications of Small-Angle X-ray Scattering/Small-Angle Neutron Scattering and Cryogenic Transmission Electron Microscopy to Understand Self-Assembly of Surfactants. Curr. Opin. Colloid Interface Sci. 2019, 42, 1–16. [Google Scholar] [CrossRef]
- Kumar, D.; Steele, E.J.; Wickramasinghe, N.C. Preface: The origin of life and astrobiology. Adv. Genet. 2020, 106, xv. [Google Scholar] [CrossRef]
- Oparin-Haldane Theory|Biology|Britannica. Available online: https://www.britannica.com/science/Oparin-Haldane-theory (accessed on 8 July 2022).
- Zhou, M. The Origin of Life Discovered:ΣRNA. OALib 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Tirard, S.J.B.S. Haldane and the Origin of Life. J. Genet. 2017, 96, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Bose, B.; Dora, T.T.-Y. Can coacervation unify disperate hypotheses in the origin of cellular life? Curr. Opin. Colloid Interface Sci. 2021, 52, 101415. [Google Scholar] [CrossRef]
- Ivinova, O.N.; Izumrudov, V.A.; Muronetz, V.I.; Galaev, I.Y.; Mattiasson, B. Influence of Complexing Polyanions on the Thermostability of Basic Proteins. Macromol. Biosci. 2003, 3, 210–215. [Google Scholar] [CrossRef]
- Hwang, D.S.; Zeng, H.; Srivastava, A.; Krogstad, D.V.; Tirrell, M.; Israelachvili, J.N.; Waite, J.H. Viscosity and Interfacial Properties in a Mussel-Inspired Adhesive Coacervate. Soft Matter 2010, 6, 3232–3236. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Weaver, J.C.; Morse, D.E.; Waite, J.H. The Tube Cement of Phragmatopoma Californica: A Solid Foam. J. Exp. Biol. 2004, 207, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, H.R.; Jackson, L.C. Colloid Science Vol. II, Vol. II; Elsevier Publ. Co: New York, NY, USA, 1949. [Google Scholar]
- Zhao, W.; Wang, Y. Coacervation with Surfactants: From Single-Chain Surfactants to Gemini Surfactants. Adv. Colloid Interface Sci. 2017, 239, 199–212. [Google Scholar] [CrossRef]
- Ou, Z.; Muthukumar, M. Entropy and Enthalpy of Polyelectrolyte Complexation: Langevin Dynamics Simulations. J. Chem. Phys. 2006, 124, 154902. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Yigit, C.; Giet, M.; van der Zidek, W.; Jankowski, J.; Dzubiella, J.; Ballauff, M. Interaction of Human Serum Albumin with Short Polyelectrolytes: A Study by Calorimetry and Computer Simulations. Soft Matter 2015, 11, 4630–4639. [Google Scholar] [CrossRef]
- Lytle, T.K.; Sing, C.E. Transfer Matrix Theory of Polymer Complex Coacervation. Soft Matter 2017, 13, 7001–7012. [Google Scholar] [CrossRef]
- Jho, Y.; Yoo, H.Y.; Lin, Y.; Han, S.; Hwang, D.S. Molecular and Structural Basis of Low Interfacial Energy of Complex Coacervates in Water. Adv. Colloid Interface Sci. 2017, 239, 61–73. [Google Scholar] [CrossRef]
- Ghasemi, M.; Larson, R.G. Future Directions in Physiochemical Modeling of the Thermodynamics of Polyelectrolyte Coacervates. AIChE J. 2022, 68, e17646. [Google Scholar] [CrossRef]
- Fu, J.; Schlenoff, J.B. Driving Forces for Oppositely Charged Polyion Association in Aqueous Solutions: Enthalpic, Entropic, but Not Electrostatic. J. Am. Chem. Soc. 2016, 138, 980–990. [Google Scholar] [CrossRef]
- Zheng, B.; Avni, Y.; Andelman, D.; Podgornik, R. Phase Separation of Polyelectrolytes: The Effect of Charge Regulation. J. Phys. Chem. B 2021, 125, 7863–7870. [Google Scholar] [CrossRef]
- Records, M.T., Jr.; Anderson, C.F.; Lohman, T.M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: The roles of ion association or release, screening and ion effects on water activity. Q. Rev. Biophys. 1978, 11, 103–178. [Google Scholar] [CrossRef]
- Mitra, D.; Chakraborty, I.; Bhattacharya, S.C.; Moulik, S.P. Interfacial and solution properties of tetraalkylammonium bromides and their sodium dodecylsulphate interacted products: A detailed physicochemical study. Langmuir 2007, 23, 3049–3061. [Google Scholar] [CrossRef]
- Overbeek, J.T.; Voorn, M.J. Phase Separation in Polyelectrolyte Solutions; Theory of Complex Coacervation. J. Cell. Physiol. Suppl. 1957, 49, 7–22. [Google Scholar] [CrossRef]
- Voorn, M.J. Complex Coacervation. III. Thermodynamic Calculations on Three-Component Systems. Recl. Des. Trav. Chim. Des. Pays-Bas 1956, 75, 427–446. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell university press: Ithaca, NY, USA, 1953; ISBN 978-0-8014-0134-3. [Google Scholar]
- Delaney, K.T.; Fredrickson, G.H. Theory of Polyelectrolyte Complexation—Complex Coacervates Are Self-Coacervates. J. Chem. Phys. 2017, 146, 224902. [Google Scholar] [CrossRef]
- Michaeli, I.; Overbeek, J.T.G.; Voorn, M.J. Phase separation of polyelectrolyte solutions. J. Polym. Sci. 1957, 23, 443–450. [Google Scholar] [CrossRef]
- Veis, A. Phase separation in polyelectrolyte solutions. II. interaction effects. J. Phys. Chem. 1961, 65, 1798–1803. [Google Scholar] [CrossRef]
- Nakajima, A.; Sato, H. Phase Relationships of an Equivalent Mixture of Sulfated Polyvinyl Alcohol and Aminoacetalyzed Polyvinyl Alcohol in Microsalt Aqueous Solution. Biopolymers 1972, 11, 1345–1355. [Google Scholar] [CrossRef]
- Sato, H.; Nakajima, A. Complex Coacervation in Sulfated Polyvinyl Alcohol-Amino Acetalyzed Polyvinyl Alcohol System. Colloid Polym. Sci. 1974, 252, 294–297. [Google Scholar] [CrossRef]
