Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Plant Material
2.3. Extract Characterization
2.4. Phenolic Compound Quantification and Antioxidant Activity
2.4.1. Phenolic Compound Quantification
2.4.2. Total Flavonoid Content
2.4.3. DPPH Radical Scavenging Activity
2.4.4. Ferric-Reducing Antioxidant Power Assay
2.5. Antibacterial Activity
2.5.1. Microorganisms and Growth Conditions
2.5.2. Minimum Inhibitory Concentration (MIC)
2.5.3. Minimum Bactericidal Concentration (MBC)
2.6. Acute Toxicity Assay
2.7. Preparation of Ultrasound-Assisted Nanoemulsions
2.8. Nanoemulsion Characterization
2.9. Storage Stability Study
2.10. Encapsulation Efficiency (EE)
2.11. In Vitro Release Study
2.12. Phenolic Compound Quantification and Antioxidant Activity of the Nanoemulsion
2.13. Antibacterial Activity of the Nanoemulsion
2.14. Effect of the Extract and Nanoemulsion on the Growth of Bacteria
2.15. Statistical Analysis
3. Results
3.1. Extract Characterization
3.2. Antimicrobial Activity of the Extracts
3.3. Brine Shrimp Bioassay
3.4. Nanoemulsion Characterization
3.5. Storage Stability Study
3.6. Encapsulation Efficiency (EE)
3.7. Drug Release Study
3.8. Phenolic Compound Quantification and Antioxidant Activity of the Nanoemulsion
3.9. Antimicrobial Activity of the Nanoemulsion
3.10. Time–Kill Kinetics Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation Techniques for Compounds and Products with Antioxidant and Antimicrobial Activity—A Critical View. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Gourama, H. Foodborne Pathogens; Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–49. [Google Scholar]
- Ji, H.-S.; Li, H.; Mo, E.-J.; Kim, U.-H.; Kim, Y.-H.; Park, H.-Y.; Jeong, T.-S. Low-Density Lipoprotein-Antioxidant Flavonoids and a Phenolic Ester from Plectranthus hadiensis Var. Tomentosus. Appl. Biol. Chem. 2019, 62, 58. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial Plant Extracts Encapsulated into Polymeric Beads for Potential Application on the Skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Ngo-Mback, M.N.L.; Famewo, E.B.; MubarakAli, D.; Eke, P.; Thajuddin, N.; Afolayan, A.J.; Jazet Dongmo, P.M.; Fekam Boyom, F. An Investigation of Chemical Composition and Antimicrobial Activity of Essential Oils Extracted from Aeollanthus and Plectranthus Species. Biocatal. Agric. Biotechnol. 2019, 22, 101412. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A Review of Ethnobotanical Uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef]
