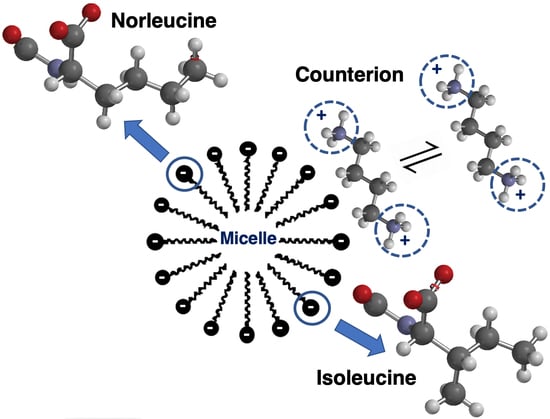
Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths
Abstract
:1. Introduction
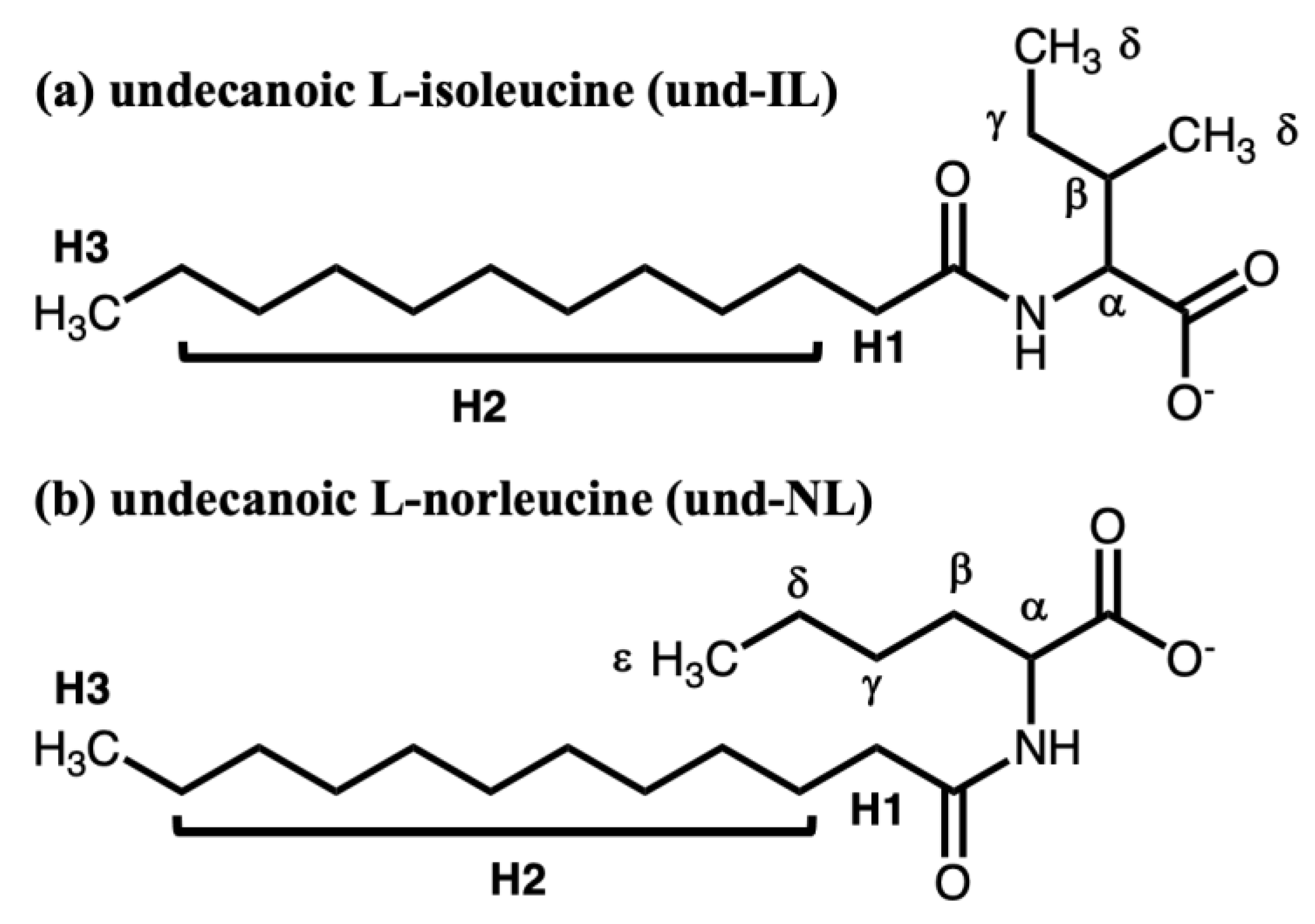
2. The Materials and Methods
3. Results and Discussion
3.1. Critical Micelle Concentrations
3.2. Hydrodynamic Radii and Mole Fraction of Bound Counterions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wieczorek, D.; Kwaśniewska, D. Novel Trends in Technology of Surfactants. In Chemical Technologies and Processes; Staszak, K., Wieszczycka, K., Tylkowski, B., Eds.; De Gruyter: Berlin, Germany, 2020; pp. 23–250. [Google Scholar]
- Bordes, R.; Holmberg, K. Amino Acid-Based Surfactants—Do They Deserve More Attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.B.; Mishra, A.; Clark, J.; Farmer, T. Synthesis, Chemistry, Physicochemical Properties and Industrial Applications of Amino Acid Surfactants: A Review. Comptes Rendus Chimie 2018, 21, 112–130. [Google Scholar] [CrossRef]
- Sharma, H.; Tyagi, R. Safer Surfactants, Based on Amino Acids for Cleaner Environment: A Review. J. Biochem. Int. 2018, 5, 28–56. Available online: https://www.ikprress.org/index.php/JOBI/article/view/4315 (accessed on 15 March 2023).
- Tackie-Otoo, B.N.; Mohammed, M.A.A.; Tze, J.Y.S.; Hassan, A.M. Experimental Investigation of N-Lauroyl Sarcosine and N-Lauroyl-L-glutamic acid as Green Surfactants for Enhanced Oil Recovery Application. J. Mol. Liq. 2022, 362, 119738–119743. [Google Scholar] [CrossRef]
- Pérez, L.; Pinazo, A.; Morán, M.C.; Pons, R. Aggregation Behavior, Antibacterial Activity and Biocompatibility of Cationic Assemblies Based Onamino Acid-Derived Surfactants. Int. J. Mol. Sci. 2020, 21, 8912. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, A.; Manresa, M.A.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino Acid-Based Surfactants: New Antimicrobial Agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Sun, L.; Zhang, F.; Sun, B.; Xu, B.; Zhou, Y. Review: Progress in Synthesis, Properties and Application of Amino Acid Surfactants. Chem. Phys. Let. 2022, 794, 139484–139499. [Google Scholar] [CrossRef]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Masahiko, A. Current Perspective of Sustainable Surfactants based on Renewable Building Blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Soni, S.; Agrawal, P.; Haider, T.; Singh, A.P.; Rohit, R.; Kumar Patra, R.K.; Dubey, H.; Namdeo, S.; Vishwakarma, M.; Soni, V. The Potential of Biosurfactants in the Pharmaceutical Industry: A Review. Bioequiv. Bioavailab. Int. J. 2022, 6, 1–15. [Google Scholar] [CrossRef]
- Rothbauer, G.A.; Rutter, E.A.; Reuter-Seng, C.; Vera, S.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Nuclear Magnetic Resonance Investigation of the Effect of pH on Micelle Formation by the Amino Acid-Based Surfactant Undecyl L-Phenylalaninate. J. Surfact. Deterg. 2018, 21, 139–153. [Google Scholar] [CrossRef]
