Experimental and Machine Learning Studies on Chitosan-Polyacrylamide Copolymers for Selective Separation of Metal Sulfides in the Froth Flotation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Samples and Reagents
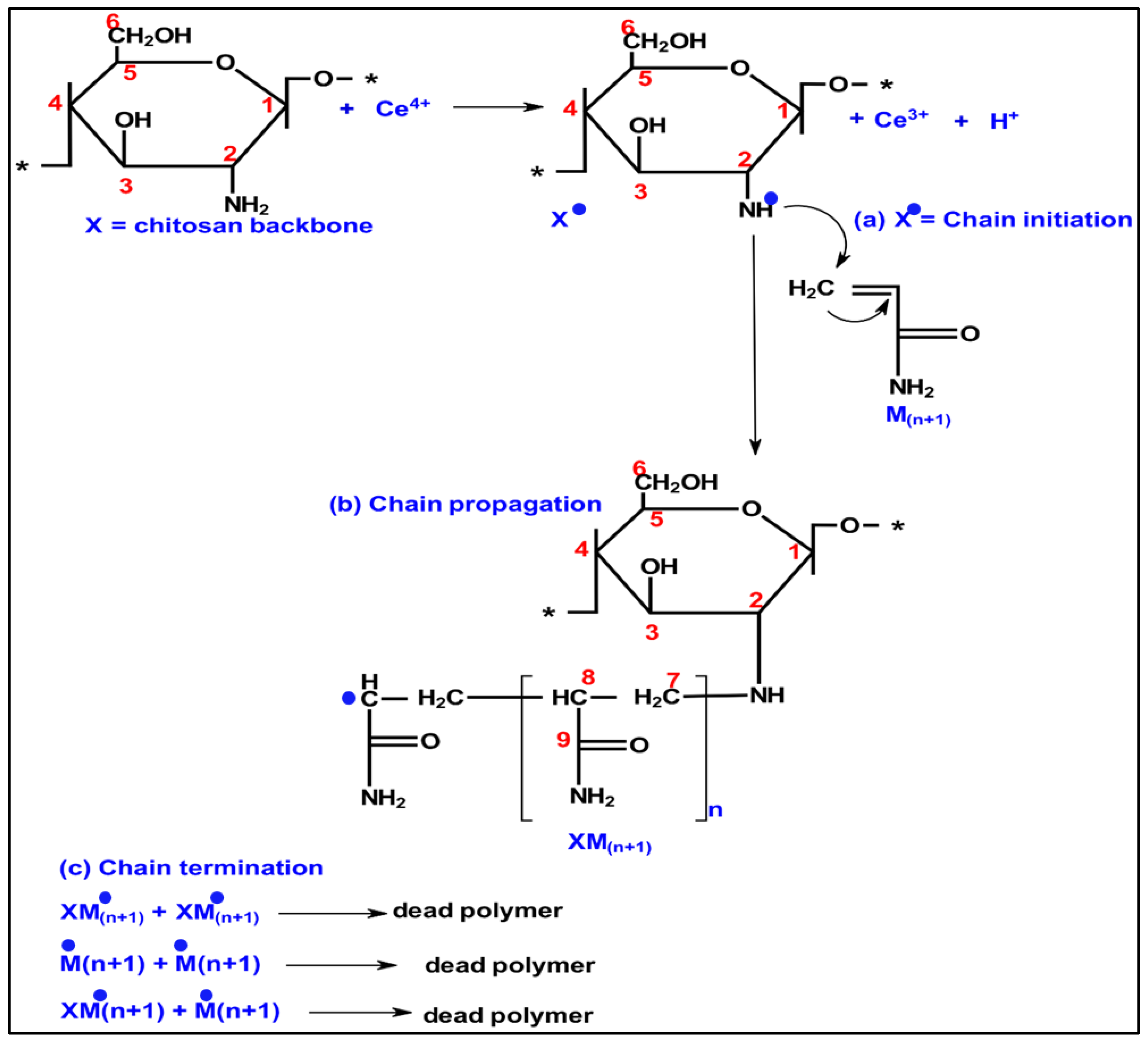
2.2. Preparation of Chitosan-Polyacrylamide Copolymers (C-PAMs)
2.3. Froth Flotation Experiments
2.4. Adsorption Mechanism Studies
2.4.1. Zeta Potential
2.4.2. Total Organic Carbon (TOC)
2.4.3. X-ray Photoelectron Spectroscopy (XPS)
2.5. Overview of the Machine Learning Models
2.5.1. Random Forest Model
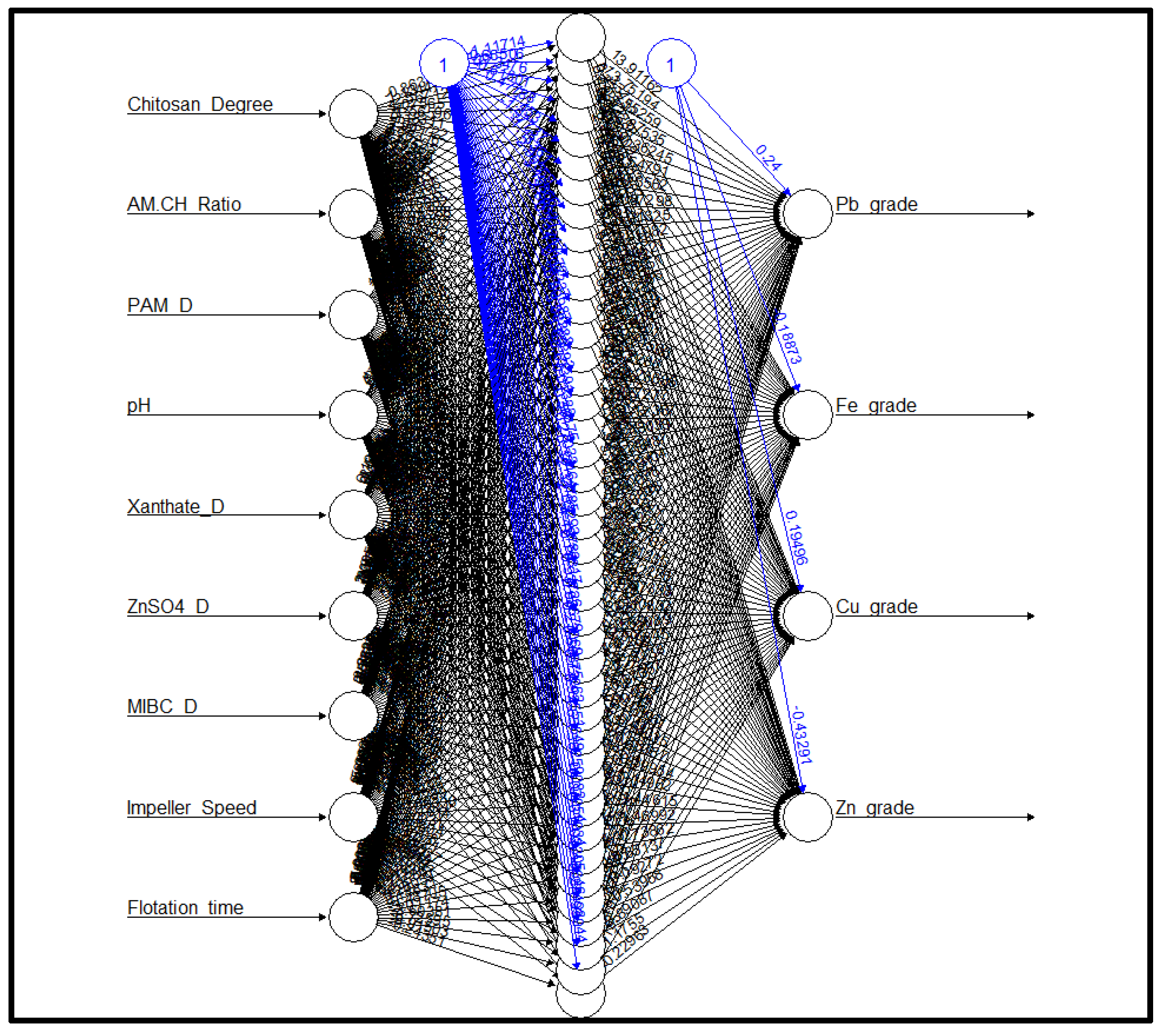
2.5.2. Artificial Neural Network (ANN)
- Wji: Connection weight connecting the neurons of jth hidden layer with the node of ith input layer;
- Wj0: Bias added to the neuron of jth hidden layer;
- fh: Activation and transfer function for each hidden neuron;
- Wkj: Connection weight connecting the node of kth output layer with the neuron of jth hidden layer;
- Wk0: Bias added to the node of output layer;
- f0: Activation and transfer function for output node;
- Xi: ith input variable;
- Zk: kth response variable;
- IN: Total input layer nodes in the model;
- HN: Total hidden layer neurons in the model.

