Enhancing Structural Stability of Oil-Shell Microbubbles via Incorporation of a Gold Nanoparticle Protective Shell for Theranostic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfluidic Device Preparation
2.2. Material
2.3. Phospholipid Formulation
2.4. Experiment Setup and Bubble Production
2.5. Imaging Setup
2.6. Image-Processing Pipeline
2.6.1. Statistical Analysis Pipeline
2.6.2. Fluorescent Intensity Measurement
2.6.3. Segmentation for Identification of Dewetting Using Machine-Learning Techniques
2.7. Microbubble Concentration Detection
3. Results and Discussion
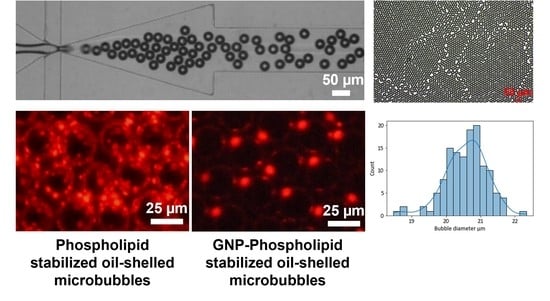
3.1. Formation of phospholipid-shell DEBs
3.2. Morphology and Stability of Phospholipid-Shell DEBs
3.3. GNP-Functionalized Phospholipid-Shell DEBs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts and Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Yang, Z.; Zang, J.; Liu, J.; Chen, X. Localized co-delivery of doxorubicin, cisplatin, and methotrexate by thermosensitive hydrogels for enhanced osteosarcoma treatment. ACS Appl. Mater. Interfaces 2015, 7, 27040–27048. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- May, J.P.; Li, S.D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013, 10, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Shevchenko, T.I.; Raju, B.I.; Seip, R.; Chin, C.T. Ultrasound-triggered release of materials entrapped in microbubble–liposome constructs: A tool for targeted drug delivery. J. Control. Release 2010, 148, 13–17. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef] [PubMed]
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble agents: New directions. Ultrasound Med. Biol. 2020, 46, 1326–1343. [Google Scholar] [CrossRef]
- Batchelor, D.V.; Abou-Saleh, R.H.; Coletta, P.L.; McLaughlan, J.R.; Peyman, S.A.; Evans, S.D. Nested nanobubbles for ultrasound-triggered drug release. ACS Appl. Mater. Interfaces 2020, 12, 29085–29093. [Google Scholar] [CrossRef]
- Unger, E.; Fritz, T.; Shen, D.K.; Lund, P.; Sahn, D.; Ramaswami, R.; Matsunaga, T.; Yellowhair, D.; Kulik, B. Gas fIIIed lipid bilayers as imaging contrast agents. J. Liposome Res. 1994, 4, 861–874. [Google Scholar] [CrossRef]
- Capece, S.; Domenici, F.; Brasili, F.; Oddo, L.; Cerroni, B.; Bedini, A.; Bordi, F.; Chiessi, E.; Paradossi, G. Complex interfaces in “phase-change” contrast agents. Phys. Chem. Chem. Phys. 2016, 18, 8378–8388. [Google Scholar] [CrossRef]
- Straub, J.A.; Chickering, D.E.; Church, C.C.; Shah, B.; Hanlon, T.; Bernstein, H. Porous PLGA microparticles: AI-700, an intravenously administered ultrasound contrast agent for use in echocardiography. J. Control. Release 2005, 108, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Angile, F.E.; Vargo, K.B.; Sehgal, C.M.; Hammer, D.A.; Lee, D. Recombinant protein-stabilized monodisperse microbubbles with tunable size using a valve-based microfluidic device. Langmuir 2014, 30, 12610–12618. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Sheng, Y.; Kamila, S.; Logan, K.; Thomas, K.; Callan, B.; Taylor, M.A.; Love, M.; O’Rourke, D.; Kelly, P.; et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J. Control. Release 2018, 279, 8–16. [Google Scholar] [CrossRef]
- Yan, F.; Li, L.; Deng, Z.; Jin, Q.; Chen, J.; Yang, W.; Yeh, C.K.; Wu, J.; Shandas, R.; Liu, X.; et al. Paclitaxel-liposome–microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J. Control. Release 2013, 166, 246–255. [Google Scholar] [CrossRef]
- Peyman, S.A.; Abou-Saleh, R.H.; McLaughlan, J.R.; Ingram, N.; Johnson, B.R.; Critchley, K.; Freear, S.; Evans, J.A.; Markham, A.F.; Coletta, P.L.; et al. Expanding 3D geometry for enhanced on-chip microbubble production and single step formation of liposome modified microbubbles. Lab Chip 2012, 12, 4544–4552. [Google Scholar] [CrossRef]