- Muthukumarana, R.; Dharmadasa, R.M. Pharmacognostical Investigation of Plectranthus Hadiensis (Forssk.) Schweinf. Ex Sprenger. and Plectranthus Amboinicus (Lour.) Spreng. World J. Agric. Res. 2014, 2, 240–246. [Google Scholar] [CrossRef]
- Kotagiri, D.; Shaik, K.B.; Chaitanya Kolluru, V.; Kotagiri, D.; Shaik, K.B.; ChaitanyaKolluru, V. Antimicrobial and Antioxidant Properties of Essential Oil Isolated from Coleus Zeylanicus under Normal and Salinity Stress Conditions. In Free Radicals, Antioxidants and Diseases; IntechOpen: New York, NY, USA, 2018; ISBN 978-1-78923-565-4. [Google Scholar]
- Sathasivampillai, S.V.; Authinarayanan, A.R.; Sebastian, P.R. Medicinal Values of a Horticultural Plant—Coleus hadiensis (Forssk.) A. J. Paton. Int. J. Sci. Lett. 2021, 3, 65–72. [Google Scholar] [CrossRef]
- Domínguez-Martín, E.M.; Magalhães, M.; Díaz-Lanza, A.M.; Marques, M.P.; Princiotto, S.; Gómez, A.M.; Efferth, T.; Cabral, C.; Rijo, P. Phytochemical Study and Antiglioblastoma Activity Assessment of Plectranthus hadiensis (Forssk.) Schweinf. Ex Sprenger Var. Hadiensis Stems. Molecules 2022, 27, 3813. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of Conventional/Non-Conventional Extraction Methods on the Untargeted Phenolic Profile of Moringa oleifera Leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. 3—Plant Extracts as Antimicrobials in Food Products: Mechanisms of Action, Extraction Methods, and Applications. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Oxford, UK, 2015; pp. 49–68. ISBN 978-1-78242-034-7. [Google Scholar]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid–Liquid Extraction Technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Silva-Jara, J. Antibacterial and Immunomodulatory Activity of Moringa (Moringa oleifera) Seed Extract in Longfin Yellowtail (Seriola rivoliana) Peripheral Blood Leukocytes. Aquac. Res. 2021, 52, 4076–4085. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An Efficient Technology to Boost the Antimicrobial Potential of Plant Essential Oils in Food System. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated Natural Antimicrobials: A Promising Way to Reduce Microbial Growth in Different Food Systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- Oprea, I.; Fărcaș, A.C.; Leopold, L.F.; Diaconeasa, Z.; Coman, C.; Socaci, S.A. Nano-Encapsulation of Citrus Essential Oils: Methods and Applications of Interest for the Food Sector. Polymers 2022, 14, 4505. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.N.; Assadpour, E.; Feng, J.; Jafari, S.M. Natural Antimicrobial-Loaded Nanoemulsions for the Control of Food Spoilage/Pathogenic Microorganisms. Adv. Colloid Interface Sci. 2021, 295, 102504. [Google Scholar] [CrossRef]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the Preparation and the Recent Advances in Their Food Applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar] [CrossRef]