- Morris, K.F.; Billiot, E.J.; Billiot, F.H.; Ingle, J.A.; Krause, K.B.; Lewis, C.R.; Lipowitz, K.D.; Southerland, W.H.; Fang, Y. Using Molecular Dynamics Simulations to Identify the Key Factors Responsible for Chiral Recognition by an Amino Acid-based Molecular Micelle. J. Dispers. Sci. 2018, 40, 716–727. [Google Scholar] [CrossRef]
- Fletcher, J.; Mahant, G.; Witzleb, T.; Busche, R.; Garcia, M.; Fang, Y.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. NMR Investigation of Counterion Binding to Undecyl LL-Leucinevalanate Micelles. J. Dispers. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Lewis, C.; Hughes, B.H.; Vasquez, M.; Wall, A.M.; Northrup, V.L.; Witzleb, T.J.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Effect of pH on the Binding of Sodium, Lysine, and Arginine Counterions to L-Undecyl Leucinate Micelles. J. Surfact. Deterg. 2016, 19, 1175–1188. [Google Scholar] [CrossRef]
- Yu, R.B.; Quirina, J.P. Chiral Selectors in Capillary Electrophoresis: Trends During 2017–2018. Molecules 2019, 24, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shils, M.E.; Shike, M.; Ross, C.A.; Caballero, B.; Cousins, R.J. Modern Nutrition in Health and Disease, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 28–30. [Google Scholar]
- Luo, S.; Levine, R.L. Methionine in Proteins Defends against Oxidative Stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Biermann, M.; Bardl, B.; Vollstädt, S.; Linnemann, J.; Knüpfer, U.; Seidel, G.; Horn, U. Simultaneous Analysis of the Non-Canonical Amino acids Norleucine and Norvaline in Biopharmaceutical-Related Fermentation Processes by a New Ultra-High Performance Liquid Chromatography Approach. Amino Acids 2013, 44, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Malik, N.A.; Uzair, S.; Ali, M. Conductometric and Fluorometric Studies of Sodium Dodecyl Sulphate in Aqueous Solution and in the Presence of Amino Acids. Mol. Phys. 2014, 112, 2681–2693. [Google Scholar] [CrossRef]
- Elarbi, F.M.; Ettarhouni, Z.O.; Abdussalam-Mohammed, W.; Mezoughi, A.B. Study on the Effects of Biologically Active Amino Acids on the Micellization of Anionic Surfactant Sodium Dodecyl Sulfate (SDS) at Different Temperatures. Chemistry 2022, 4, 146–155. [Google Scholar] [CrossRef]
- Patra, N.; Ray, D.; Aswal, V.K.; Ghosh, S. Exploring Physicochemical Interactions of Different Salts with Sodium N-Dodecanoyl Sarcosinate in Aqueous Solution. ACS Omega 2018, 3, 9256–9266. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zou, A.; Ye, R.; Mu, B. Counterion-Induced Changes to the Micellization of Surfactin-C16 Aqueous Solution. J. Phys. Chem B. 2009, 113, 15272–15277. [Google Scholar] [CrossRef]