- Tainaka, K.-I. Effect of Counterions on Complex Coacervation. Biopolymers 1980, 19, 1289–1298. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Cohen Stuart, M.A. Cylindrical Cell Model for the Electrostatic Free Energy of Polyelectrolyte Complexes. Langmuir 2004, 20, 4764–4770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shklovskii, B.I. Phase Diagram of Solution of Oppositely Charged Polyelectrolytes. Phys. A Stat. Mech. Its Appl. 2005, 352, 216–238. [Google Scholar] [CrossRef]
- Borue, V.Y.; Erukhimovich, I.Y. A Statistical Theory of Globular Polyelectrolyte Complexes. Macromolecules 1990, 23, 3625–3632. [Google Scholar] [CrossRef]
- Castelnovo, M.; Joanny, J.-F. Complexation between Oppositely Charged Polyelectrolytes: Beyond the Random Phase Approximation. Eur. Phys. J. E 2001, 6, 377–386. [Google Scholar] [CrossRef]
- Kudlay, A.; Ermoshkin, A.V.; Olvera de la Cruz, M. Complexation of Oppositely Charged Polyelectrolytes: Effect of Ion Pair Formation. Macromolecules 2004, 37, 9231–9241. [Google Scholar] [CrossRef]
- Castelnovo, M.; Joanny, J.F. Phase Diagram of Diblock Polyampholyte Solutions. Macromolecules 2002, 35, 4531–4538. [Google Scholar] [CrossRef]
- Perry, S.L.; Sing, C.E. PRISM-Based Theory of Complex Coacervation: Excluded Volume versus Chain Correlation. Macromolecules 2015, 48, 5040–5053. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Song, J.; Forman-Kay, J.D.; Chan, H.S. Random-Phase-Approximation Theory for Sequence-Dependent, Biologically Functional Liquid-Liquid Phase Separation of Intrinsically Disordered Proteins. J. Mol. Liq. 2017, 228, 176–193. [Google Scholar] [CrossRef] [Green Version]
- Sing, C.E.; Perry, S.L. Recent Progress in the Science of Complex Coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef]
- Qin, J.; de Pablo, J.J. Criticality and Connectivity in Macromolecular Charge Complexation. Macromolecules 2016, 49, 8789–8800. [Google Scholar] [CrossRef]
- Rumyantsev, A.M.; de Pablo, J.J. Liquid Crystalline and Isotropic Coacervates of Semiflexible Polyanions and Flexible Polycations. Macromolecules 2019, 52, 5140–5156. [Google Scholar] [CrossRef]
- Priftis, D.; Xia, X.; Margossian, K.O.; Perry, S.L.; Leon, L.; Qin, J.; de Pablo, J.J.; Tirrell, M. Ternary, Tunable Polyelectrolyte Complex Fluids Driven by Complex Coacervation. Macromolecules 2014, 47, 3076–3085. [Google Scholar] [CrossRef]
- Chollakup, R.; Smitthipong, W.; Eisenbach, C.D.; Tirrell, M. Phase Behavior and Coacervation of Aqueous Poly(Acrylic Acid)−Poly(Allylamine) Solutions. Macromolecules 2010, 43, 2518–2528. [Google Scholar] [CrossRef]
- Perry, S.L.; Li, Y.; Priftis, D.; Leon, L.; Tirrell, M. The Effect of Salt on the Complex Coacervation of Vinyl Polyelectrolytes. Polymers 2014, 6, 1756–1772. [Google Scholar] [CrossRef]
- Fares, H.M.; Ghoussoub, Y.E.; Delgado, J.D.; Fu, J.; Urban, V.S.; Schlenoff, J.B. Scattering Neutrons along the Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2018, 51, 4945–4955. [Google Scholar] [CrossRef]
- Wang, Q.; Schlenoff, J.B. The Polyelectrolyte Complex/Coacervate Continuum. Macromolecules 2014, 47, 3108–3116. [Google Scholar] [CrossRef]
- Radhakrishna, M.; Basu, K.; Liu, Y.; Shamsi, R.; Perry, S.L.; Sing, C.E. Molecular Connectivity and Correlation Effects on Polymer Coacervation. Macromolecules 2017, 50, 3030–3037. [Google Scholar] [CrossRef]
- Liu, Y.; Momani, B.; Winter, H.H.; Perry, S.L. Rheological Characterization of Liquid-to-Solid Transitions in Bulk Polyelectrolyte Complexes. Soft Matter 2017, 13, 7332–7340. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Bleuel, M.; Prabhu, V.M. Lower Critical Solution Temperature in Polyelectrolyte Complex Coacervates. ACS Macro Lett. 2019, 8, 289–293. [Google Scholar] [CrossRef]
- Wei, M.-T.; Elbaum-Garfinkle, S.; Holehouse, A.S.; Chen, C.C.-H.; Feric, M.; Arnold, C.B.; Priestley, R.D.; Pappu, R.V.; Brangwynne, C.P. Phase Behaviour of Disordered Proteins Underlying Low Density and High Permeability of Liquid Organelles. Nat. Chem. 2017, 9, 1118–1125. [Google Scholar] [CrossRef]
- Lou, J.; Friedowitz, S.; Qin, J.; Xia, Y. Tunable Coacervation of Well-Defined Homologous Polyanions and Polycations by Local Polarity. ACS Cent. Sci. 2019, 5, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, E.; Westphal, A.H.; Borst, J.W.; Cohen Stuart, M.A.; van der Gucht, J. Binodal Compositions of Polyelectrolyte Complexes. Macromolecules 2010, 43, 6476–6484. [Google Scholar] [CrossRef]
- Li, L.; Srivastava, S.; Andreev, M.; Marciel, A.B.; de Pablo, J.J.; Tirrell, M.V. Phase Behavior and Salt Partitioning in Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 2988–2995. [Google Scholar] [CrossRef]
- Fu, J.; Fares, H.M.; Schlenoff, J.B. Ion-Pairing Strength in Polyelectrolyte Complexes. Macromolecules 2017, 50, 1066–1074. [Google Scholar] [CrossRef]
- Sadman, K.; Wang, Q.; Chen, Y.; Keshavarz, B.; Jiang, Z.; Shull, K.R. Influence of Hydrophobicity on Polyelectrolyte Complexation. Macromolecules 2017, 50, 9417–9426. [Google Scholar] [CrossRef]
- Lim, S.; Moon, D.; Kim, H.J.; Seo, J.H.; Kang, I.S.; Cha, H.J. Interfacial Tension of Complex Coacervated Mussel Adhesive Protein According to the Hofmeister Series. Langmuir 2014, 30, 1108–1115. [Google Scholar] [CrossRef]