- Bourn, M.D.; Mohajerani, S.Z.; Mavria, G.; Ingram, N.; Coletta, P.L.; Evans, S.; Peyman, S.A. Tumour associated vasculature-on-a-chip for the evaluation of microbubble-mediated delivery of targeted liposomes. Lab Chip 2023, 23, 1674–1693. [Google Scholar] [CrossRef]
- Hettiarachchi, K.; Zhang, S.; Feingold, S.; Lee, A.P.; Dayton, P.A. Controllable microfluidic synthesis of multiphase drug-carrying lipospheres for site-targeted therapy. Biotechnol. Prog. 2009, 25, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Postema, M.; Gilja, O.H. Ultrasound-directed drug delivery. Curr. Pharm. Biotechnol. 2007, 8, 355–361. [Google Scholar] [CrossRef]
- Sanwal, R.; Joshi, K.; Ditmans, M.; Tsai, S.S.; Lee, W.L. Ultrasound and Microbubbles for Targeted Drug Delivery to the Lung Endothelium in ARDS: Cellular Mechanisms and Therapeutic Opportunities. Biomedicines 2021, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Domenici, F.; Capocefalo, A.; Brasili, F.; Bedini, A.; Giliberti, C.; Palomba, R.; Silvestri, I.; Scarpa, S.; Morrone, S.; Paradossi, G.; et al. Ultrasound delivery of Surface Enhanced InfraRed Absorption active gold-nanoprobes into fibroblast cells: A biological study via Synchrotron-based InfraRed microanalysis at single cell level. Sci. Rep. 2019, 9, 11845. [Google Scholar] [CrossRef]
- Domenici, F.; Brasili, F.; Giantulli, S.; Cerroni, B.; Bedini, A.; Giliberti, C.; Palomba, R.; Silvestri, I.; Morrone, S.; Paradossi, G.; et al. Differential effects on membrane permeability and viability of human keratinocyte cells undergoing very low intensity megasonic fields. Sci. Rep. 2017, 7, 16536. [Google Scholar] [CrossRef]
- Gao, Y.; Chan, C.U.; Gu, Q.; Lin, X.; Zhang, W.; Yeo, D.C.L.; Alsema, A.M.; Arora, M.; Chong, M.S.K.; Shi, P.; et al. Controlled nanoparticle release from stable magnetic microbubble oscillations. NPG Asia Mater. 2016, 8, e260. [Google Scholar] [CrossRef]
- Dove, J.D.; Murray, T.W.; Borden, M.A. Enhanced photoacoustic response with plasmonic nanoparticle-templated microbubbles. Soft Matter 2013, 9, 7743–7750. [Google Scholar] [CrossRef]
- Meng, Z.; Zhou, X.; She, J.; Zhang, Y.; Feng, L.; Liu, Z. Ultrasound-responsive conversion of microbubbles to nanoparticles to enable background-free in vivo photoacoustic imaging. Nano Lett. 2019, 19, 8109–8117. [Google Scholar] [CrossRef] [PubMed]
- Talu, E.; Hettiarachchi, K.; Powell, R.L.; Lee, A.P.; Dayton, P.A.; Longo, M.L. Maintaining monodispersity in a microbubble population formed by flow-focusing. Langmuir 2008, 24, 1745–1749. [Google Scholar] [CrossRef]
- Hettiarachchi, K.; Talu, E.; Longo, M.L.; Dayton, P.A.; Lee, A.P. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip 2007, 7, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.; Bardin, D.; Martz, T.D.; Sheeran, P.S.; Dayton, P.A.; Lee, A.P. Flow-focusing regimes for accelerated production of monodisperse drug-loadable microbubbles toward clinical-scale applications. Lab Chip 2013, 13, 4816–4826. [Google Scholar] [CrossRef]
- Churchman, A.H.; Mico, V.; De Pablo, J.G.; Peyman, S.A.; Freear, S.; Evans, S.D. Combined flow-focus and self-assembly routes for the formation of lipid stabilized oil-shell microbubbles. Microsystems Nanoeng. 2018, 4, 17087. [Google Scholar] [CrossRef]
- Hayward, R.C.; Utada, A.S.; Dan, N.; Weitz, D.A. Dewetting instability during the formation of polymersomes from block-copolymer-stabilized double emulsions. Langmuir 2006, 22, 4457–4461. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Vallejo, D.; Lee, S.; Lee, D.; Zhang, C.; Rapier, C.; Chessler, S.; Lee, A. Cell-sized lipid vesicles for cell–cell synaptic therapies. Technology 2017, 5, 201–213. [Google Scholar] [CrossRef]