- Ochoa-Flores, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; Soto-Rodríguez, I.; Sanchez-Otero, M.G.; Vernon-Carter, E.J.; García, H.S. Enhanced Bioavailability of Curcumin Nanoemulsions Stabilized with Phosphatidylcholine Modified with Medium Chain Fatty Acids. Curr. Drug Deliv. 2017, 14, 377–385. [Google Scholar] [CrossRef]
- da Silva, B.D.; do Rosário, D.K.A.; Neto, L.T.; Lelis, C.A.; Conte-Junior, C.A. Antioxidant, Antibacterial and Antibiofilm Activity of Nanoemulsion-Based Natural Compound Delivery Systems Compared with Non-Nanoemulsified Versions. Foods 2023, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 29, e47. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Mahomoodally, M.F. Essential Oils from 9 Exotic and Endemic Medicinal Plants from Mauritius Shows in Vitro Antibacterial and Antibiotic Potentiating Activities. S. Afr. J. Bot. 2020, 132, 355–362. [Google Scholar] [CrossRef]
- Cavazos-Garduño, A.; Ochoa Flores, A.A.; Serrano-Niño, J.C.; Martínez-Sanchez, C.E.; Beristain, C.I.; García, H.S. Preparation of Betulinic Acid Nanoemulsions Stabilized by ω-3 Enriched Phosphatidylcholine. Ultrason. Sonochemistry 2015, 24, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.; Al-Keridis, L.A.; Malik, M.; Haq, A.; Ahmad, S.; Kaur, J.; Adnan, M.; Alshammari, N.; Ashraf, S.A.; Panda, B.P. Preparation, Modelling, Characterization and Release Profile of Vitamin D3 Nanoemulsion. LWT 2022, 169, 113980. [Google Scholar] [CrossRef]
- Pathania, R.; Najda, A.; Chawla, P.; Kaushik, R.; Khan, M.A. Low-Energy Assisted Sodium Alginate Stabilized Phyllanthus Niruri Extract Nanoemulsion: Characterization, in Vitro Antioxidant and Antimicrobial Application. Biotechnol. Rep. 2022, 33, e00711. [Google Scholar] [CrossRef]
- Qi, H.; Chen, S.; Zhang, J.; Liang, H. Robust Stability and Antimicrobial Activity of D-Limonene Nanoemulsion by Sodium Caseinate and High Pressure Homogenization. J. Food Eng. 2022, 334, 111159. [Google Scholar] [CrossRef]
- Manikandan, S.; Alagu Lakshmanan, G.M.; Ansarali, S. Identification of Bioactive Compounds from Selected Plectranthus Species by Gas Chromatography-Mass Spectroscopy and Fourier Transform Infra-Red Spectroscopy. J. Biol. Act. Prod. Nat. 2017, 7, 438–451. [Google Scholar] [CrossRef]