- Spruijt, E.; Sprakel, J.; Stuart, M.A.C.; Gucht, J. van der Interfacial Tension between a Complex Coacervate Phase and Its Coexisting Aqueous Phase. Soft Matter 2009, 6, 172–178. [Google Scholar] [CrossRef]
- Priftis, D.; Farina, R.; Tirrell, M. Interfacial Energy of Polypeptide Complex Coacervates Measured via Capillary Adhesion. Langmuir 2012, 28, 8721–8729. [Google Scholar] [CrossRef]
- Sun, J.; Perry, S.L.; Schiffman, J.D. Electrospinning Nanofibers from Chitosan/Hyaluronic Acid Complex Coacervates. Biomacromolecules 2019, 20, 4191–4198. [Google Scholar] [CrossRef]
- Huang, J.; Morin, F.J.; Laaser, J.E. Charge-Density-Dominated Phase Behavior and Viscoelasticity of Polyelectrolyte Complex Coacervates. Macromolecules 2019, 52, 4957–4967. [Google Scholar] [CrossRef]
- Suarez-Martinez, P.C.; Batys, P.; Sammalkorpi, M.; Lutkenhaus, J.L. Time–Temperature and Time–Water Superposition Principles Applied to Poly(Allylamine)/Poly(Acrylic Acid) Complexes. Macromolecules 2019, 52, 3066–3074. [Google Scholar] [CrossRef]
- Heo, T.-Y.; Kim, S.; Chen, L.; Sokolova, A.; Lee, S.; Choi, S.-H. Molecular Exchange Kinetics in Complex Coacervate Core Micelles: Role of Associative Interaction. ACS Macro Lett. 2021, 10, 1138–1144. [Google Scholar] [CrossRef]
- Markarian, M.Z.; Hariri, H.H.; Reisch, A.; Urban, V.S.; Schlenoff, J.B. A Small-Angle Neutron Scattering Study of the Equilibrium Conformation of Polyelectrolytes in Stoichiometric Saloplastic Polyelectrolyte Complexes. Macromolecules 2012, 45, 1016–1024. [Google Scholar] [CrossRef]
- Spruijt, E.; Leermakers, F.A.M.; Fokkink, R.; Schweins, R.; van Well, A.A.; Cohen Stuart, M.A.; van der Gucht, J. Structure and Dynamics of Polyelectrolyte Complex Coacervates Studied by Scattering of Neutrons, X-rays, and Light. Macromolecules 2013, 46, 4596–4605. [Google Scholar] [CrossRef]
- Joshi, N.; Rawat, K.; Bohidar, H.B. PH and Ionic Strength Induced Complex Coacervation of Pectin and Gelatin A. Food Hydrocoll. 2018, 74, 132–138. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Iyilik, E.K. Complex Coacervation of Hyaluronic Acid and Chitosan: Effects of PH, Ionic Strength, Charge Density, Chain Length and the Charge Ratio. Soft Matter 2015, 11, 8605–8612. [Google Scholar] [CrossRef]
- Chollakup, R.; Beck, J.B.; Dirnberger, K.; Tirrell, M.; Eisenbach, C.D. Polyelectrolyte Molecular Weight and Salt Effects on the Phase Behavior and Coacervation of Aqueous Solutions of Poly(Acrylic Acid) Sodium Salt and Poly(Allylamine) Hydrochloride. Macromolecules 2013, 46, 2376–2390. [Google Scholar] [CrossRef]
- Priftis, D.; Laugel, N.; Tirrell, M. Thermodynamic Characterization of Polypeptide Complex Coacervation. Langmuir 2012, 28, 15947–15957. [Google Scholar] [CrossRef]
- Perry, S.L.; Leon, L.; Hoffmann, K.Q.; Kade, M.J.; Priftis, D.; Black, K.A.; Wong, D.; Klein, R.A.; Pierce, C.F.; Margossian, K.O.; et al. Chirality-Selected Phase Behaviour in Ionic Polypeptide Complexes. Nat. Commun. 2015, 6, 6052. [Google Scholar] [CrossRef]
- Ali, S.; Prabhu, V.M. Relaxation behaviour by time-salt and time-temperature superposition of polyelectrolyte complexes from coacervate to precipitate. Gels 2018, 4, 11. [Google Scholar] [CrossRef]
- Kuroyanagi, S.; Shimada, N.; Fujii, S.; Furuta, T.; Harada, A.; Sakurai, K.; Maruyama, A. Highly Ordered Polypeptide with UCST Phase Separation Behavior. J. Am. Chem. Soc. 2019, 141, 1261–1268. [Google Scholar] [CrossRef]
- Vieregg, J.R.; Lueckheide, M.; Marciel, A.B.; Leon, L.; Bologna, A.J.; Rivera, J.R.; Tirrell, M.V. Oligonucleotide–Peptide Complexes: Phase Control by Hybridization. J. Am. Chem. Soc. 2018, 140, 1632–1638. [Google Scholar] [CrossRef]
- Cummings, C.S.; Obermeyer, A.C. Phase Separation Behavior of Supercharged Proteins and Polyelectrolytes. Biochemistry 2018, 57, 314–323. [Google Scholar] [CrossRef]
- Aumiller, W.M.; Keating, C.D. Phosphorylation-Mediated RNA/Peptide Complex Coacervation as a Model for Intracellular Liquid Organelles. Nat. Chem. 2016, 8, 129–137. [Google Scholar] [CrossRef]
- Anvari, M.; Pan, C.-H.; Yoon, W.-B.; Chung, D. Characterization of Fish Gelatin–Gum Arabic Complex Coacervates as Influenced by Phase Separation Temperature. Int. J. Biol. Macromol. 2015, 79, 894–902. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.; Kim, S.; Jho, Y.; Hwang, D.S. Upper Critical Solution Temperature (UCST) Behavior of Coacervate of Cationic Protamine and Multivalent Anions. Polymers 2019, 11, 691. [Google Scholar] [CrossRef]
- Nakashima, K.K.; van Haren, M.H.I.; André, A.A.M.; Robu, I.; Spruijt, E. Active Coacervate Droplets Are Protocells That Grow and Resist Ostwald Ripening. Nat. Commun. 2021, 12, 3819. [Google Scholar] [CrossRef]
- Sofronova, A.A.; Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Protein-Polyelectrolyte Complexes: Molecular Dynamics Simulations and Experimental Study. Polymer 2017, 113, 39–45. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Y.; Yeo, J.; Ting, J.M.; Tirrell, M.V. Effect of mixed solvents on polyelectrolyte complexes with salt. Colloid Polym. Sci. 2020, 298, 887–894. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Nguyen, T.-Q.; Fredrickson, G.H.; Segalman, R.A. Complexation of a Conjugated Polyelectrolyte and Impact on Optoelectronic Properties. ACS Macro Lett. 2019, 8, 88–94. [Google Scholar] [CrossRef]