- Segers, T.; Lassus, A.; Bussat, P.; Gaud, E.; Frinking, P. Improved coalescence stability of monodisperse phospholipid-coated microbubbles formed by flow-focusing at elevated temperatures. Lab Chip 2019, 19, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Segers, T.; Lohse, D.; Versluis, M.; Frinking, P. Universal equations for the coalescence probability and long-term size stability of phospholipid-coated monodisperse microbubbles formed by flow focusing. Langmuir 2017, 33, 10329–10339. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Microbubble dissolution in a multigas environment. Langmuir 2010, 26, 6542–6548. [Google Scholar] [CrossRef]
- Pham, D.L.; Xu, C.; Prince, J.L. Current methods in medical image segmentation. Annu. Rev. Biomed. Eng. 2000, 2, 315–337. [Google Scholar] [CrossRef]
- Pal, N.R.; Pal, S.K. A review on image segmentation techniques. Pattern Recognit. 1993, 26, 1277–1294. [Google Scholar] [CrossRef]
- Seo, H.; Badiei Khuzani, M.; Vasudevan, V.; Huang, C.; Ren, H.; Xiao, R.; Jia, X.; Xing, L. Machine learning techniques for biomedical image segmentation: An overview of technical aspects and introduction to state-of-art applications. Med Phys. 2020, 47, e148–e167. [Google Scholar] [CrossRef]
- Arzt, M.; Deschamps, J.; Schmied, C.; Pietzsch, T.; Schmidt, D.; Tomancak, P.; Haase, R.; Jug, F. LABKIT: Labeling and segmentation toolkit for big image data. Front. Comput. Sci. 2022, 4, 10. [Google Scholar] [CrossRef]
- Schroff, F.; Criminisi, A.; Zisserman, A. Object Class Segmentation using Random Forests. In Proceedings of the BMVC, Leeds, UK, 1–4 September 2008; pp. 1–10. [Google Scholar]
- Shum, H.C.; Santanach-Carreras, E.; Kim, J.W.; Ehrlicher, A.; Bibette, J.; Weitz, D.A. Dewetting-induced membrane formation by adhesion of amphiphile-laden interfaces. J. Am. Chem. Soc. 2011, 133, 4420–4426. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.N.; Yelleswarapu, M.; Huck, W.T. Monodisperse uni-and multicompartment liposomes. J. Am. Chem. Soc. 2016, 138, 7584–7591. [Google Scholar] [CrossRef] [PubMed]
- Segers, T.; De Rond, L.; De Jong, N.; Borden, M.; Versluis, M. Stability of monodisperse phospholipid-coated microbubbles formed by flow-focusing at high production rates. Langmuir 2016, 32, 3937–3944. [Google Scholar] [CrossRef]
- Segers, T.; Gaud, E.; Casqueiro, G.; Lassus, A.; Versluis, M.; Frinking, P. Foam-free monodisperse lipid-coated ultrasound contrast agent synthesis by flow-focusing through multi-gas-component microbubble stabilization. Appl. Phys. Lett. 2020, 116, 173701. [Google Scholar] [CrossRef]
- Zalloum, I.O.; Paknahad, A.A.; Kolios, M.C.; Karshafian, R.; Tsai, S.S. Controlled Shrinkage of Microfluidically Generated Microbubbles by Tuning Lipid Concentration. Langmuir 2022, 38, 13021–13029. [Google Scholar] [CrossRef]
- Cerezo, J.; Zuniga, J.; Bastida, A.; Requena, A.; Ceron-Carrasco, J.P. Atomistic molecular dynamics simulations of the interactions of oleic and 2-hydroxyoleic acids with phosphatidylcholine bilayers. J. Phys. Chem. B 2011, 115, 11727–11738. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, J.; Suga, K.; Kuhl, T.L. Interaction forces and membrane charge tunability: Oleic acid containing membranes in different pH conditions. Biochim. Biophys. Acta-(BBA)-Biomembr. 2017, 1859, 211–217. [Google Scholar] [CrossRef]
- Fredrick, E.; Walstra, P.; Dewettinck, K. Factors governing partial coalescence in oil-in-water emulsions. Adv. Colloid Interface Sci. 2010, 153, 30–42. [Google Scholar] [CrossRef]
