Biocomposite Films of Amylose Reinforced with Polylactic Acid by Solvent Casting Method Using a Pickering Emulsion Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CNF Grafting
2.2.2. Characterization of Grafted CNF
2.2.3. Blends Preparation Using a Pickering Emulsion Approach
2.2.4. Films Production and Characterization
Fourier Transform Infra-Red (FT-IR) Spectroscopy
Wide-Angle X-ray Scattering (WAXS)
Moisture Content
Swelling and Water Solubility
Mechanical Properties
Scanning Electron Microscopy (SEM)
Wettability by Contact Angle
Statistical Analysis
3. Results
3.1. Grafting of CNF Align Margins the Same Everywhere, See Below for Example
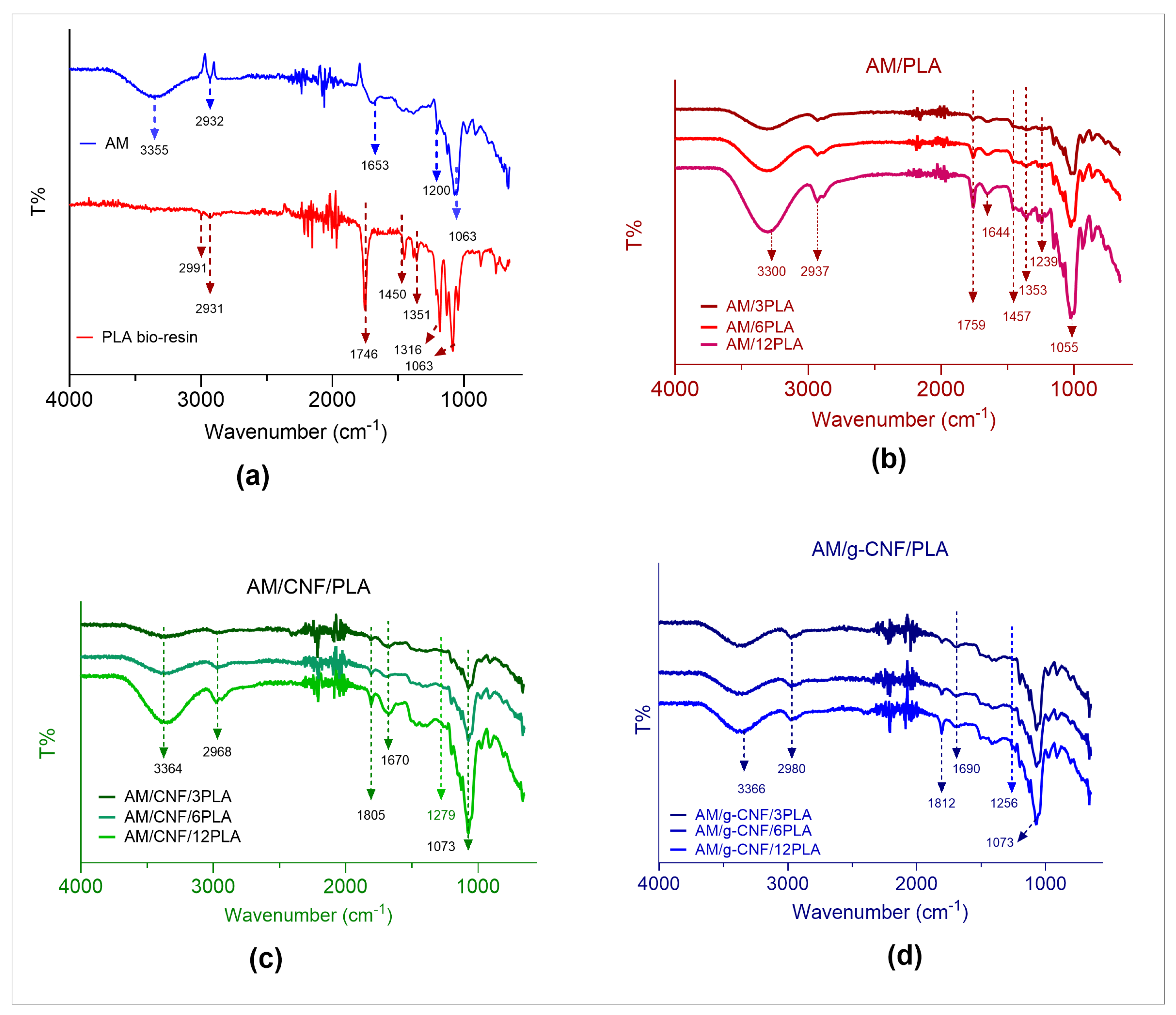
3.2. FTIR of the Composite Films Align Margins the Same Everywhere