- Khavani, M.; Batys, P.; Lalwani, S.M.; Eneh, C.I.; Leino, A.; Lutkenhaus, J.L.; Sammalkorpi, M. Effect of Ethanol and Urea as Solvent Additives on PSS–PDADMA Polyelectrolyte Complexation. Macromolecules 2022, 55, 3140–3150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, Y.; Liu, Z.; Chen, L.; Spruijt, E.; Wang, Y. A Multiresponsive Transformation between Surfactant-Based Coacervates and Vesicles. CCS Chem. 2021, 3, 358–366. [Google Scholar] [CrossRef]
- Menger, F.M.; Seredyuk, V.A.; Apkarian, R.P.; Wright, E.R. Colloidal Assemblies of Branched Geminis Studied by Cryo-Etch-HRSEM. J. Am. Chem. Soc. 2002, 124, 12408–12409. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cao, M.; Wang, J.; Wang, Y. Controllable Organization of a Carboxylic Acid Type Gemini Surfactant at Different PH Values by Adding Copper(II) Ions. J. Phys. Chem. B 2006, 110, 19479–19486. [Google Scholar] [CrossRef]
- Mohanty, B.; Bohidar, H.B. Systematic of Alcohol-Induced Simple Coacervation in Aqueous Gelatin Solutions. Biomacromolecules 2003, 4, 1080–1086. [Google Scholar] [CrossRef]
- Manaf, M.A.; Subuki, I.; Jai, J.; Raslan, R.; Mustapa, A.N. Encapsulation of Volatile Citronella Essential Oil by Coacervation: Efficiency and Release Study. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012072. [Google Scholar] [CrossRef]
- Perro, A.; Giraud, L.; Coudon, N.; Shanmugathasan, S.; Lapeyre, V.; Goudeau, B.; Douliez, J.-P.; Ravaine, V. Self-Coacervation of Ampholyte Polymer Chains as an Efficient Encapsulation Strategy. J. Colloid Interface Sci. 2019, 548, 275–283. [Google Scholar] [CrossRef]
- Lazko, J.; Popineau, Y.; Legrand, J. Soy Glycinin Microcapsules by Simple Coacervation Method. Colloids Surf. B Biointerfaces 2004, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Patel, U.; Ianiro, A.; Hurst, P.J.; Merham, J.G.; Patterson, J.P. Nonionic Block Copolymer Coacervates. Macromolecules 2020, 53, 6078–6086. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, Z.; Wang, Y.; Dimova, R. Resolving the Mechanisms of Soy Glycinin Self-Coacervation and Hollow-Condensate Formation. ACS Macro Lett. 2020, 9, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Burke, N.A.D.; Mazumder, M.A.J.; Hanna, M.; Stöver, H.D.H. Polyelectrolyte Complexation between Poly(Methacrylic Acid, Sodium Salt) and Poly(Diallyldimethylammonium Chloride) or Poly [2-(Methacryloyloxyethyl) Trimethylammonium Chloride]. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4129–4143. [Google Scholar] [CrossRef]
- Lappan, U.; Scheler, U. Influence of the Nature of the Ion Pairs on the Segmental Dynamics in Polyelectrolyte Complex Coacervate Phases. Macromolecules 2017, 50, 8631–8636. [Google Scholar] [CrossRef]
- Salehi, A.; Desai, P.S.; Li, J.; Steele, C.A.; Larson, R.G. Relationship between Polyelectrolyte Bulk Complexation and Kinetics of Their Layer-by-Layer Assembly. Macromolecules 2015, 48, 400–409. [Google Scholar] [CrossRef]
- Zhao, M.; Zacharia, N.S. Sequestration of Methylene Blue into Polyelectrolyte Complex Coacervates. Macromol. Rapid Commun. 2016, 37, 1249–1255. [Google Scholar] [CrossRef]
- Vitorazi, L.; Ould-Moussa, N.; Sekar, S.; Fresnais, J.; Loh, W.; Chapel, J.-P.; Berret, J.-F. Evidence of a Two-Step Process and Pathway Dependency in the Thermodynamics of Poly(Diallyldimethylammonium Chloride)/Poly(Sodium Acrylate) Complexation. Soft Matter 2014, 10, 9496–9505. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of Polyelectrolyte Structure on Protein−Polyelectrolyte Coacervates: Coacervates of Bovine Serum Albumin with Poly(Diallyldimethylammonium Chloride) versus Chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Leisner, D.; Imae, T. Interpolyelectrolyte Complex and Coacervate Formation of Poly(Glutamic Acid) with a Dendrimer Studied by Light Scattering and SAXS. J. Phys. Chem. B 2003, 107, 8078–8087. [Google Scholar] [CrossRef]
- Li, H.; Fauquignon, M.; Haddou, M.; Schatz, C.; Chapel, J.-P. Interfacial Behavior of Solid- and Liquid-like Polyelectrolyte Complexes as a Function of Charge Stoichiometry. Polymers 2021, 13, 3848. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Digby, Z.A.; Schlenoff, J.B. Precision Doping of Polyelectrolyte Complexes: Insight on the Role of Ions. Macromolecules 2020, 53, 5465–5474. [Google Scholar] [CrossRef]
- Swanson, J.P.; Monteleone, L.R.; Haso, F.; Costanzo, P.J.; Liu, T.; Joy, A. A Library of Thermoresponsive, Coacervate-Forming Biodegradable Polyesters. Macromolecules 2015, 48, 3834–3842. [Google Scholar] [CrossRef]
- Cruz, M.A.; Morris, D.L.; Swanson, J.P.; Kundu, M.; Mankoci, S.G.; Leeper, T.C.; Joy, A. Efficient Protein Encapsulation within Thermoresponsive Coacervate-Forming Biodegradable Polyesters. ACS Macro Lett. 2018, 7, 477–481. [Google Scholar] [CrossRef]
- Kundu, M.; Morris, D.L.; Cruz, M.A.; Miyoshi, T.; Leeper, T.C.; Joy, A. Elucidating the Molecular Interactions of Encapsulated Doxorubicin within a Nonionic, Thermoresponsive Polyester Coacervate. ACS Appl. Bio Mater. 2020, 3, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Menefee, J.R.; Liu, Q.; Dhinojwala, A.; Joy, A. Lower Critical Solution Temperature-Driven Self-Coacervation of Nonionic Polyester Underwater Adhesives. ACS Nano 2020, 14, 8359–8367. [Google Scholar] [CrossRef]