- LIMEM, S.; Radhouan, M.; Mani Kongnine, D.; Férid, M.; Karmous, T. Preliminary Identification of Citrullus colocynthis from Togo by FT-IR and Raman Spectroscopy. Int. J. Adv. Res. 2015, 3, 354–360. [Google Scholar]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.W.; Morton, D. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Hssaini, L.; Razouk, R.; Bouslihim, Y. Rapid prediction of fig phenolic acids and flavonoids using mid-infrared spectroscopy combined with partial least square regression. Front. Plant Sci. 2022, 13, 429. [Google Scholar] [CrossRef]
- Ntungwe, E.; Domínguez-Martín, E.M.; Teodósio, C.; Teixidó-Trujillo, S.; Armas Capote, N.; Saraiva, L.; Díaz-Lanza, A.M.; Duarte, N.; Rijo, P. Preliminary Biological Activity Screening of Plectranthus Spp. Extracts for the Search of Anticancer Lead Molecules. Pharmaceuticals 2021, 14, 402. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, E.; Mudili, V.; Kalagatur, N.K.; Anthuvan, A.J.; Krishna, K.; Thangaraj, P.; Natarajan, G. Antioxidant and Anticancer Activities of Plectranthus stocksii Hook. f. Leaf and Stem Extracts. Agric. Nat. Resour. 2017, 51, 63–73. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Menon, D.; Sasikumar, J. Pharmacognostic Study and Phytochemical Investigation of Plectranthus Hadiensis. Int. J. Pharm. Pharm. Sci. 2011, 3, 300–304. [Google Scholar]
- Schultz, F.; Osuji, O.F.; Wack, B.; Anywar, G.; Garbe, L.-A. Antiinflammatory Medicinal Plants from the Ugandan Greater Mpigi Region Act as Potent Inhibitors in the COX-2/PGH2 Pathway. Plants 2021, 10, 351. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Sánchez-Vioque, R.; Santana-Méridas, O.; Martín-Bejerano, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. A Potential Use of Vine-Shoot Wastes: The Antioxidant, Antifeedant and Phytotoxic Activities of Their Aqueous Extracts. Ind. Crops Prod. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Abdo, S.A.A.; Hasson, S.; Althawab, F.M.N.; Alaghbari, S.A.Z.; Lindequist, U. Antimicrobial, Antioxidant and Cytotoxic Activities and Phytochemical Screening of Some Yemeni Medicinal Plants. Evid.-Based Complement. Altern. Med. 2010, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Batista, M.; Matos, M.; Rocha, H.; Jesus, S.; Simões, M.F. Screening of antioxidant and antimicrobial activities on Plectranthus spp. extracts: Pesquisa das actividades antioxidante e antimicrobiana em extractos de plantas do género Plectranthus. Iomed. Biopharm. Res. 2018, 9, 225–235. [Google Scholar] [CrossRef]
- Sripathi, R.; Ravi, S. Chemical Composition and Antibacterial Activity of the Essential Oil from the Seeds of Plectranthus hadiensis. Phyto 2017, 9, 637–639. [Google Scholar] [CrossRef]