- Vu, T.; Koenig, P.; Weaver, M.; Hutton, H.D.; Kasting, G.B. Effects of Cationic Counterions and Surfactant on Viscosity of an Amino Acid-Based Surfactant System. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127040–127043. [Google Scholar] [CrossRef]
- Sow-Kebe, K.; Wunsch, K.; Camblong, A.; Fameau, A. Cosmetic Compositions Comprising 12-Hydroxystearic Acid and an Organic Amine and a Liquid Fatty Substance; World Intellectual Property Organization: Geneva, Switzerland, 2020; WO2020/260629. [Google Scholar]
- Bodet, J.F.; Scheper, W.M.; Oglesby, J.L.; Murch, B.P.; Kacher, M.L. Dishwashing Detergent Compositions Containing Organic Polyamines; World Intellectual Property Organization: Geneva, Switzerland, 2000; WO2000063334. [Google Scholar]
- Gao, P.; Karim, A.; Hassan, F.; Forbes, J.C. Oral Pharmaceutical Compositions Comprising a Low-Water-Soluble Drug, a Solvent, a Fatty Acid and an Organic Amine; World Intellectual Property Organization: Geneva, Switzerland, 2002; WO2002083177. [Google Scholar]
- Rumble, J. (Ed.) CRC Handbook of Chemistry and Physics, 103rd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Johnson, C.S. Diffusion Ordered Nuclear Magnetic Resonance Spectroscopy: Principles and Applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Stilbs, P. Fourier Transform Pulsed-Gradient Spin-Echo Studies of Molecular Diffusion. Prog. Nucl. Mag. Reason. Spectrosc. 1987, 19, 1–45. [Google Scholar] [CrossRef]
- Jansson, M.; Stilbs, P. A Comparative Study of Organic Counterion Binding to Micelles with the Fourier transform NMR Self-Diffusion Technique. J. Phys. Chem. 1985, 89, 4868–4873. [Google Scholar] [CrossRef]
- Stilbs, P. Diffusion and Electrophoretic NMR; Walter de Gruyter: Boston, MA, USA, 2019. [Google Scholar] [CrossRef]
- Evans, R.; Day, I.J. Matrix-Assisted Diffusion-Ordered Spectroscopy. RSC Adv. 2016, 6, 47010–47022. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.G.S.; Gramosa, N.V.; Ricardo, N.M.; Morris, G.A.; Adams, R.W.; Nilsson, M. Natural Product Mixture Analysis by Matrix-Assisted DOSY using Brij Surfactants in Mixed Solvents. RSC Adv. 2014, 4, 42029–42034. [Google Scholar] [CrossRef] [Green Version]
- Lipidot, Y.; Rappoport, S.; Wolman, Y.J. Use of Esters of N-hydroxysuccinimide in the Synthesis of N-acylamino Acids. J. Lipid Res. 1967, 8, 142–145. [Google Scholar] [CrossRef]
- Piotto, M.; Saudek, V.; Skienar, V. Gradient-Tailored Excitation for Single-Quantum NMR Spectroscopy of Aqueous Solutions. J. Biomol. NMR 1992, 2, 661–665. [Google Scholar] [CrossRef]