- Li, Y.; Dubin, P.L.; Havel, H.A.; Edwards, S.L.; Dautzenberg, H. Complex Formation between Polyelectrolyte and Oppositely Charged Mixed Micelles: Soluble Complexes vs. Coacervation. Langmuir 1995, 11, 2486–2492. [Google Scholar] [CrossRef]
- Kizilay, E.; Dinsmore, A.D.; Hoagland, D.A.; Sun, L.; Dubin, P.L. Evolution of Hierarchical Structures in Polyelectrolyte–Micelle Coacervates. Soft Matter 2013, 9, 7320–7332. [Google Scholar] [CrossRef]
- Wang, Y.; Kimura, K.; Dubin, P.L.; Jaeger, W. Polyelectrolyte−Micelle Coacervation: Effects of Micelle Surface Charge Density, Polymer Molecular Weight, and Polymer/Surfactant Ratio. Macromolecules 2000, 33, 3324–3331. [Google Scholar] [CrossRef]
- Kizilay, E.; Maccarrone, S.; Foun, E.; Dinsmore, A.D.; Dubin, P.L. Cluster Formation in Polyelectrolyte−Micelle Complex Coacervation. J. Phys. Chem. B 2011, 115, 7256–7263. [Google Scholar] [CrossRef]
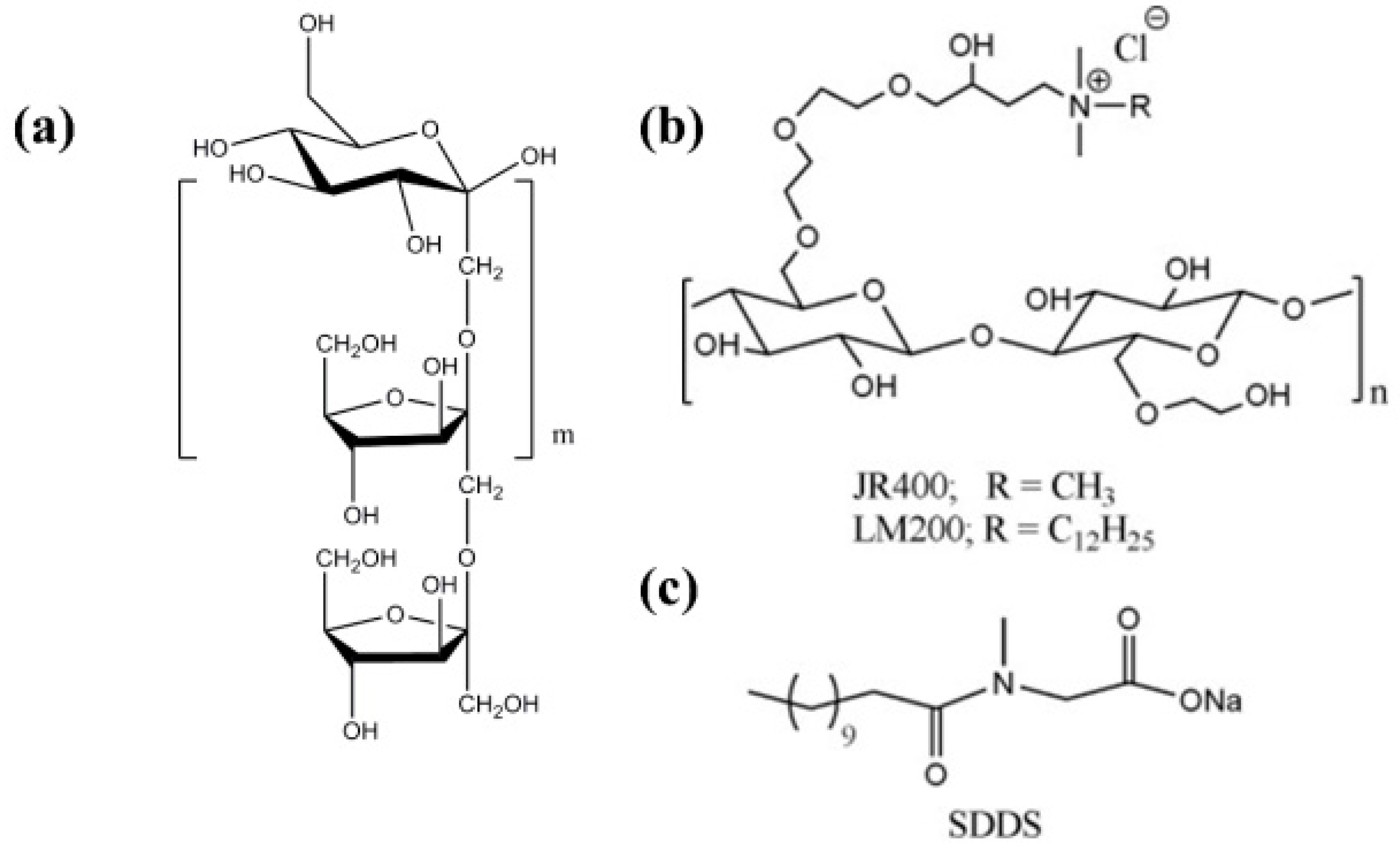
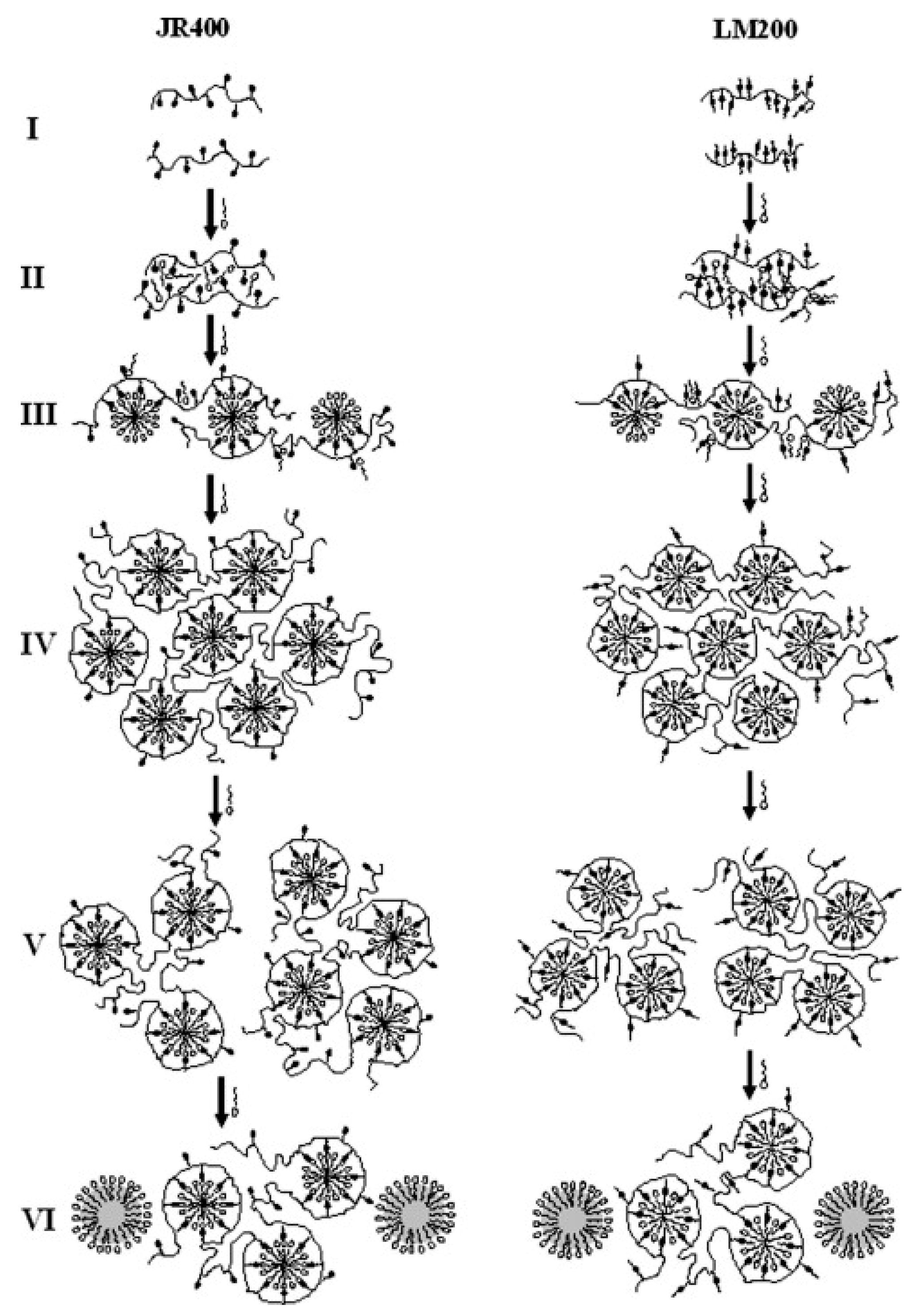
- Dan, A.; Ghosh, S.; Moulik, S.P. Interaction of Cationic Hydroxyethylcellulose (JR400) and Cationic Hydrophobically Modified Hydroxyethylcellulose (LM200) with the Amino-Acid Based Anionic Amphiphile Sodium N-Dodecanoyl Sarcosinate(SDDS) in Aqueous Medium. Carbohydr. Polym. 2010, 80, 44–52. [Google Scholar] [CrossRef]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemistry of the Interaction between Inulin and Alkyltrimethylammonium Bromides in Aqueous Medium and the Formed Coacervates. J. Phys. Chem. B 2009, 113, 8505–8513. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Ghosh, S.; Moulik, S.P. Interaction between a Bio-Tolerable Amino-Acid Based Amphiphile (N-Dodecanoylsarcosinate, SDDS) and Modified Cationic Polymers, Hydroxyethylcelluloses (JR 400, and LM 200) in Isopropanol-Water Medium. Colloids Surf. A Physicochem. Eng. Asp. 2019, 566, 156–165. [Google Scholar] [CrossRef]
- Mandal, B.; Mondal, S.; Pan, A.; Moulik, S.P.; Ghosh, S. Physicochemical Study of the Interaction of Lysozyme with Surface Active Ionic Liquid 1-Butyl-3-Methylimidazolium Octylsulfate [BMIM] [OS] in Aqueous and Buffer Media. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 345–353. [Google Scholar] [CrossRef]
- Mitra, D.; Bhattacharya, S.C.; Moulik, S.P. Physicochemical Studies on the Interaction of Gelatin with Cationic Surfactants Alkyltrimethylammonium Bromides (ATABs) with Special Focus on the Behavior of the Hexadecyl Homologue. J. Phys. Chem. B 2008, 112, 6609–6619. [Google Scholar] [CrossRef]
- Mitra, D.; Moulik, S.P. Physicochemistry of HexadecylammoniumBromide and Its Methyl and Ethanolic Head Group Analogues in Buffered Aqueous and Gelatin Solution. J. Chem. Sci. 2010, 122, 349–362. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Miyake, M. Creation of New Functions by Combination of Surfactant and Polymer—Complex Coacervation with Oppositely Charged Polymer and Surfactant for Shampoo and Body Wash. J. Oleo Sci. 2019, 68, 525–539. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Schwarzenberger, K.; Huang, M.; Javadi, A.; Eckert, K. Formation of Structured Membranes by Coacervation of Xanthan Gum with CnTAB Surfactants. Langmuir 2019, 35, 13624–13635. [Google Scholar] [CrossRef]
- Hansson, P. Phase Behavior of Aqueous Polyion–Surfactant Ion Complex Salts: A Theoretical Analysis. J. Colloid Interface Sci. 2009, 332, 183–193. [Google Scholar] [CrossRef]
- Allen, R.J.; Warren, P.B. Phase Behaviour of Oppositely Charged Polymer/Surfactant Mixtures. EPL 2003, 64, 468. [Google Scholar] [CrossRef]
- Maiti, K.; Bhattacharya, S.C.; Moulik, S.P.; Panda, A.K. Effect of Bovine Serum Albumin on the Functionality and Structure of Catanionic Surfactant at Air–Buffer Interface. Mater. Sci. Eng. C 2013, 33, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Douliez, J.-P.; Martin, N.; Gaillard, C.; Beneyton, T.; Baret, J.-C.; Mann, S.; Beven, L. Catanionic Coacervate Droplets as a Surfactant-Based Membrane-Free Protocell Model. Angew. Chem. Int. Ed. 2017, 56, 13689–13693. [Google Scholar] [CrossRef] [PubMed]
- Dzuricky, M.; Rogers, B.A.; Shahid, A.; Cremer, P.S.; Chilkoti, A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 2020, 12, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, F.P.; Choi, S.; Meyer, M.O.; Bevilacqua, P.C.; Keating, C.D. rebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments. Nat. Commun. 2020, 11, 5949. [Google Scholar] [CrossRef]
- Te Brinke, E.; Groen, J.; Herrmann, A.; Heus, H.A.; Rivas, G.; Spruijt, E.; Huck, W.T.S. Dissipative adaptation in driven self-assembly leading to self-dividing fibrils. Nat. Nanotechnol. 2018, 13, 849–855. [Google Scholar] [CrossRef]
- Ma, C.; Malessa, A.; Boersma, A.J.; Liu, K.; Herrmann, A. Supercharged Proteins and Polypeptides. Adv. Mater. 2020, 32, e1905309. [Google Scholar] [CrossRef]
- Kumar, R.K.; Harniman, R.L.; Patil, A.J.; Mann, S. Self-transformation and structural reconfiguration in coacervate-based protocells. Chem. Sci. 2016, 7, 5879–5887. [Google Scholar] [CrossRef]
- Gabryelczyk, B.; Cai, H.; Shi, X.; Sun, Y.; Swinkels, P.J.M.; Salentinig, S.; Pervushin, K.; Miserez, A. Hydrogen bond guidance and aromatic stacking drive liquid-liquid phase separation of intrinsically disordered histidine-rich peptides. Nat. Commun. 2019, 10, 5465. [Google Scholar] [CrossRef]