- Sripathi, R.; Jayagopal, D.; Ravi, S. A Study on the Seasonal Variation of the Essential Oil Composition from Plectranthus hadiensis and Its Antibacterial Activity. Nat. Prod. Res. 2018, 32, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; Lorenz, S.; De Soyza, S.; Qader, M.; Nagahawatte, A.; Wijayaratne, G.B.; Schneider, B.; Svatoš, A.; et al. Plectranthus zeylanicus: A Rich Source of Secondary Metabolites with Antimicrobial, Disinfectant and Anti-Inflammatory Activities. Pharmaceuticals 2022, 15, 436. [Google Scholar] [CrossRef]
- Zarshenas, M.M.; Samani, S.M.; Petramfar, P.; Moein, M. Analysis of the Essential Oil Components from Different Carum copticum L. Samples from Iran. Pharmacogn. Res. 2014, 6, 62–66. [Google Scholar] [CrossRef]
- Soliman, F.M.; Yousif, M.F.; Zaghloul, S.S.; Okba, M.M. Seasonal Variation in the Essential Oil Composition of Origanum majorana L. Cultivated in Egypt. Z. Naturforschung C 2009, 64, 611–614. [Google Scholar] [CrossRef]
- Ashaari, N.S.; Mohamad, N.E.; Afzinizam, A.H.A.B.; Rahim, M.-H.; Lai, K.S.; Ong Abdullah, J. Chemical Composition of Hexane-Extracted Plectranthus amboinicus Leaf Essential Oil: Maximizing Contents on Harvested Plant Materials. Appl. Sci. 2021, 11, 10838. [Google Scholar] [CrossRef]
- Carpenter, J.; Saharan, V.K. Ultrasonic Assisted Formation and Stability of Mustard Oil in Water Nanoemulsion: Effect of Process Parameters and Their Optimization. Ultrason. Sonochemistry 2017, 35, 422–430. [Google Scholar] [CrossRef]
- Lago, A.M.T.; Neves, I.C.O.; Oliveira, N.L.; Botrel, D.A.; Minim, L.A.; de Resende, J.V. Ultrasound-Assisted Oil-in-Water Nanoemulsion Produced from Pereskia aculeata Miller Mucilage. Ultrason. Sonochemistry 2019, 50, 339–353. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, G.; Khatkar, S.K. Optimization of Emulsification Conditions for Designing Ultrasound Assisted Curcumin Loaded Nanoemulsion: Characterization, Antioxidant Assay and Release Kinetics. LWT 2021, 141, 110962. [Google Scholar] [CrossRef]
- Gorjian, H.; Mihankhah, P.; Khaligh, N.G. Influence of Tween Nature and Type on Physicochemical Properties and Stability of Spearmint Essential Oil (Mentha spicata L.) Stabilized with Basil Seed Mucilage Nanoemulsion. J. Mol. Liq. 2022, 359, 119379. [Google Scholar] [CrossRef]
- Chu, Y.; Cheng, W.; Feng, X.; Gao, C.; Wu, D.; Meng, L.; Zhang, Y.; Tang, X. Fabrication, Structure and Properties of Pullulan-Based Active Films Incorporated with Ultrasound-Assisted Cinnamon Essential Oil Nanoemulsions. Food Packag. Shelf Life 2020, 25, 100547. [Google Scholar] [CrossRef]