- Scholz, N.; Behnke, T.; Resch-Gengerry, U. Determination of the Critical Micelle Concentration of Neutral and Ionic Surfactants with Fluorometry, Conductivity, and Surface Tension-A Method Comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Szutkowski, K.; Kołodziejska, Z.; Pietralik, Z.; Zhukov, I.; Skrzypczak, A.; Materna, K.; Kozak, M. Clear Distinction between CAC and CMC Revealed by High-Resolution NMR Diffusometry for a Series of Bis-Imidazolium Gemini Surfactants in Aqueous Solutions. RSC Adv. 2018, 38470–38482. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.C. Micellar Systems: Nuclear Magnetic Resonance Spectroscopy. In Encyclopedia of Surface and Colloid Science; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Evans, R.; Hernandez-Cid, A.; Dal Poggetto, G.; Vesty, A.; Haiber, S.; Morris, G.A.; Nilsson, M. Matrix-Assisted Diffusion-Ordered NMR Spectroscopy with an Invisible Matrix: A Vanishing Surfactant. RSC Adv. 2017, 7, 449–452. [Google Scholar] [CrossRef] [Green Version]
- D’Errico, G.; Ortona, O.; Paduano, L.; Vitagliano, V. Transport Properties of Aqueous Solutions of Alkyltrimethylammonium Bromide Surfactants at 25 °C. J. Colloid Interface Sci. 2001, 239, 264–271. [Google Scholar] [CrossRef]
- Chachaty, C. Applications of NMR Methods to the Physical Chemistry of Micellar Solutions. Prog. Nucl. Magn. Reason. Spectrosc. 1987, 19, 183–222. [Google Scholar] [CrossRef]
- Wu, D.; Chen, A.; Johnson, C.S., Jr. An Improved Diffusion-Ordered Spectroscopy Experiment Incorporating Bipolar-Gradient Pulses. J. Magn. Reson. 1995, 115, 260–264. [Google Scholar] [CrossRef]
- Morris, K.F.; Froberg, A.L.; Becker, B.A.; Almeida, V.K.; Tarus, J.; Larive, C.K. Using NMR to Develop Insights into Electrokinetic Chromatography. Anal. Chem. 2005, 77, 254A–264A. [Google Scholar] [CrossRef] [Green Version]
- Arkhipov, V.P.; Arkhipov, R.V.; Petrova, E.V.; Fillippov, A. Micellar and Solubilizing Properties of Rhamnolipids. Mag. Reason. Chem. 2023, in press. [Google Scholar] [CrossRef]
- Koyama, M. Effect of Arginine as a Counterion on Surfactant Properties of Fatty Acid Salts. J. Dispers. Sci. Technol. 2005, 26, 785–789. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, S.Y.; Huang, C.C. Effect of Hydrophobic Chain Length of Surfactants on Enthalpy−Entropy Compensation of Micellization. J. Phys. Chem. B 1998, 102, 4350–4356. [Google Scholar] [CrossRef]
- Kronberg, B. The Hydrophobic Effect. Curr. Opin. Colloid Interface Sci. 2016, 22, 14–22. [Google Scholar] [CrossRef]
- Inoue, T.; Yamakawa, H. Micelle Formation of Nonionic Surfactants in a Room Temperature Ionic Liquid, 1-Butyl-3-Methylimidazolium Tetrafluoroborate: Surfactant Chain Length Dependence of the Critical Micelle Concentration. J. Colloid Interface Sci. 2011, 356, 798–802. [Google Scholar] [CrossRef]