2.6. Data Collection and Dataset Preparation
- = Scaled/Normalized value for input variable ‘’;
- = Highest value of input variable ‘’;
- = Minimum value of input variable ‘’;
- Z = Real value of input variable ‘’.
| Attribute | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|
| Chitosan Degree of deacetylation (%) | 75.00 | 95.00 | 85.00 | 5.55 |
| Chitosan: AM Ratio (g/g) | 3.00 | 7.00 | 5.00 | 1.11 |
| C-PAM dosage (g/ton) | 75.00 | 125.00 | 100.00 | 13.87 |
| Slurry pH (unitless) | 7.00 | 10.00 | 8.50 | 0.83 |
| Xanthate Dosage (g/ton) | 300.00 | 500.00 | 400.00 | 55.47 |
| ZnSO4 (g/ton) | 500.00 | 700.00 | 600.00 | 55.47 |
| MIBC Dosage (g/ton) | 50.00 | 100.00 | 75.00 | 13.87 |
| Impeller Speed (RPM) | 1000.00 | 1500.00 | 1250.00 | 138.68 |
| Flotation time (min) | 3.00 | 8.00 | 5.50 | 1.39 |
| Pb grade (%) | 7.29 | 24.39 | 15.76 | 4.00 |
| Pb recovery (%) | 19.87 | 99.94 | 64.77 | 22.68 |
| Fe grade (%) | 0.65 | 11.36 | 7.63 | 1.91 |
| Fe recovery (%) | 2.43 | 64.97 | 35.00 | 11.21 |
| Cu grade (%) | 1.14 | 2.81 | 1.93 | 0.32 |
| Cu recovery (%) | 23.74 | 85.75 | 55.97 | 16.45 |
| Zn grade (%) | 0.50 | 7.22 | 2.31 | 1.78 |
| Zn recovery (%) | 1.09 | 22.82 | 8.45 | 5.05 |
3. Results
3.1. Adsorption Mechanism of C-PAM on Mineral Surfaces
3.1.1. Zeta Potential
3.1.2. Adsorption Density Analysis
3.1.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.1.4. Mechanism of C-PAM Adsorption on Pyrite
3.2. Machine Learning Models Comparison
3.2.1. Random Forest (RF) Model
3.2.2. Artificial Neural Network (ANN) Model
3.3. Discussion on the Models’ Prediction Performance
4. Optimization Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arbiter, N.; Harris, C.C. Flotation Kinetics. In Froth Flotation; Edward Brothers Inc.: New York, NY, USA, 1962; pp. 215–246. [Google Scholar]
- Shean, B.J.; Cilliers, J.J. A review of froth flotation control. Int. J. Miner. Process. 2011, 100, 57–71. [Google Scholar] [CrossRef]
- Laurila, H.; Karesvuori, J.; Tiili, O. Strategies for instrumentation and control of flotation circuits. In Mineral Processing Plant Design; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2002; pp. 2174–2195. [Google Scholar]
- Wright, B. The Development of a Vision-Based Flotation Froth Analysis System. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 1999. [Google Scholar]
- Jovanović, I.; Miljanović, I. Contemporary advanced control techniques for flotation plants with mechanical flotation cells—A review. Miner. Eng. 2015, 70, 228–249. [Google Scholar] [CrossRef]
- Aldrich, C.; Marais, C.; Shean, B.J.; Cilliers, J.J. Online monitoring and control of froth flotation systems with machine vision: A review. Int. J. Miner. Process. 2010, 96, 1–13. [Google Scholar] [CrossRef]
- Zhang, P. Advanced Industrial Control Technology; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Monyake, K.C.; Alagha, L. Evaluation of Functionalized Chitosan Polymers for Pyrite’s Depression in Pb-Cu Sulfide Flotation Using Response Surface Methodology. Min. Metall. Explor. 2022, 39, 1205–1218. [Google Scholar] [CrossRef]
- Pilkington, J.L.; Preston, C.; Gomes, R.L. Comparison of response surface methodology (RSM) and artificial neural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind. Crop. Prod. 2014, 58, 15–24. [Google Scholar] [CrossRef]