- Liu, M.; Pan, Y.; Feng, M.; Guo, W.; Fan, X.; Feng, L.; Huang, J.; Cao, Y. Garlic Essential Oil in Water Nanoemulsion Prepared by High-Power Ultrasound: Properties, Stability and Its Antibacterial Mechanism against MRSA Isolated from Pork. Ultrason. Sonochem. 2022, 90, 106201. [Google Scholar] [CrossRef] [PubMed]
- Bazana, M.T.; da Silva, S.S.; Codevilla, C.F.; de Deus, C.; Lucas, B.N.; Ugalde, G.A.; Mazutti, M.A.; Moraes Flores, E.M.; Barin, J.S.; de Bona da Silva, C.; et al. Development of Nanoemulsions Containing Physalis Peruviana Calyx Extract: A Study on Stability and Antioxidant Capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef] [PubMed]
- Barden, L.; Barouh, N.; Villeneuve, P.; Decker, E. Impact of Hydrophobicity on Antioxidant Efficacy in Low-Moisture Food. J. Agric. Food Chem. 2015, 63, 5821–5827. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of droplet size on the interfacial concentrations of antioxidants in fish and olive oil-in-water emulsions and nanoemulsions and on their oxidative stability. J. Colloid Interface Sci. 2020, 562, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Lin, Y.; Li, Z. Ultrasonic-Assisted Preparation of Eucalyptus Oil Nanoemulsion: Process Optimization, in Vitro Digestive Stability, and Anti-Escherichia Coli Activity. Ultrason. Sonochem. 2022, 82, 105904. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Antimicrobial Activity of Nanoemulsions of Cinnamon, Rosemary, and Oregano Essential Oils on Fresh Celery. LWT 2019, 112, 108247. [Google Scholar] [CrossRef]
- Imam, S.S.; Gilani, S.J.; Zafar, A.; bin Jumah, M.N.; Ali, R.; Ahmed, M.M.; Alshehri, S. Preparation and Optimization of Naringin Oral Nanocarrier: In Vitro Characterization and Antibacterial Activity. Coatings 2022, 12, 1230. [Google Scholar] [CrossRef]
- Badr, M.M.; Badawy, M.E.I.; Taktak, N.E.M. Characterization, Antimicrobial Activity, and Antioxidant Activity of the Nanoemulsions of Lavandula Spica Essential Oil and Its Main Monoterpenes. J. Drug Deliv. Sci. Technol. 2021, 65, 102732. [Google Scholar] [CrossRef]
- Doost, A.s.; Van Camp, J.; Dewettinck, K.; Van der Meeren, P. Production of Thymol Nanoemulsions Stabilized Using Quillaja Saponin as a Biosurfactant: Antioxidant Activity Enhancement. Food Chem. 2019, 293, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Chen, J.; Yu, F.; Wang, H.; Kou, X.; Ma, C.; Zhu, S. The Antioxidant, Antibacterial, Antibiofilm Activity of Essential Oil from Citrus medica L. Var. Sarcodactylis and Its Nanoemulsion. LWT—Food Sci. Technol. 2017, 80, 371–377. [Google Scholar] [CrossRef]