- Wilkins, D.K.; Grimshaw, S.B.; Recson, V.; Jones, J.A.; Smith, L.J. Hydrodynamic Radii of Native and Denatured Proteins Measured by Pulse Field Gradient NMR Techniques. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.B. Advances in the Synthesis, Molecular Architectures and Potential Applications of Gemini Surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Pinazo, A.; Infante, M.R.; Pons, R. Investigation of the Micellization Process of Single and Gemini Surfactants from Arginine by SAXS, NMR Self-Diffusion, and Light Scattering. J. Phys. Chem. B 2007, 111, 11379–11387. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, M.T.; Hermann, M.R.; Wojcik, M.; Perez, J.; Jochen, S. Small-Angle X-ray Scattering Curves of Detergent Micelles: Effects of Asymmetry, Shape Fluctuations, Disorder, and Atomic Details. J. Phys. Chem. Lett. 2020, 11, 945–951. [Google Scholar] [CrossRef] [PubMed]






| Counterion | Structure | pKa1 | pKa2 |
|---|---|---|---|
| 1,2-ethylenediamine |  | 6.86 | 9.92 |
| 1,3-diaminopropane |  | 8.88 | 10.55 |
| 1,4-diaminobutane |  | 9.63 | 10.8 |
| 1,5-diaminopentane |  | 10.05 | 10.93 |
| 1,6-diaminohexane |  | 10.76 | 11.86 |
| Counterion | Undecanoic L-Isoleucine CMC (mM) | Undecanoic L-Norleucine CMC (mM) |
|---|---|---|
| Na+ | 13.0 ± 0.1 | 12.8 ± 0.1 |
| 1,2-ethylenediamine | 12.2 ± 0.1 | 11.5 ± 0.8 |
| 1,3-diaminopropane | 10.9 ± 0.2 | 9.0 ± 0.1 |
| 1,4-diaminobutane | 5.8 ± 0.3 | 7.2 ± 0.5 |
| 1,5-diaminopentane | 3.0 ± 0.1 | 6.1 ± 0.7 |
| 1,6-diaminohexane | 2.0 ± 0.1 | 4.9 ± 0.9 |
| Counterion | Und-IL Rh (Å) | Und-IL fb | Und-NL Rh (Å) | Und-NL fb |
|---|---|---|---|---|
| Na+ | 9.7 ± 0.2 | n/a | 11.9 ± 0.1 | n/a |
| 1,2-ethylenediamine | 9.8 ± 0.2 | 0.22 ± 0.01 | 9.2 ± 0.1 | 0.24 ± 0.01 |
| 1,3-diaminopropane | 12.3 ± 0.2 | 0.34 ± 0.01 | 19.6 ± 0.8 | 0.10 ± 0.01 |
| 1,4-diaminobutane | 15.6 ± 0.1 | 0.39 ± 0.01 | 39.4 ± 0.3 | 0.45 ± 0.02 |
| 1,5-diaminopentane | 14.7 ± 0.3 | 0.29 ± 0.01 | 20.0 ± 2.0 | 0.28 ± 0.02 |
| 1,6-diaminohexane | 11.0 ± 1.0 | 0.34 ± 0.02 | 24.5 ± 0.1 | 0.32 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maynard-Benson, A.; Alekisch, M.; Wall, A.; Billiot, E.J.; Billiot, F.H.; Morris, K.F. Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths. Colloids Interfaces 2023, 7, 28. https://doi.org/10.3390/colloids7020028
Maynard-Benson A, Alekisch M, Wall A, Billiot EJ, Billiot FH, Morris KF. Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths. Colloids and Interfaces. 2023; 7(2):28. https://doi.org/10.3390/colloids7020028
Chicago/Turabian StyleMaynard-Benson, Amber, Mariya Alekisch, Alyssa Wall, Eugene J. Billiot, Fereshteh H. Billiot, and Kevin F. Morris. 2023. "Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths" Colloids and Interfaces 7, no. 2: 28. https://doi.org/10.3390/colloids7020028
APA StyleMaynard-Benson, A., Alekisch, M., Wall, A., Billiot, E. J., Billiot, F. H., & Morris, K. F. (2023). Characterization of Micelle Formation by the Single Amino Acid-Based Surfactants Undecanoic L-Isoleucine and Undecanoic L-Norleucine in the Presence of Diamine Counterions with Varying Chain Lengths. Colloids and Interfaces, 7(2), 28. https://doi.org/10.3390/colloids7020028