- Khodakarami, M.; Molatlhegi, O.; Alagha, L. Evaluation of Ash and Coal Response to Hybrid Polymeric Nanoparticles in Flotation Process: Data Analysis Using Self-Learning Neural Network. Int. J. Coal Prep. Util. 2019, 39, 199–218. [Google Scholar] [CrossRef]
- Jorjani, E.; Chehreh Chelgani, S.; Mesroghli, S. Prediction of microbial desulfurization of coal using artificial neural networks. Miner. Eng. 2007, 20, 1285–1292. [Google Scholar] [CrossRef]
- Labidi, J.; Pèlach, M.À.; Turon, X.; Mutjé, P. Predicting flotation efficiency using neural networks. Chem. Eng. Process. Process Intensif. 2007, 46, 314–322. [Google Scholar] [CrossRef]
- Mohanty, S. Artificial neural network based system identification and model predictive control of a flotation column. J. Process Control 2009, 19, 991–999. [Google Scholar] [CrossRef]
- Çilek, E.C. Application of neural networks to predict locked cycle flotation test results. Miner. Eng. 2002, 15, 1095–1104. [Google Scholar] [CrossRef]
- Nakhaei, F.; Mosavi, M.R.; Sam, A.; Vaghei, Y. Recovery and grade accurate prediction of pilot plant flotation column concentrate: Neural network and statistical techniques. Int. J. Miner. Process. 2012, 110–111, 140–154. [Google Scholar] [CrossRef]
- Allahkarami, E.; Nuri, O.S.; Abdollahzadeh, A.; Rezai, B.; Chegini, M. Estimation of Copper and Molybdenum Grades and Recoveries in the Industrial Flotation Plant Using the Artificial Neural Network. Int. J. Nonferrous Metall. 2016, 5, 23–32. [Google Scholar] [CrossRef]
- Ali, D.; Hayat, M.B.; Alagha, L.; Molatlhegi, O.K. An evaluation of machine learning and artificial intelligence models for predicting the flotation behavior of fine high-ash coal. Adv. Powder Technol. 2018, 29, 3493–3506. [Google Scholar] [CrossRef]
- Shahbazi, B.; Chehreh Chelgani, S.; Matin, S.S. Prediction of froth flotation responses based on various conditioning parameters by Random Forest method. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 936–941. [Google Scholar] [CrossRef]
- Cook, R.; Monyake, K.C.; Hayat, M.B.; Kumar, A.; Alagha, L. Prediction of Flotation Efficiency of Metal Sulfides Using an Original Hybrid Machine Learning Model. Eng. Rep. 2020, 2, e12167. [Google Scholar] [CrossRef]
- Monyake, K.C.; Alagha, L. Enhanced Separation of Base Metal Sulfides in Flotation Systems Using Chitosan-grafted-Polyacrylamides. Sep. Purif. Technol. 2021, 281, 119818. [Google Scholar] [CrossRef]
- Monyake, K.C. Depression of Pyrite in Polymetallic Sulfide Flotation Using Chitosan-Grafted-Polyacrylamide Polymers. Ph.D. Dissertation, Missouri University of Science and Technology ProQuest Dissertations Publishing, Rolla, MO, USA, 2022. [Google Scholar]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by Random Forests. R News 2001, 3, 18–22. [Google Scholar]
- Biau, G.; Devroye, L.; Lugosi, G. Consistency of Random Forests and Other Averaging Classifiers. J. Mach. Learn. Res. 2008, 9, 2015–2033. [Google Scholar]
- Cook, R.; Lapeyre, J.; Ma, H.; Kumar, A. Prediction of Compressive Strength of Concrete: Critical Comparison of Performance of a Hybrid Machine Learning Model with Standalone Models. J. Mater. Civ. Eng. 2019, 31, 04019255. [Google Scholar] [CrossRef]
- Schaffer, C. Selecting a classification method by cross-validation. Mach. Learn. 1993, 13, 135–143. [Google Scholar] [CrossRef]