- Araujo, T.D.S.; da Costa, J.M.A.R.; de Oliveira Silva Ribeiro, F.; de Jesus Oliveira, A.C.; do Nascimento Dias, J.; de Araujo, A.R.; Barros, A.B.; da Paixão Brito, M.; de Oliveira, T.M.; de Almeida, M.P.; et al. Nanoemulsion of Cashew Gum and Clove Essential Oil (Ocimum gratissimum Linn) Potentiating Antioxidant and Antimicrobial Activity. Int. J. Biol. Macromol. 2021, 193, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; dos Santos Almeida, J.C.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of Propolis Nanoemulsion with Antioxidant and Antimicrobial Activity for Use as a Potential Natural Preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Deepika; Prasad, J.; Dwivedy, A.K.; Dubey, N.K. Improvement of in Vitro and in Situ Antifungal, AFB1 Inhibitory and Antioxidant Activity of Origanum Majorana L. Essential Oil through Nanoemulsion and Recommending as Novel Food Preservative. Food Chem. Toxicol. 2020, 143, 111536. [Google Scholar] [CrossRef]
- Kang, Z.; Chen, S.; Zhou, Y.; Ullah, S.; Liang, H. Rational Construction of Citrus Essential Oil Nanoemulsion with Robust Stability and High Antimicrobial Activity Based on Combination of Emulsifiers. Innov. Food Sci. Emerg. Technol. 2022, 80, 103110. [Google Scholar] [CrossRef]
- Sharma, M.; Mann, B.; Pothuraju, R.; Sharma, R.; Kumar, R. Physico-Chemical Characterization of Ultrasound Assisted Clove Oil-Loaded Nanoemulsion: As Enhanced Antimicrobial Potential. Biotechnol. Rep. 2022, 34, e00720. [Google Scholar] [CrossRef]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial Effect of Laurel Essential Oil Nanoemulsion on Food-Borne Pathogens and Fish Spoilage Bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef]
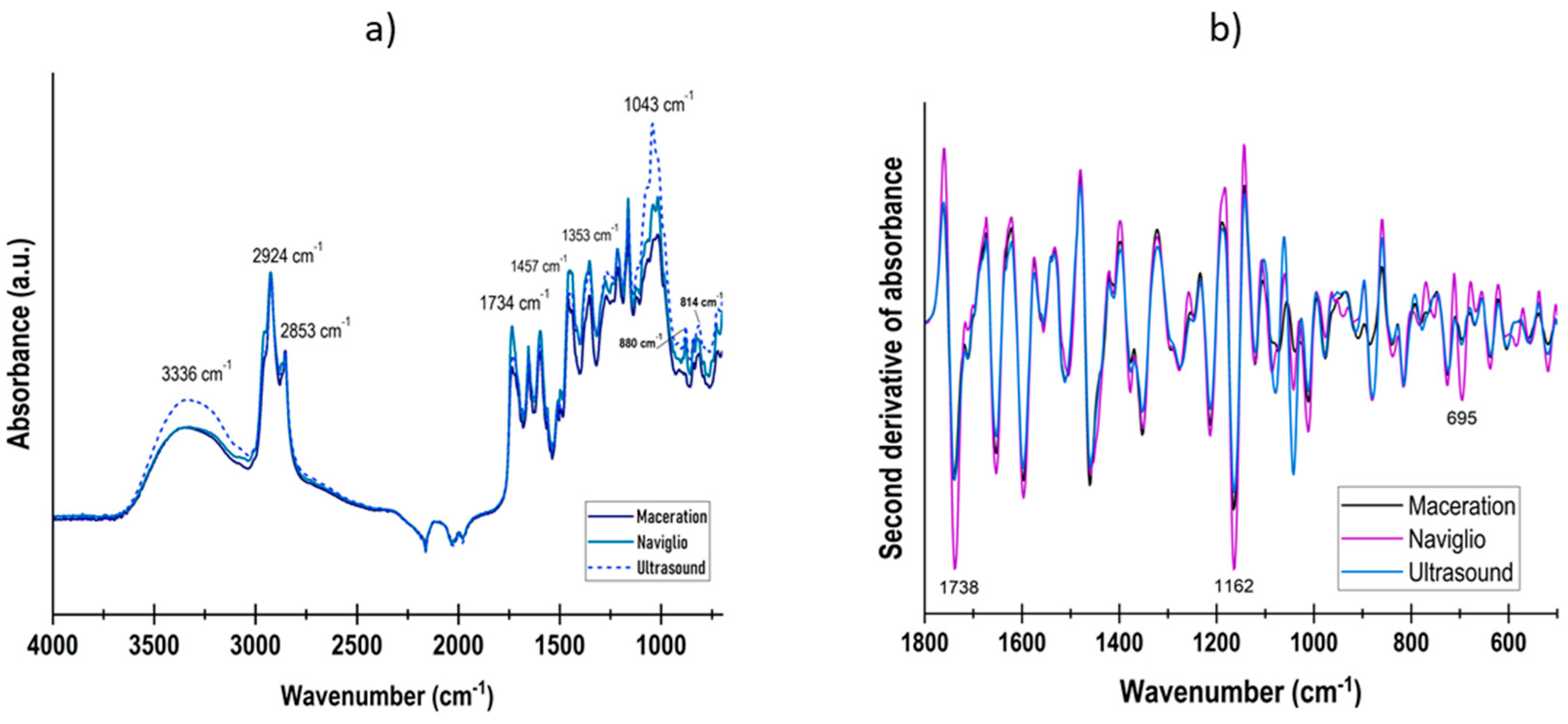


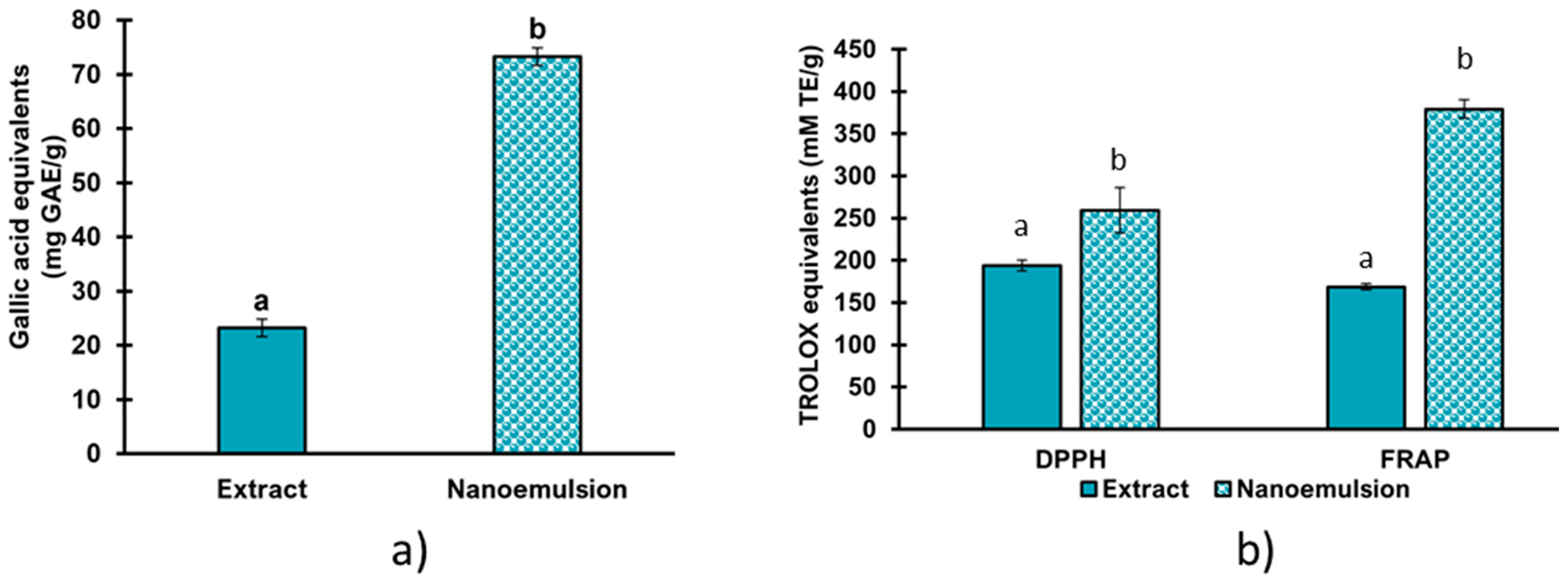

| Treatment | Extract Concentration (%) | Amplitude (%) |
|---|---|---|
| NE1 | 5 | 40 |
| NE2 | 5 | 50 |
| NE3 | 10 | 40 |
| NE4 | 10 | 50 |
| Extraction Method | Polyphenols 1 | Flavonoids 2 | Antioxidant Capacity | |
|---|---|---|---|---|
| DPPH 3 | FRAP 4 | |||
| Maceration | 95.49 ±5.3 a | 5.77 ±1.02 a | 295.72 ±75.8 a | 527.31 ±23.1 a |
| Naviglio | 104.9 ±6.8 b | 4.57 ±0.63 b | 258.03 ±74.8 a | 359.64 ±23.4 b |
| Ultrasound | 97.58 ±9.0 ab | 5.98 ± 0.79 a | 494.96 ±68.9 b | 665.81 ±49.1 c |
| Bacteria | MIC (mg/mL) 1 | MBC (mg/mL) 1 | ||||
|---|---|---|---|---|---|---|
| Maceration | Ultrasound | Naviglio | Maceration | Ultrasound | Naviglio | |