- Wei, W.; Petrone, L.; Tan, Y.; Cai, H.; Israelachvili, J.N.; Miserez, A.; Waite, J.H. An Underwater Surface-Drying Peptide Inspired by a Mussel Adhesive Protein. Adv. Funct. Mater. 2016, 26, 3496–3507. [Google Scholar] [CrossRef]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef]
- Abbas, M.; Lipin’ski, W.P.; Wang, J.; Spruijt, E. Peptide-based coacervates as biomimetic Protocells. Chem. Soc. Rev. 2021, 50, 3690–3705. [Google Scholar] [CrossRef] [PubMed]
- Kapelner, R.A.; Yeong, V.; Obermeyer, A.C. Molecular determinants of protein-based coacervates. Curr. Opin. Colloid Interface Sci. 2021, 52, 10140. [Google Scholar] [CrossRef]
- Melnyk, A.; Namieśnik, J.; Wolska, L. Theory and Recent Applications of Coacervate-Based Extraction Techniques. TrAC Trends Anal. Chem. 2015, 71, 282–292. [Google Scholar] [CrossRef]
- Wang, Y.; Banziger, J.; Dubin, P.L.; Filippelli, G.; Nuraje, N. Adsorptive Partitioning of an Organic Compound onto Polyelectrolyte-Immobilized Micelles on Porous Glass and Sand. Environ. Sci. Technol. 2001, 35, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fan, Y.; Wang, H.; Wang, Y. Coacervate of Polyacrylamide and Cationic Gemini Surfactant for the Extraction of Methyl Orange from Aqueous Solution. Langmuir 2017, 33, 6846–6856. [Google Scholar] [CrossRef]
- Chiappisi, L.; Simon, M.; Gradzielski, M. Toward Bioderived Intelligent Nanocarriers for Controlled Pollutant Recovery and PH-Sensitive Binding. ACS Appl. Mater. Interfaces 2015, 7, 6139–6145. [Google Scholar] [CrossRef]
- Valley, B.; Jing, B.; Ferreira, M.; Zhu, Y. Rapid and Efficient Coacervate Extraction of Cationic Industrial Dyes from Wastewater. ACS Appl. Mater. Interfaces 2019, 11, 7472–7478. [Google Scholar] [CrossRef]
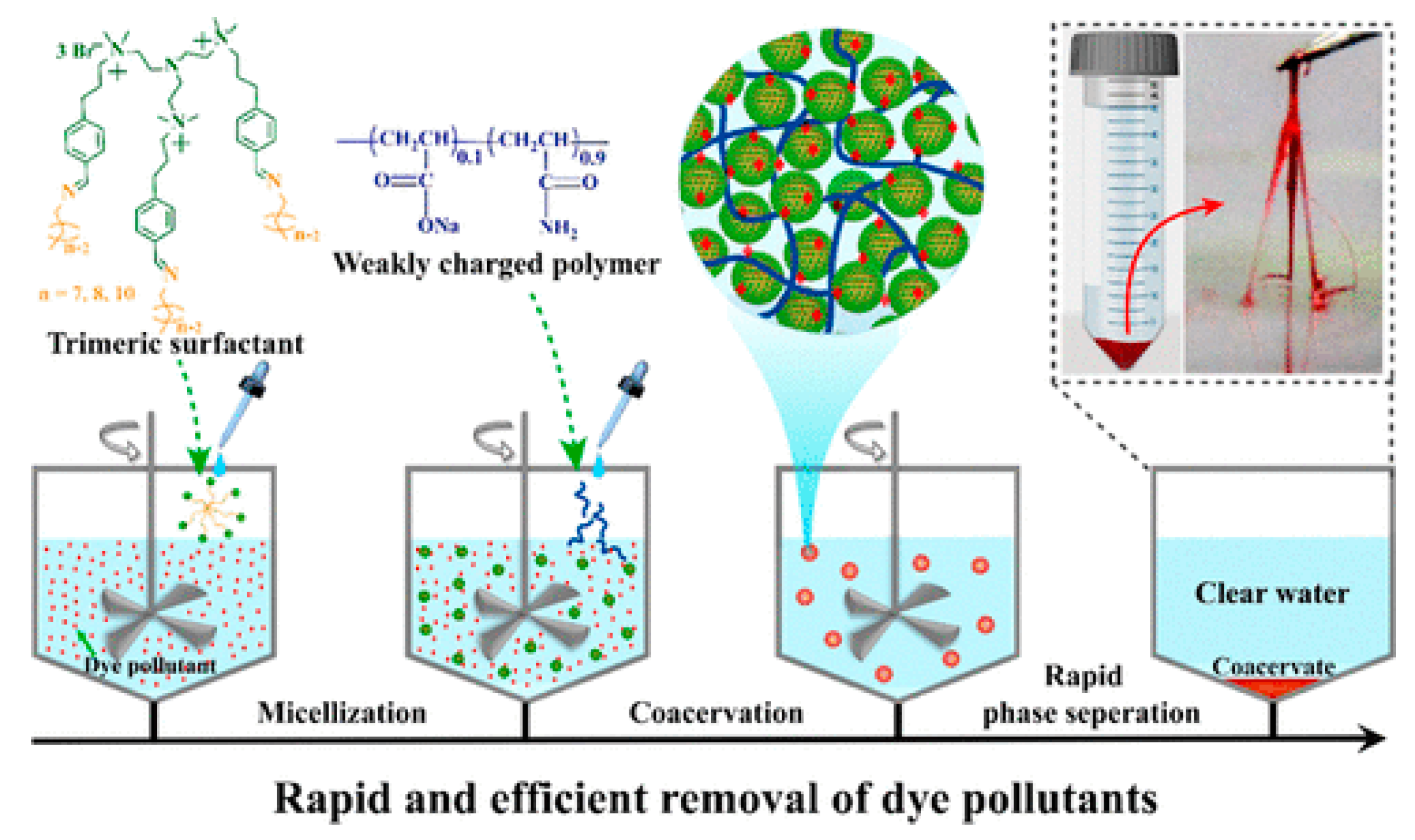
- Liu, B.; Zhao, W.; Shen, Y.; Fan, Y.; Wang, Y. Trimeric Cationic Surfactant Coacervation as a Versatile Approach for Removing Organic Pollutants. Langmuir 2021, 37, 5993–6001. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Caballero-Casero, N.; García-Fonseca, S.; Lunar, L.; Rubio, S. Multifunctional Vesicular Coacervates as Engineered Supramolecular Solvents for Wastewater Treatment. Chemosphere 2019, 223, 569–576. [Google Scholar] [CrossRef]
- Sieberz, J.; Wohlgemuth, K.; Schembecker, G. The Influence of Impurity Proteins on the Precipitation of a Monoclonal Antibody with an Anionic Polyelectrolyte. Sep. Purif. Technol. 2015, 146, 252–260. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Faisal, M.; Si, Y.; Guo, Y. Selective Protein Complexation and Coacervation by Polyelectrolytes. Adv. Colloid Interface Sci. 2017, 239, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mazzawi, M.; Chen, K.; Sun, L.; Dubin, P.L. Protein Purification by Polyelectrolyte Coacervation: Influence of Protein Charge Anisotropy on Selectivity. Biomacromolecules 2011, 12, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dubin, P.L.; Hoagland, D.A.; Sun, L. Protein-Selective Coacervation with Hyaluronic Acid. Biomacromolecules 2014, 15, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Selective Complex Coacervation of Pea Whey Proteins with Chitosan To Purify Main 2S Albumins. J. Agric. Food Chem. 2020, 68, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rawat, K.; Aswal, V.K.; Bohidar, H.B. Interactions in Globular Proteins with Polyampholyte: Coacervation Route for Protein Separation. RSC Adv. 2015, 5, 13579–13589. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Hamon, P.; Carvalho, A.F.; Bouhallab, S. Selective Coacervation between Lactoferrin and the Two Isoforms ofβ-Lactoglobulin. Food Hydrocoll. 2015, 48, 238–247. [Google Scholar] [CrossRef]
- A.Kapelner, R.; C.Obermeyer, A. Ionic Polypeptide Tags for Protein Phase Separation. Chem. Sci. 2019, 10, 2700–2707. [CrossRef]
- Lyons, R.E.; Elvin, C.M.; Taylor, K.; Lekieffre, N.; Ramshaw, J.A.M. Purification of Recombinant Protein by Cold-Coacervation of Fusion Constructs Incorporating Resilin-Inspired Polypeptides. Biotechnol. Bioeng. 2012, 109, 2947–2954. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of Active Ingredients in Polysaccharide–Protein Complex Coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex Coacervation: Principles, Mechanisms and Applications in Microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A. Development of Vanillin/β-Cyclodexterin Inclusion Microcapsules Using Flax Seed Gum-Rice Bran Protein Complex Coacervates. Int. J. Biol. Macromol. 2019, 131, 60–66. [Google Scholar] [CrossRef]
- Yuan, Y.; Kong, Z.-Y.; Sun, Y.-E.; Zeng, Q.-Z.; Yang, X.-Q. Complex Coacervation of Soy Protein with Chitosan: Constructing Antioxidant Microcapsule for Algal Oil Delivery. LWT 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Ach, D.; Briançon, S.; Broze, G.; Puel, F.; Rivoire, A.; Galvan, J.-M.; Chevalier, Y. Formation of Microcapsules by Complex Coacervation. Can. J. Chem. Eng. 2015, 93, 183–191. [Google Scholar] [CrossRef]