- Wang, S.C. Artificial Neural Network; The Springer International Series in Engineering and Computer Science; Springer: Boston, MA, USA, 2003; ISBN 978-1-4615-0377-4. [Google Scholar]
- Ali, D.; Frimpong, S. DeepHaul: A deep learning and reinforcement learning-based smart automation framework for dump trucks. Prog. Artif. Intell. 2021, 10, 157–180. [Google Scholar] [CrossRef]
- Sheela, K.G.; Deepa, S.N. Review on Methods to Fix Number of Hidden Neurons in Neural Networks. Math. Probl. Eng. 2013, 2013, 425740. [Google Scholar] [CrossRef]
- Ali, D.; Frimpong, S. Artificial intelligence models for predicting the performance of hydro-pneumatic suspension struts in large capacity dump trucks. Int. J. Ind. Ergon. 2018, 67, 283–295. [Google Scholar] [CrossRef]
- Alsafasfeh, A.; Alagha, L.; Alzidaneen, A.; Nadendla, S. Optimization of Flotation Efficiency of Phosphate Minerals in Mine Tailings using Polymeric Depressants: Experiments and Machine Learning, Physicochem. Probl. Miner. Process. 2022, 58, 150477. [Google Scholar] [CrossRef]
- Hornik, K.; Stinchcombe, M.; White, H. Multilayer feedforward networks are universal approximators. Neural Netw. 1989, 2, 359–366. [Google Scholar] [CrossRef]
- Nourani, V.; Baghanam, A.H.; Adamowski, J.; Gebremichael, M. Using self-organizing maps and wavelet transforms for space–time pre-processing of satellite precipitation and runoff data in neural network based rainfall–runoff modeling. J. Hydrol. 2013, 476, 228–243. [Google Scholar] [CrossRef]
- Barzegar, R.; Asghari Moghaddam, A. Combining the advantages of neural networks using the concept of committee machine in the groundwater salinity prediction. Model. Earth Syst. Environ. 2016, 2, 26. [Google Scholar] [CrossRef]
- Barzegar, R.; Sattarpour, M.; Nikudel, M.R.; Moghaddam, A.A. Comparative evaluation of artificial intelligence models for prediction of uniaxial compressive strength of travertine rocks, Case study: Azarshahr area, NW Iran. Model. Earth Syst. Environ. 2016, 2, 76. [Google Scholar] [CrossRef]
- Ali, D. Computational Intelligent Impact Force Modeling and Monitoring in HISLO Conditions for Maximizing Surface Mining Efficiency, Safety, and Health. Ph.D. Thesis, Missouri University of Science & Technology, Rolla, MO, USA, 2021. [Google Scholar]
- Ali, D.; Frimpong, S. DeepImpact: A deep learning model for whole body vibration control using impact force monitoring. Neural Comput. Appl. 2020, 33, 3521–3544. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Interaction of sphalerite with potassium n-butyl xanthate and copper sulfate solutions studied by XPS of fast-frozen samples and zeta-potential measurement. Vacuum 2016, 125, 98–105. [Google Scholar] [CrossRef]
- Wang, L. The Use of Polyacrylamide as a Selective Depressant in the Separation of Chalcopyrite and Galena. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2013. [Google Scholar]
- Huang, P. Chitosan in Differential Flotation of Base Metal Sulfides. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2013. [Google Scholar]
- Wills, B.A.; Napier-Munn, T. Wills’ Mineral Processing Technology—An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2006; ISBN 9780750644501. [Google Scholar]