- Chen, W.; Yu, B.; Wei, Z.; Mao, S.; Li, T. The creation of raspberry-like droplets and their coalescence dynamics: An ideal model for certain biological processes. J. Colloid Interface Sci. 2022, 615, 752–758. [Google Scholar] [CrossRef]
- Pickering, S.U. Cxcvi.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Pan, M.; Rosenfeld, L.; Kim, M.; Xu, M.; Lin, E.; Derda, R.; Tang, S.K. Fluorinated pickering emulsions impede interfacial transport and form rigid interface for the growth of anchorage-dependent cells. ACS Appl. Mater. Interfaces 2014, 6, 21446–21453. [Google Scholar] [CrossRef] [PubMed]
- Azmin, M.; Mohamedi, G.; Edirisinghe, M.; Stride, E. Dissolution of coated microbubbles: The effect of nanoparticles and surfactant concentration. Mater. Sci. Eng. C 2012, 32, 2654–2658. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of Pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Mohamedi, G.; Azmin, M.; Pastoriza-Santos, I.; Huang, V.; Perez-Juste, J.; Liz-Marzan, L.M.; Edirisinghe, M.; Stride, E. Effects of gold nanoparticles on the stability of microbubbles. Langmuir 2012, 28, 13808–13815. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Tay, L.M.; Xu, C. Coating microbubbles with nanoparticles for medical imaging and drug delivery. Nanomedicine 2017, 12, 91–94. [Google Scholar] [CrossRef]
- Palmieri, D.; Brasili, F.; Capocefalo, A.; Bizien, T.; Angelini, I.; Oddo, L.; Toumia, Y.; Paradossi, G.; Domenici, F. Improved hybrid-shell perfluorocarbon microdroplets as ultrasound-and laser-activated phase-change platform. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 641, 128522. [Google Scholar] [CrossRef]









| Formulation Reference | Total Lipid Concentration | GNP Concentration |
|---|---|---|
| F1 | 0.83 mg/mL | 0 Particles/mL |
| F2 | 1.19 mg/mL | 0 Particles/mL |
| F3 | 1.19 mg/mL | 1013 Particles/mL |
| F4 | 1.56 mg/mL | 0 Particles/mL |
| F5 | 1.56 mg/mL | 1013 Particles/mL |
| F6 | 2.45 mg/mL | 0 Particles/mL |
| F7 | 2.45 mg/mL | 1013 Particles/mL |
| Solution | Pressure/Flow Rate |
|---|---|
| Lipid phase | 40–45 µL/min |
| Gas phase | 27.5–34.5 KPa |
| Oil phase | 0.1 µL/min |
| Fluorophore Type | Fluorophore Purpose | Excitation/Emission Wavelength (nm) |
|---|---|---|
| Nile red | Visualization of the oil layer, Wavelength and DEB structural integrity | 559/635 |
| CY3 | Visualization of the GNP protective Wavelength shell on the GNP-DEBs | 555/570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataei, M.; Yi, H.-P.; Taravatfard, A.Z.; Lin, K.Y.; Lee, A.P. Enhancing Structural Stability of Oil-Shell Microbubbles via Incorporation of a Gold Nanoparticle Protective Shell for Theranostic Applications. Colloids Interfaces 2023, 7, 34. https://doi.org/10.3390/colloids7020034
Ataei M, Yi H-P, Taravatfard AZ, Lin KY, Lee AP. Enhancing Structural Stability of Oil-Shell Microbubbles via Incorporation of a Gold Nanoparticle Protective Shell for Theranostic Applications. Colloids and Interfaces. 2023; 7(2):34. https://doi.org/10.3390/colloids7020034
Chicago/Turabian StyleAtaei, Marzieh, Hsiu-Ping Yi, Aida Zahra Taravatfard, Ken Young Lin, and Abraham Phillip Lee. 2023. "Enhancing Structural Stability of Oil-Shell Microbubbles via Incorporation of a Gold Nanoparticle Protective Shell for Theranostic Applications" Colloids and Interfaces 7, no. 2: 34. https://doi.org/10.3390/colloids7020034
APA StyleAtaei, M., Yi, H.-P., Taravatfard, A. Z., Lin, K. Y., & Lee, A. P. (2023). Enhancing Structural Stability of Oil-Shell Microbubbles via Incorporation of a Gold Nanoparticle Protective Shell for Theranostic Applications. Colloids and Interfaces, 7(2), 34. https://doi.org/10.3390/colloids7020034