- Alexandre, J.D.B.; Barroso, T.L.C.T.; Oliveira, M.D.A.; Mendes, F.R.D.S.; Costa, J.M.C.D.; Moreira, R.D.A.; Furtado, R.F. Cross-Linked Coacervates of Cashew Gum and Gelatin in the Encapsulation of Pequi Oil. Cienc. Rural 2019, 49. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, B.; Akanbi, T.O.; Li, R.; Yang, W.; Adhikari, B.; Barrow, C.J. Microencapsulation of Lipase Produced Omega-3 Concentrates Resulted in Complex Coacervates with Unexpectedly High Oxidative Stability. J. Funct. Foods 2017, 35, 499–506. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolini, H.M.A.; Fávaro-Trindade, C.S. Microencapsulation of Aspartame by Double Emulsion Followed by Complex Coacervation to Provide Protection and Prolong Sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Pedroza-Islas, R.; Escalona-Buendía, H.B.; Pedraza-Chaverri, J.; Ponce-Alquicira, E. Comparative Study of the Microencapsulation by Complex Coacervation of Nisin in Combination with an Avocado Antioxidant Extract. Food Hydrocoll. 2017, 62, 49–57. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Microencapsulated Cells of Lactobacillus Paracasei Subsp. Paracasei in Biopolymer Complex Coacervates and Their Function in a Yogurt Matrix. Food Funct. 2017, 8, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. What Lava Lamps and Vinaigrette Can Teach Us about Cell Biology. Nature 2018, 555, 300–302. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein Intrinsic Disorder-Based Liquid–Liquid Phase Transitions in Biological Systems: Complex Coacervates and Membrane-Less Organelles. Adv. Colloid Interface Sci. 2017, 239, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Huck, W.T.S. Microfluidic Formation of Monodisperse Coacervate Organelles in Liposomes. Angew. Chem. 2017, 129, 9868–9872. [Google Scholar] [CrossRef]
- Drobot, B.; Iglesias-Artola, J.M.; Le Vay, K.; Mayr, V.; Kar, M.; Kreysing, M.; Mutschler, H.; Tang, T.-Y.D. Compartmentalised RNA Catalysis in Membrane-Free Coacervate Protocells. Nat. Commun. 2018, 9, 3643. [Google Scholar] [CrossRef]
- Kojima, T.; Takayama, S. Membraneless Compartmentalization Facilitates Enzymatic Cascade Reactions and Reduces Substrate Inhibition. ACS Appl. Mater. Interfaces 2018, 10, 32782–32791. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, M.; Qiu, D.; Mann, S. Response-Retaliation Behavior in Synthetic Protocell Communities. Angew. Chem. 2019, 131, 17922–17927. [Google Scholar] [CrossRef]
- Aumiller, W.M.; Keating, C.D. Experimental Models for Dynamic Compartmentalization of Biomolecules in Liquid Organelles: Reversible Formation and Partitioning in Aqueous Biphasic Systems. Adv. Colloid Interface Sci. 2017, 239, 75–87. [Google Scholar] [CrossRef]
- Cinar, S.; Cinar, H.; Chan, H.S.; Winter, R. Pressure-Sensitive and Osmolyte-Modulated Liquid–Liquid Phase Separation of Eye-Lens γ-Crystallins. J. Am. Chem. Soc. 2019, 141, 7347–7354. [Google Scholar] [CrossRef]
- Schuster, B.S.; Reed, E.H.; Parthasarathy, R.; Jahnke, C.N.; Caldwell, R.M.; Bermudez, J.G.; Ramage, H.; Good, M.C.; Hammer, D.A. Controllable Protein Phase Separation and Modular Recruitment to Form Responsive Membraneless Organelles. Nat. Commun. 2018, 9, 2985. [Google Scholar] [CrossRef]
- Banerjee, P.R.; Milin, A.N.; Moosa, M.M.; Onuchic, P.L.; Deniz, A.A. Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew. Chem. 2017, 129, 11512–11517. [Google Scholar] [CrossRef]
- Martin, N.; Tian, L.; Spencer, D.; Coutable-Pennarun, A.; Anderson, J.L.R.; Mann, S. Photoswitchable Phase Separation and Oligonucleotide Trafficking in DNA Coacervate Microdroplets. Angew. Chem. 2019, 131, 14736–14740. [Google Scholar] [CrossRef]
- Magana, J.R.; Sproncken, C.C.M.; Voets, I.K. On Complex Coacervate Core Micelles: Structure-Function Perspectives. Polymers 2020, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. Synthesis of Gelatin Nanoparticles via Simple Coacervation. J. Surf. Sci. Technol. 2005, 21, 149–160. [Google Scholar]
- Barthold, S.; Kletting, S.; Taffner, J.; Carvalho-Wodarz, C.D.S.; Lepeltier, E.; Loretz, B.; Lehr, C.-M. Preparation of Nanosized Coacervates of Positive and Negative Starch Derivatives Intended for Pulmonary Delivery of Proteins. J. Mater. Chem. B 2016, 4, 2377–2386. [Google Scholar] [CrossRef]
- Patra, S.; Basak, P.; Tibarewala, D.N. Synthesis of Gelatin Nano/Submicron Particles by Binary Nonsolvent Aided Coacervation (BNAC) Method. Mater. Sci. Eng. C 2016, 59, 310–318. [Google Scholar] [CrossRef]
- Awada, H.K.; Hwang, M.P.; Wang, Y. Towards Comprehensive Cardiac Repair and Regeneration after Myocardial Infarction: Aspects to Consider and Proteins to Deliver. Biomaterials 2016, 82, 94–112. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex Coacervate-Based Materials for Biomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef]
- Park, U.; Lee, M.S.; Jeon, J.; Lee, S.; Hwang, M.P.; Wang, Y.; Yang, H.S.; Kim, K. Coacervate-Mediated Exogenous Growth Factor Delivery for Scarless Skin Regeneration. Acta Biomater. 2019, 90, 179–191. [Google Scholar] [CrossRef]
- Johnson, N.R.; Kruger, M.; Goetsch, K.P.; Zilla, P.; Bezuidenhout, D.; Wang, Y.; Davies, N.H. Coacervate Delivery of Growth Factors Combined with a Degradable Hydrogel Preserves Heart Function after Myocardial Infarction. ACS Biomater. Sci. Eng. 2015, 1, 753–759. [Google Scholar] [CrossRef]
- Wang, Z.; Long, D.W.; Huang, Y.; Khor, S.; Li, X.; Jian, X.; Wang, Y. Fibroblast Growth Factor-1 Released from a Heparin Coacervate Improves Cardiac Function in a Mouse Myocardial Infarction Model. ACS Biomater. Sci. Eng. 2017, 3, 1988–1999. [Google Scholar] [CrossRef]