- Boulton, A.; Fornasiero, D.; Ralston, J. Selective depression of pyrite with polyacrylamide polymers. Int. J. Miner. Process. 2001, 61, 13–22. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444530295. [Google Scholar]
- Li, Y.; Quanjun, L.; Shimei, L.; Chao, S.; Yang, G.; Jianwen, S. The synergetic depression effect of KMnO 4 and CMC on the depression of galena flotation. Chem. Eng. Commun. 2019, 206, 581–591. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Liu, R.; Tian, M.; Sun, W.; Gao, Y.; Han, H.; Gao, Z. Selective depression of pyrite with a novel functionally modified biopolymer in a Cu–Fe flotation system. Miner. Eng. 2019, 135, 55–63. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Lyu, F.; Liu, R.; Sun, W. Selective separation of chalcopyrite from pyrite with a novel non-hazardous biodegradable depressant. J. Clean. Prod. 2019, 232, 888–897. [Google Scholar] [CrossRef]
- Ge, W.; Li, H.; Ren, Y.; Zhao, F.; Song, S. Flocculation of pyrite fines in aqueous suspensions with corn starch to eliminate mechanical entrainment in flotation. Minerals 2015, 5, 654–664. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Chen, Y.; Guo, M.; Wang, H. Flotation separation of molybdenite and talc using tragacanth gum as depressant and potassium butyl xanthate as collector. Trans. Nonferrous Met. Soc. China 2021, 31, 3879–3890. [Google Scholar] [CrossRef]
- Chen, X.; Ishwaran, H. Random forests for genomic data analysis. Genomics 2012, 99, 323–329. [Google Scholar] [CrossRef]
- Svetnik, V.; Liaw, A.; Tong, C.; Culberson, J.C.; Sheridan, R.P.; Feuston, B.P. Random forest: A classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comput. Sci. 2003, 43, 1947–1958. [Google Scholar] [CrossRef]
- Gomaa, E.; Han, T.; ElGawady, M.; Huang, J.; Kumar, A. Machine learning to predict properties of fresh and hardened alkali-activated concrete. Cem. Concr. Compos. 2021, 115, 103863. [Google Scholar] [CrossRef]
- Belayneh, A.; Adamowski, J.; Khalil, B.; Ozga-Zielinski, B. Long-term SPI drought forecasting in the Awash River Basin in Ethiopia using wavelet neural network and wavelet support vector regression models. J. Hydrol. 2014, 508, 418–429. [Google Scholar] [CrossRef]
- Cai, R.; Han, T.; Liao, W.; Huang, J.; Li, D.; Kumar, A.; Ma, H. Prediction of surface chloride concentration of marine concrete using ensemble machine learning. Cem. Concr. Res. 2020, 136, 106164. [Google Scholar] [CrossRef]
- Moretti, J.F.; Minussi, C.R.; Akasaki, J.L.; Fioriti, C.F.; Melges, J.L.P.; Tashima, M.M. Previsão do módulo de elasticidade e da resistência à compressão de corpos de prova de concreto por meio de redes neurais artificiais. Acta Sci.—Technol. 2016, 38, 65–70. [Google Scholar] [CrossRef]
- Dou, X.; Yang, Y. Comprehensive evaluation of machine learning techniques for estimating the responses of carbon fluxes to climatic forces in different terrestrial ecosystems. Atmosphere 2018, 9, 83. [Google Scholar] [CrossRef]
- Ali, D.; Frimpong, S. Artificial intelligence, machine learning and process automation: Existing knowledge frontier and way forward for mining sector. Artif. Intell. Rev. 2020, 53, 6025–6042. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Selective depression of pyrite with chitosan in Pb-Fe sulfide flotation. Miner. Eng. 2013, 46–47, 45–51. [Google Scholar] [CrossRef]