| Staphylococcus aureus | 25 a | 25 a | 12.5 b | 50 a | 50 a | 25 b |
| Listeria monocytogenes | 25 a | 25 a | 12.5 b | 25 a | 25 a | 12.5 b |
| Escherichia coli | 25 a | 25 a | 12.5 b | 25 a | 25 a | 25 b |
| Salmonella enterica | 25 a | 25 a | 12.5 b | 25 a | 25 a | 25 b |
| Treatment | Extract Concentration (%) | Amplitude (%) | Droplet Size (nm) 1,2 | Polydispersity Index 1,2 | Zeta Potential (mV) 1,2 |
|---|---|---|---|---|---|
| NE1 | 5 | 40 | 13.5 ± 3.7 a | 0.85 ± 0.08 a | 0.03 ± 0.15 a |
| NE2 | 5 | 50 | 4.4 ± 1.3 b | 0.48 ± 0.03 b | −0.08 ± 0.20 a |
| NE3 | 10 | 40 | 5.8 ± 1.2 bc | 0.51 ± 0.03 b | 0.04 ± 0.20 a |
| NE4 | 10 | 50 | 7.7 ± 0.6 c | 0.59 ± 0.07 c | 0.06 ± 0.17 a |
| Treatment | Total Polyphenols (mg GAE/g) 1 | Antioxidant Capacity (mM TE/g) | ||
|---|---|---|---|---|
| Day 1 | Day 30 | Day 1 | Day 30 | |
| NE1 | 28 ± 0.9 a | 31 ± 1.1 b | 62 ± 2.7 a | 79 ± 15 b |
| NE2 | 27 ± 1.7 a | 27 ± 1.8 a | 60 ± 2.0 a | 74 ± 3.7 b |
| NE3 | 30 ± 1.6 a | 22 ± 0.5 b | 72 ± 5.7 a | 94 ± 5.9 b |
| NE4 | 27 ± 1.0 a | 22 ± 0.4 b | 67 ± 3.8 a | 94 ± 4.1 b |
| Bacteria | MIC (mg/mL) 1 | MBC (mg/mL) | ||
|---|---|---|---|---|
| Extract | Nanoemulsion | Extract | Nanoemulsion | |
| Staphylococcus aureus | >25 | 25 | n.d. 2 | >25 |
| Listeria monocytogenes | >25 | 25 | n.d. | 25 |
| Salmonella enterica | >25 | 25 | n.d. | 25 |
| Escherichia coli | >25 | 25 | n.d. | >25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Hernández, L.C.; Serrano-Niño, J.C.; Velázquez-Carriles, C.A.; Martínez-Preciado, A.H.; Cavazos-Garduño, A.; Silva-Jara, J.M. Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals. Colloids Interfaces 2024, 8, 3. https://doi.org/10.3390/colloids8010003
Vega-Hernández LC, Serrano-Niño JC, Velázquez-Carriles CA, Martínez-Preciado AH, Cavazos-Garduño A, Silva-Jara JM. Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals. Colloids and Interfaces. 2024; 8(1):3. https://doi.org/10.3390/colloids8010003
Chicago/Turabian StyleVega-Hernández, Lucía Carolina, Julio César Serrano-Niño, Carlos Arnulfo Velázquez-Carriles, Alma H. Martínez-Preciado, Adriana Cavazos-Garduño, and Jorge Manuel Silva-Jara. 2024. "Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals" Colloids and Interfaces 8, no. 1: 3. https://doi.org/10.3390/colloids8010003
APA StyleVega-Hernández, L. C., Serrano-Niño, J. C., Velázquez-Carriles, C. A., Martínez-Preciado, A. H., Cavazos-Garduño, A., & Silva-Jara, J. M. (2024). Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals. Colloids and Interfaces, 8(1), 3. https://doi.org/10.3390/colloids8010003