- Li, R.; Ma, J.; Wu, Y.; Nangle, M.; Zou, S.; Li, Y.; Yin, J.; Zhao, Y.; Xu, H.; Zhang, H.; et al. Dual Delivery of NGF and BFGF Coacervater Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Schwann Cells Apoptosis. Int. J. Biol. Sci. 2017, 13, 640–651. [Google Scholar] [CrossRef] [PubMed]
- De Silva, U.K.; Brown, J.L.; Lapitsky, Y. Poly(Allylamine)/Tripolyphosphate Coacervates Enable High Loading and Multiple-Month Release of Weakly Amphiphilic Anionic Drugs: An in Vitro Study with Ibuprofen. RSC Adv. 2018, 8, 19409–19419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, H.; Kasmi, S.; Van Herck, S.; Deswarte, K.; Lambrecht, B.N.; Hoogenboom, R.; Nuhn, L.; De Geest, B.G. A Synthetic, Transiently Thermoresponsive Homopolymer with UCST Behaviour within a Physiologically Relevant Window. Angew. Int. Ed. 2019, 58, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Tamura, A.; Yui, N. PH-Responsive Coacervate Droplets Formed from Acid-Labile Methylated Polyrotaxanes as an Injectable Protein Carrier. Biomacromolecules 2018, 19, 2238–2247. [Google Scholar] [CrossRef]
- Lim, Z.W.; Ping, Y.; Miserez, A. Glucose-Responsive Peptide Coacervates with High Encapsulation Efficiency for Controlled Release of Insulin. Bioconjugate Chem. 2018, 29, 2176–2180. [Google Scholar] [CrossRef]
- Chenglong, W.; Shuhan, X.; Jiayi, Y.; Wencai, G.; Guoxiong, X.; Hongjing, D. Dextran-Based Coacervate Nanodroplets as Potential Gene Carriers for Efficient Cancer Therapy. Carbohydr. Polym. 2020, 231, 115687. [Google Scholar] [CrossRef] [PubMed]
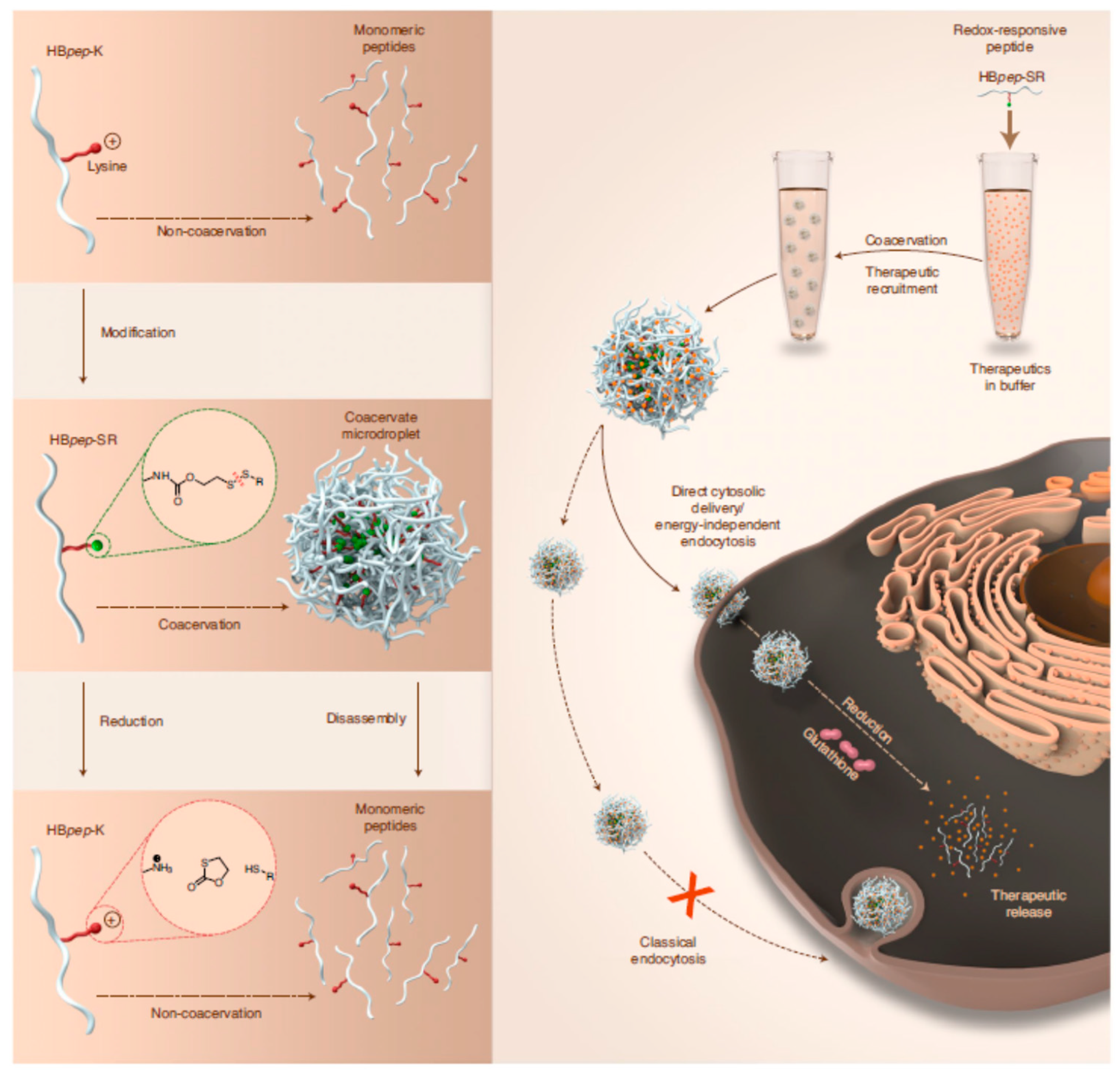
- Sun, Y.; Lau, S.Y.; Lim, Z.W.; Chang, S.C.; Ghadessy, F.; Partridge, A.; Miserez, A. Phase-Separating Peptides for Direct Cytosolic Delivery and Redox-Activated Release of Macromolecular Therapeutics. Nat. Chem. 2022, 14, 274–283. [Google Scholar] [CrossRef]
- Jing, H.; Du, X.; Mo, L.; Wang, H. Self-Coacervation of Carboxymethyl Chitosan as a PH-Responsive Encapsulation and Delivery Strategy. Int. J. Biol. Macromol. 2021, 192, 1169–1177. [Google Scholar] [CrossRef]
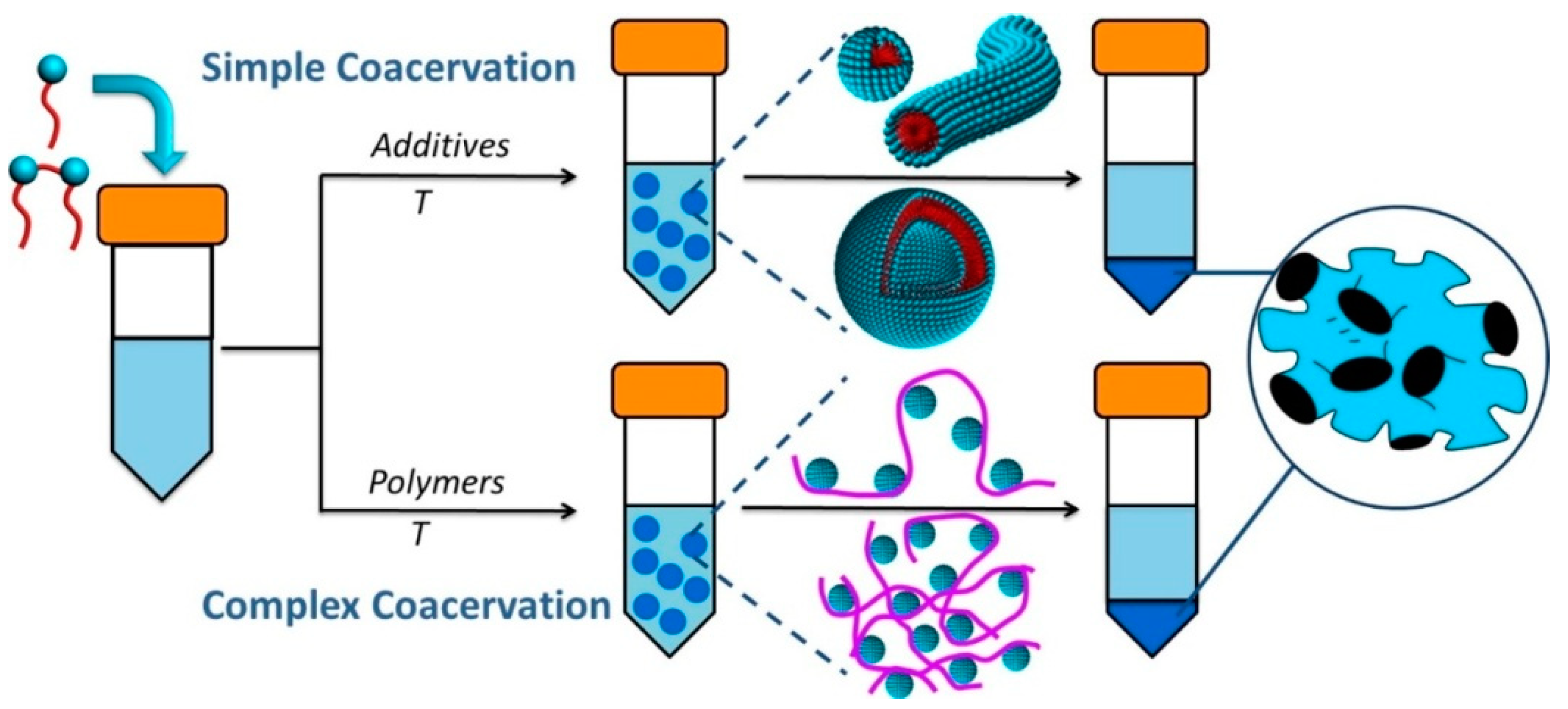

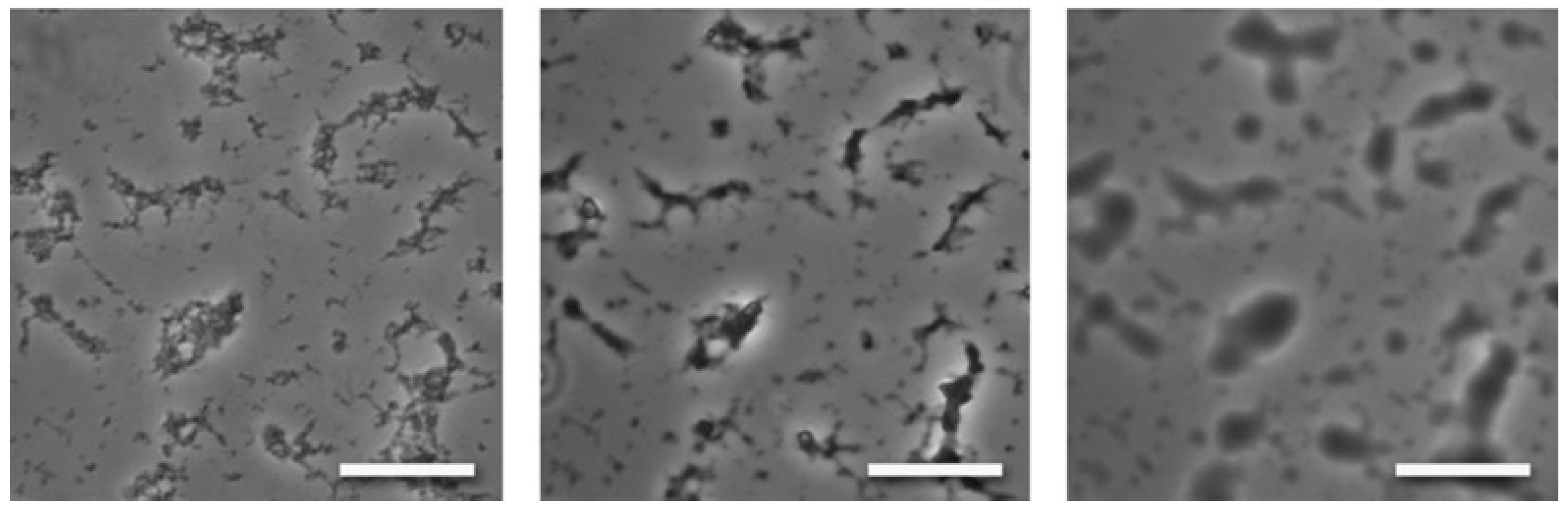

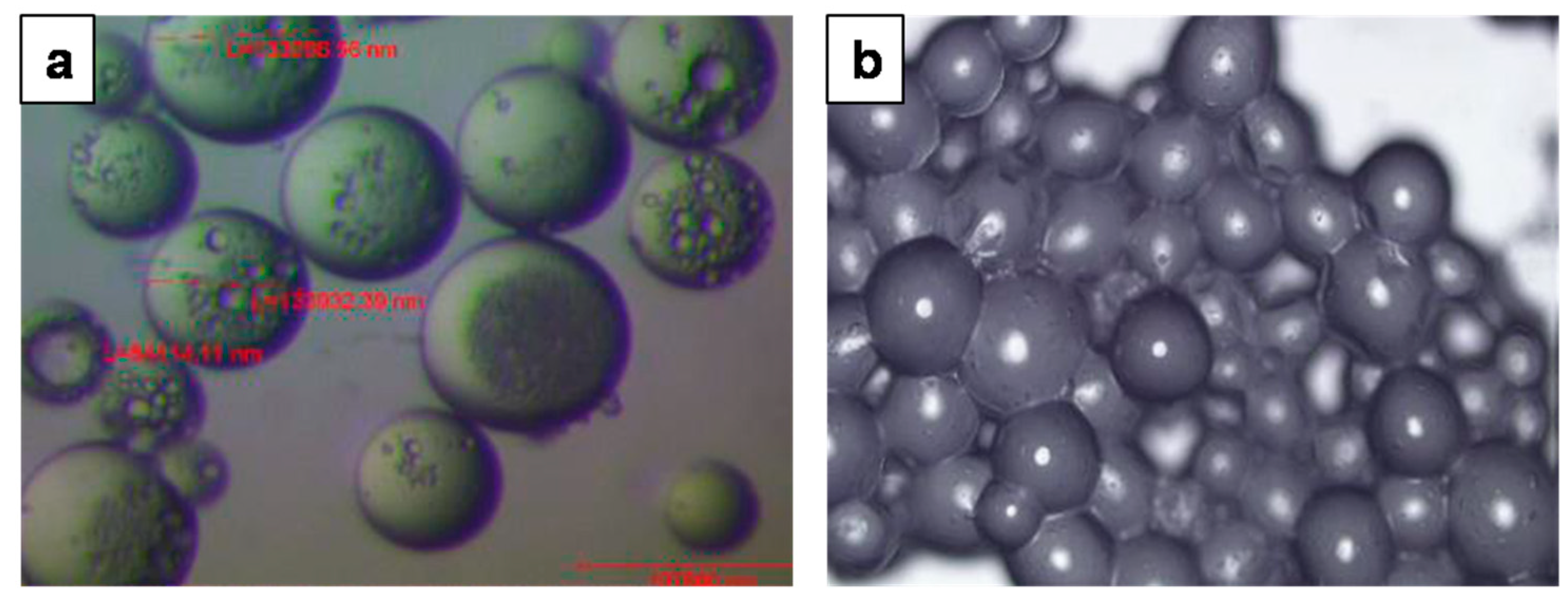
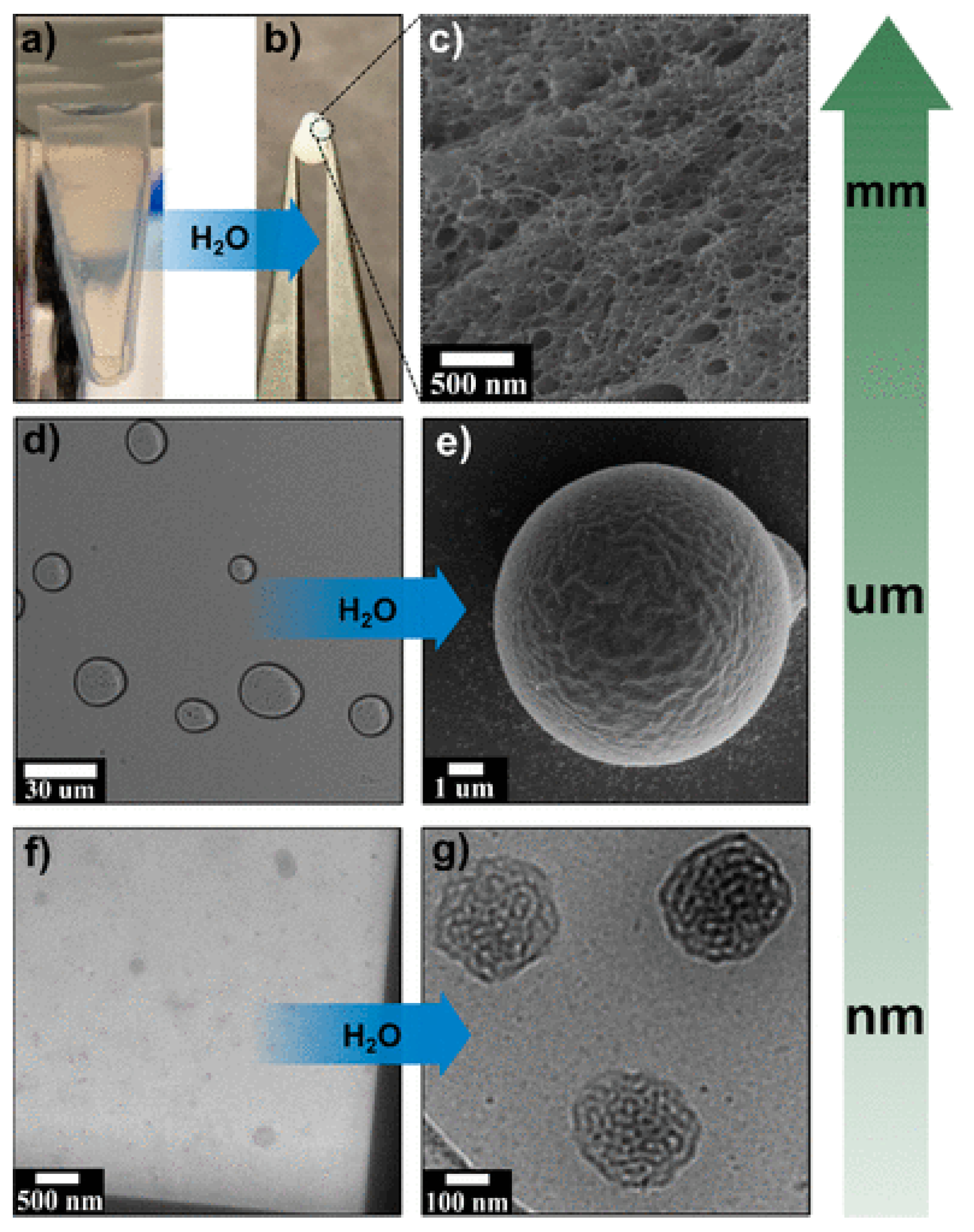


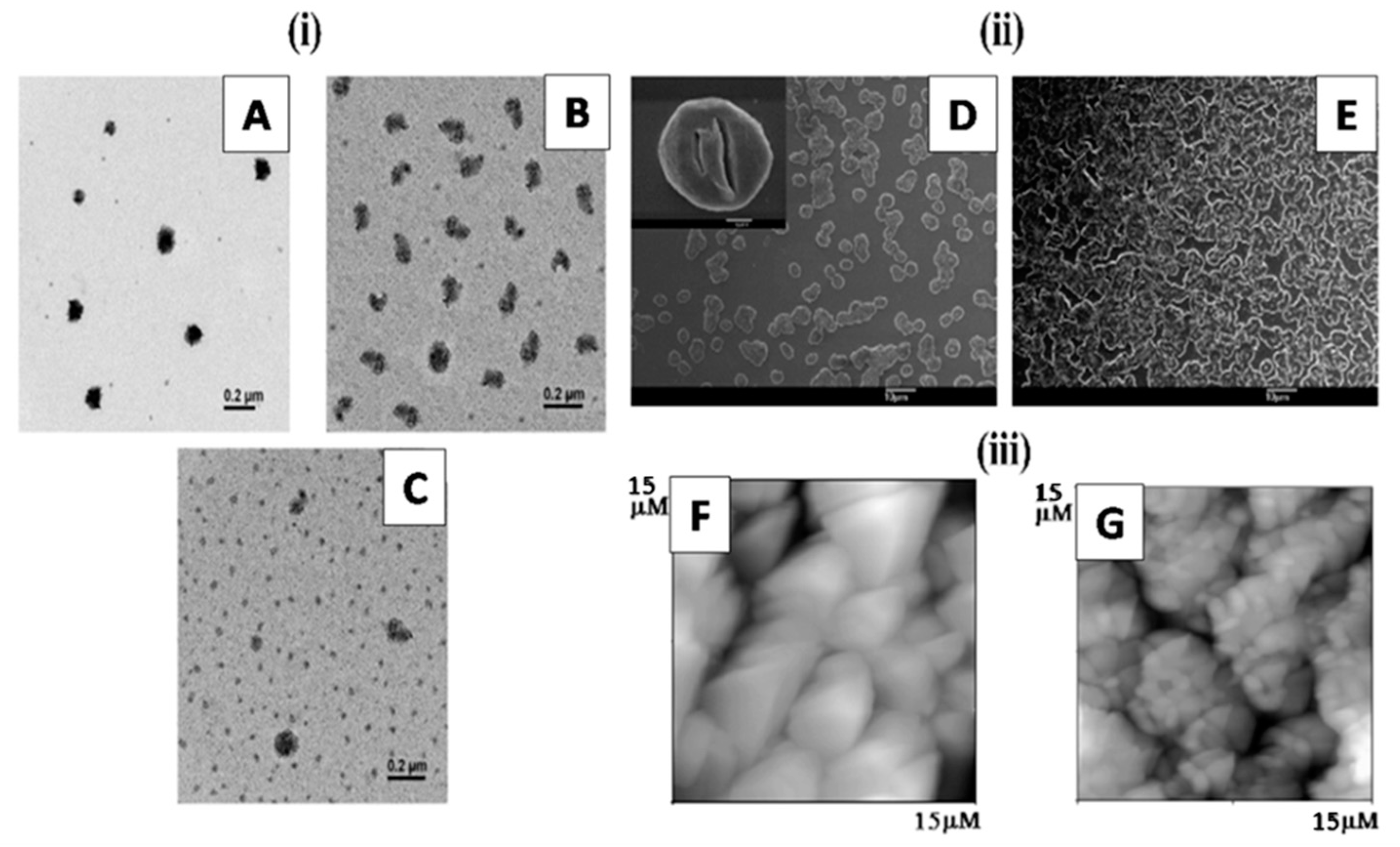


| Simple Coacervation | Complex Coacervation | |||
|---|---|---|---|---|
| Polymer | Coacervating agent | Polymer 1 | Polymer 2 | Coacervating agent |
| Bovine Serum albumin | Ethanol | Gum Arabic | Gelatin | |
| Chitosan | NaOH | Gum Arabic | Chitosan | |
| Ethyl Cellulose | Water | Chitosan | Gelatin | |
| Gelatin | Acetone | Poly(lactide) | Poly(lactide-co-glycolide) | Silicone oil |
| Gelatin | Acetone + ethanol | Chitosan | Hyaluronic acid | Na-acetate |
| Soy glycinin | Acidic water | Hydroxyethyl– cellulose | Sodiumsarcosinate | Isopropanol– water |
| Polyelectrolyte Pairs a | Ionic Strength [×10−3 M] | pH Range | Reference |
|---|---|---|---|
| PDADMAC/PMAA | 0–400 | - | [97] |
| PDADMAC/PSS | 1300–1800 | - | [52] |
| PDADMAC/P(E-alt-MA) | - | >6 | [98] |
| PDADMAC/PAA | 300–3000 | 3–9 | [99] |
| PAH/PAA | 0–4700 | 5–9 | [49] |
| PEI/PAA | - | 2–7 | [100] |
| PDMAEMA/PAA | 50–3000 | 3–8 | [99] |
| PMAA/PMOETAC | 200–500 | - | [97] |
| PEI/PVS | - | 2–10 | [100] |
| PAH/P(E-alt-MA) | - | 6–9 | [98] |
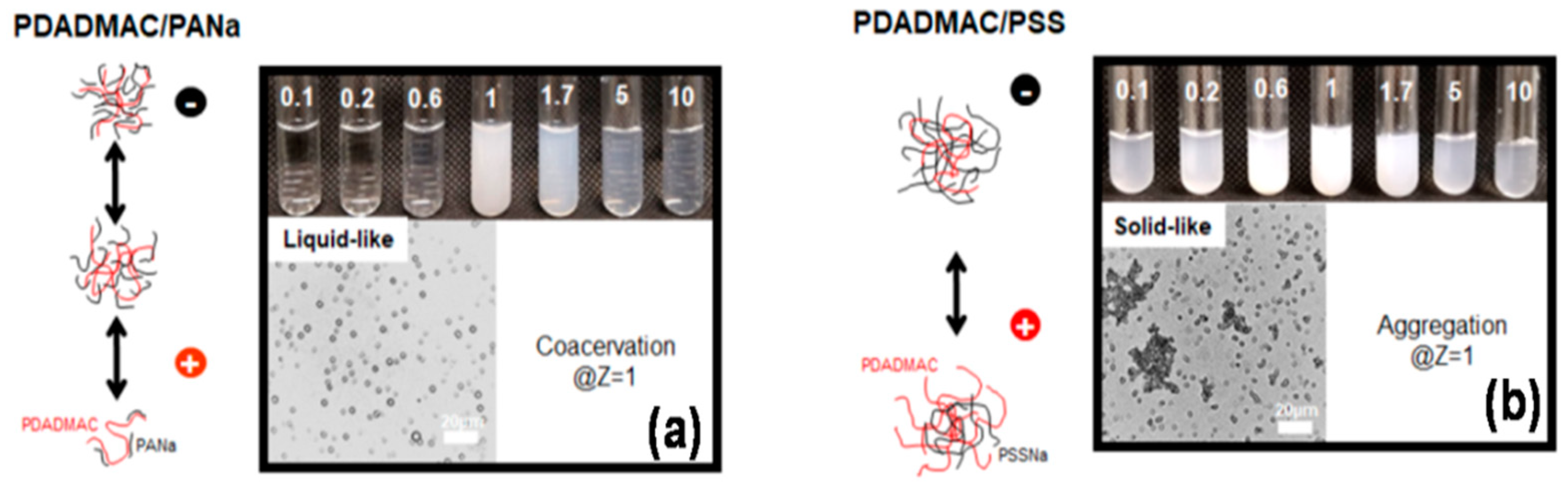
| PDADMAC/PANa | - | 7, 10 | [101] |
| PDADMAC/BSA | 100 | 7.7 | [102] |
| Chitosan/BSA | 100 | 4.0 | [102] |
| PAAD/PGNa | 250 | 9.1 | [103] |
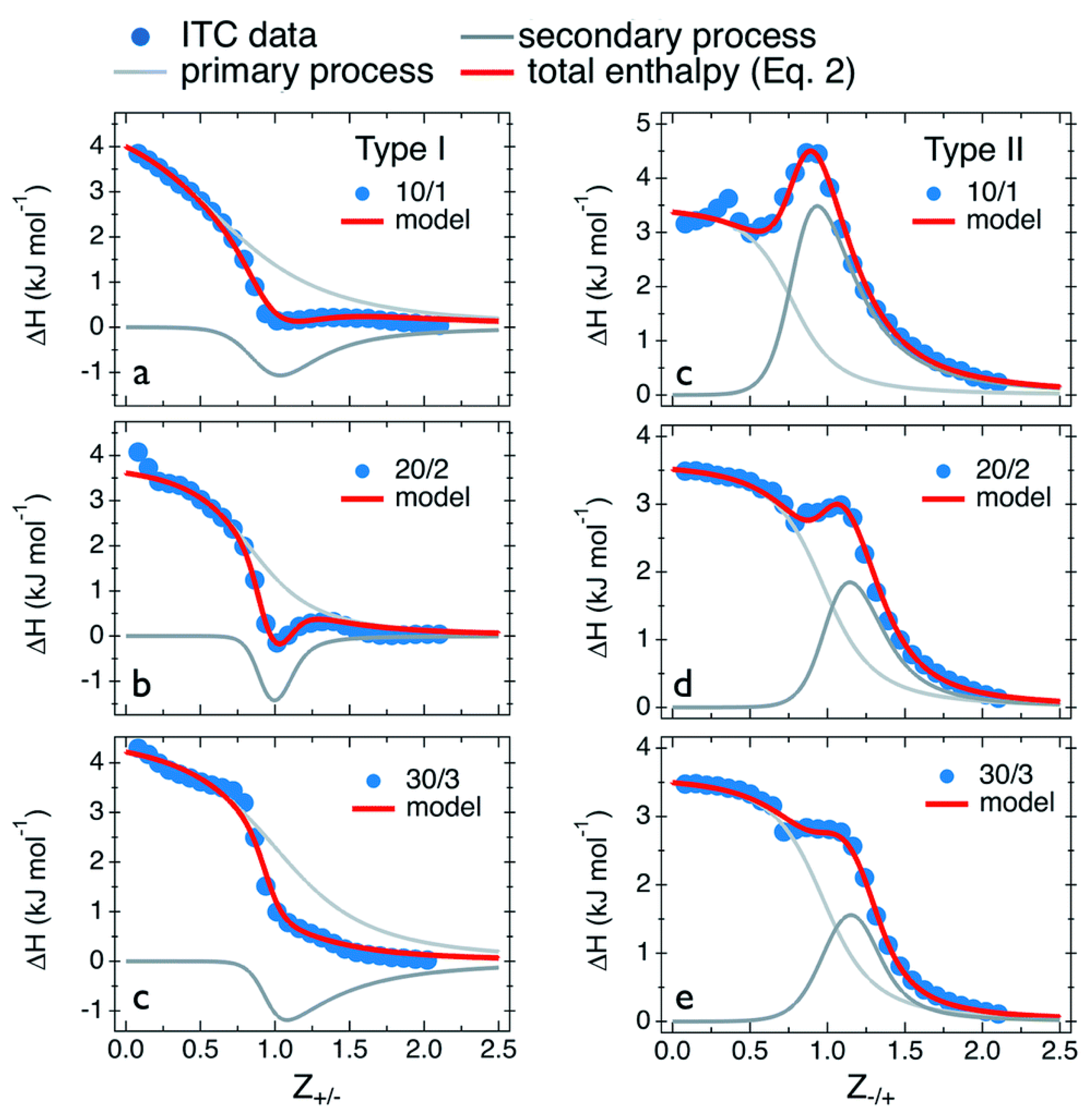
| Primary Process | (kJ mol−1) | (M−1) | (kJ mol−1) | (J mol−1 K−1) | |
|---|---|---|---|---|---|
| Type I: PDADMAC in PANa | |||||
| 10/1 | 5.0 | 5.0 × 103 | 0.8 | −21.1 | 87.5 |
| 20/2 | 3.8 | 8.3 × 103 | 0.9 | −22.4 | 87.9 |
| 30/3 | 4.6 | 3.3 × 103 | 1.1 | −20.1 | 82.8 |
| Type II: PANa in PDADMAC | |||||
| 10/1 | 3.5 | 3.3 × 104 | 0.8 | −25.8 | 98.3 |
| 20/2 | 3.4 | 1.6 × 104 | 1.0 | −24.1 | 92.0 |
| 30/3 | 3.6 | 1.1 × 104 | 1.0 | −23.1 | 89.5 |
| Secondary Process | (kJ mol−1) | (M−1) | (kJ mol−1) | (J mol−1 K−1) | |
| Type I: PDADMAC in PANa | |||||
| 10/1 | −2.6 | 2.0 × 104 | 1.1 | −24.5 | 73.5 |
| 20/2 | −2.1 | 1.0 × 105 | 1.1 | −28.5 | 88.6 |
| 30/3 | −3.0 | 3.3 × 103 | 1.1 | −20.1 | 57.3 |
| Type II: PANa in PDADMAC | |||||
| 10/1 | 7.0 | 2.5 × 104 | 1.05 | −25.1 | 107.6 |
| 20/2 | 3.9 | 3.3 × 104 | 1.3 | −25.8 | 99.6 |
| 30/3 | 2.5 | 3.3 × 104 | 1.3 | −25.8 | 94.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulik, S.P.; Rakshit, A.K.; Pan, A.; Naskar, B. An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution. Colloids Interfaces 2022, 6, 45. https://doi.org/10.3390/colloids6030045
Moulik SP, Rakshit AK, Pan A, Naskar B. An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution. Colloids and Interfaces. 2022; 6(3):45. https://doi.org/10.3390/colloids6030045
Chicago/Turabian StyleMoulik, Satya Priya, Animesh Kumar Rakshit, Animesh Pan, and Bappaditya Naskar. 2022. "An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution" Colloids and Interfaces 6, no. 3: 45. https://doi.org/10.3390/colloids6030045
APA StyleMoulik, S. P., Rakshit, A. K., Pan, A., & Naskar, B. (2022). An Overview of Coacervates: The Special Disperse State of Amphiphilic and Polymeric Materials in Solution. Colloids and Interfaces, 6(3), 45. https://doi.org/10.3390/colloids6030045