- Hayat, M.B.; Alagha, L.; Sannan, S.M. Flotation Behavior of Complex Sulfide Ores in the Presence of Biodegradable Polymeric Depressants. Int. J. Polym. Sci. 2017, 2017, 4835842. [Google Scholar] [CrossRef]
- Han, T.; Siddique, A.; Khayat, K.; Huang, J.; Kumar, A. An ensemble machine learning approach for prediction and optimization of modulus of elasticity of recycled aggregate concrete. Constr. Build. Mater. 2020, 244, 118271. [Google Scholar] [CrossRef]














| Input Name | Input Code | Input Levels | Outputs | ||
|---|---|---|---|---|---|
| Low | Center | High | |||
| Chitosan Degree of deacetylation (%) | X1 | 75 | 85 | 95 | Fe grade (%) Fe recovery (%) Pb grade (%) Pb recovery (%) Cu grade (%) Cu recovery (%) Zn grade (%) Zn recovery (%) |
| Chitosan: AM Ratio (g/g) | X2 | 3 | 5 | 7 | |
| C-PAM dosage (g/ton) | X3 | 75 | 100 | 125 | |
| Slurry pH (unitless) | X4 | 7 | 8.5 | 10 | |
| Xanthate Dosage (g/ton) | X5 | 300 | 400 | 500 | |
| ZnSO4 (g/ton) | X6 | 500 | 600 | 700 | |
| MIBC Dosage (g/ton) | X7 | 50 | 75 | 100 | |
| Impeller Speed (RPM) | X8 | 1000 | 1250 | 1500 | |
| Flotation time (min) | X9 | 3 | 5.5 | 8 | |
| pH | Zeta Potential Shifts (Δζ), mV | ||
|---|---|---|---|
| Galena | Chalcopyrite | Pyrite | |
| 4 | +0.8 | +18.2 | +0.8 |
| 6 | +11.2 | +23.3 | +5.9 |
| 8 | +9.3 | +22.5 | +28.6 |
| 10 | +17.3 | +20.5 | +34.4 |
| 12 | +24.8 | +20.2 | +35.5 |
| Training | Testing | |||
|---|---|---|---|---|
| Model | R2 | RMSE | R2 | RMSE |
| RF | 0.883 | 4.380 | 0.901 | 3.780 |
| ANN | 0.789 | 3.868 | 0.717 | 4.349 |
| Training | Testing | ||||
|---|---|---|---|---|---|
| Response | ML Model | R2 (Unitless) | RMSE (%) | R2 (Unitless) | RMSE (%) |
| Pb grade | RF ANN | 0.75 0.81 | 2.077 1.77 | 0.79 0.53 | 1.962 2.43 |
| Pb recovery | RF ANN | 0.70 0.73 | 13.288 12.02 | 0.63 0.55 | 12.402 13.44 |
| Cu grade | RF ANN | 0.77 0.80 | 0.164 0.14 | 0.80 0.68 | 0.141 0.22 |
| Cu recovery | RF ANN | 0.70 0.75 | 9.298 8.14 | 0.73 0.69 | 8.268 9.77 |
| Fe grade | RF ANN | 0.61 0.71 | 1.143 1.02 | 0.87 0.64 | 0.834 1.11 |
| Fe recovery | RF ANN | 0.65 0.75 | 6.288 5.41 | 0.91 0.82 | 4.196 5.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monyake, K.; Han, T.; Ali, D.; Alagha, L.; Kumar, A. Experimental and Machine Learning Studies on Chitosan-Polyacrylamide Copolymers for Selective Separation of Metal Sulfides in the Froth Flotation Process. Colloids Interfaces 2023, 7, 41. https://doi.org/10.3390/colloids7020041
Monyake K, Han T, Ali D, Alagha L, Kumar A. Experimental and Machine Learning Studies on Chitosan-Polyacrylamide Copolymers for Selective Separation of Metal Sulfides in the Froth Flotation Process. Colloids and Interfaces. 2023; 7(2):41. https://doi.org/10.3390/colloids7020041
Chicago/Turabian StyleMonyake, Keitumetse, Taihao Han, Danish Ali, Lana Alagha, and Aditya Kumar. 2023. "Experimental and Machine Learning Studies on Chitosan-Polyacrylamide Copolymers for Selective Separation of Metal Sulfides in the Froth Flotation Process" Colloids and Interfaces 7, no. 2: 41. https://doi.org/10.3390/colloids7020041
APA StyleMonyake, K., Han, T., Ali, D., Alagha, L., & Kumar, A. (2023). Experimental and Machine Learning Studies on Chitosan-Polyacrylamide Copolymers for Selective Separation of Metal Sulfides in the Froth Flotation Process. Colloids and Interfaces, 7(2), 41. https://doi.org/10.3390/colloids7020041