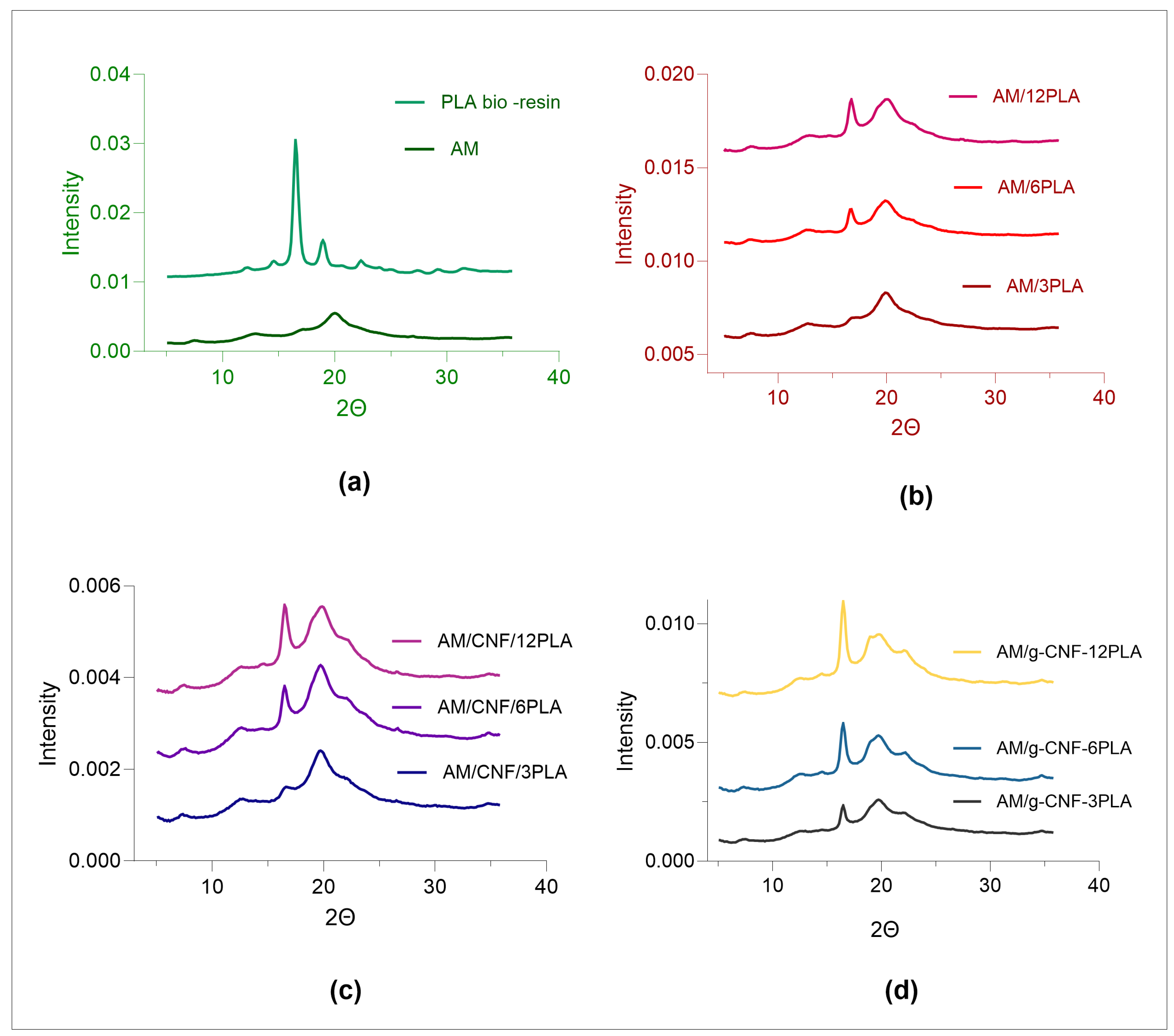
3.3. Crystallinity
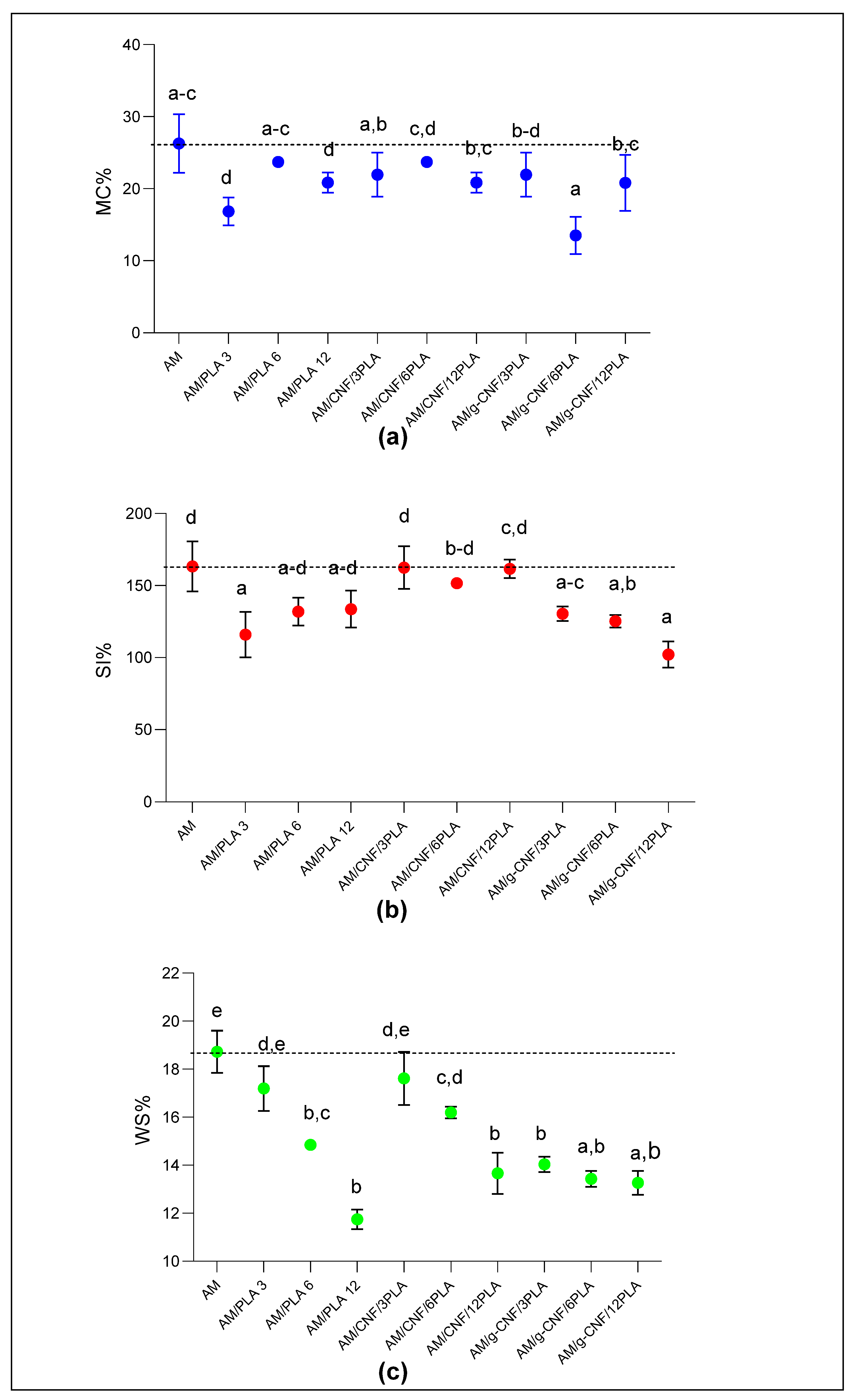
3.4. Moisture Content
3.5. Swelling Index and Water Solubility
3.6. Mechanical Properties
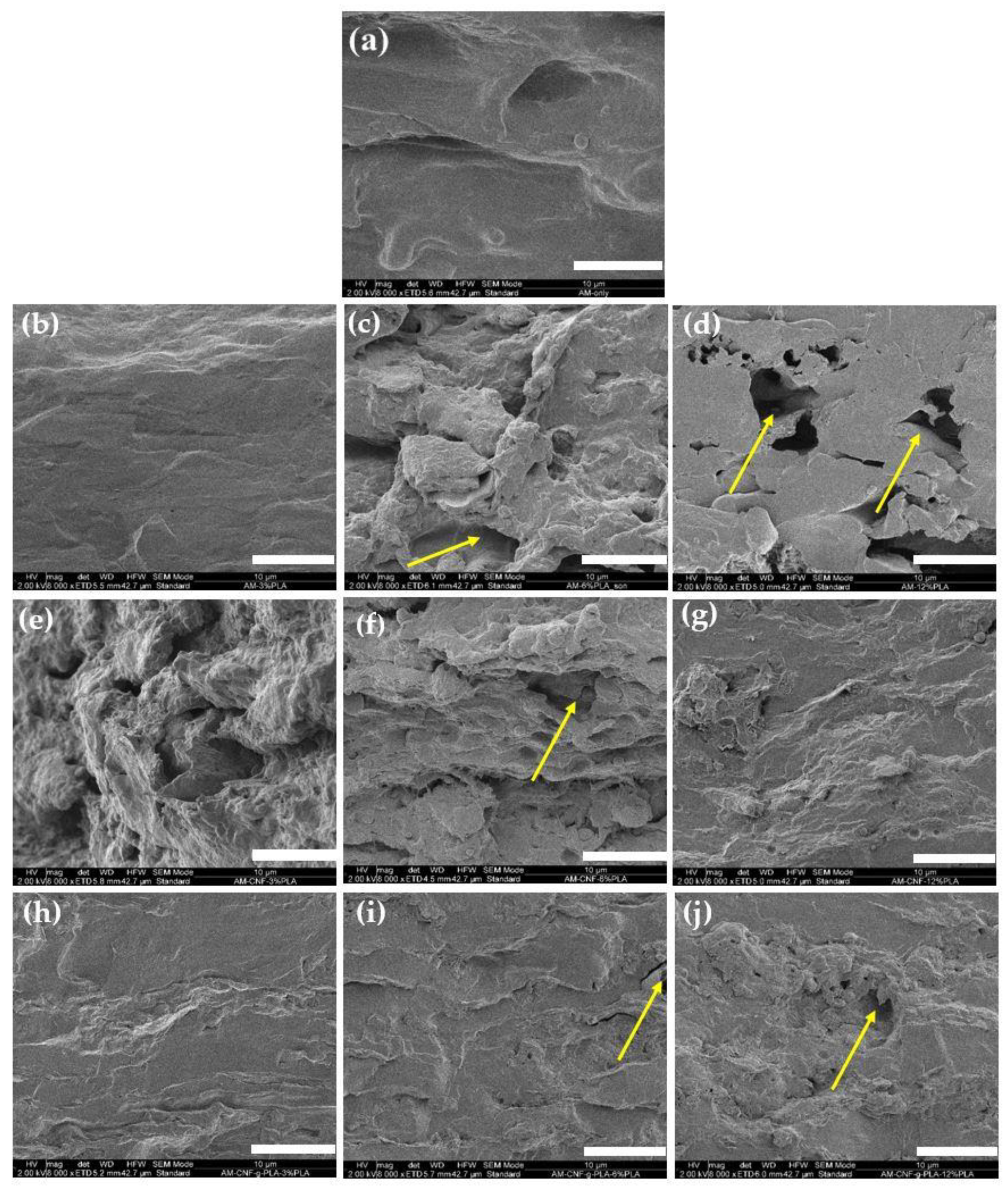
3.7. SEM
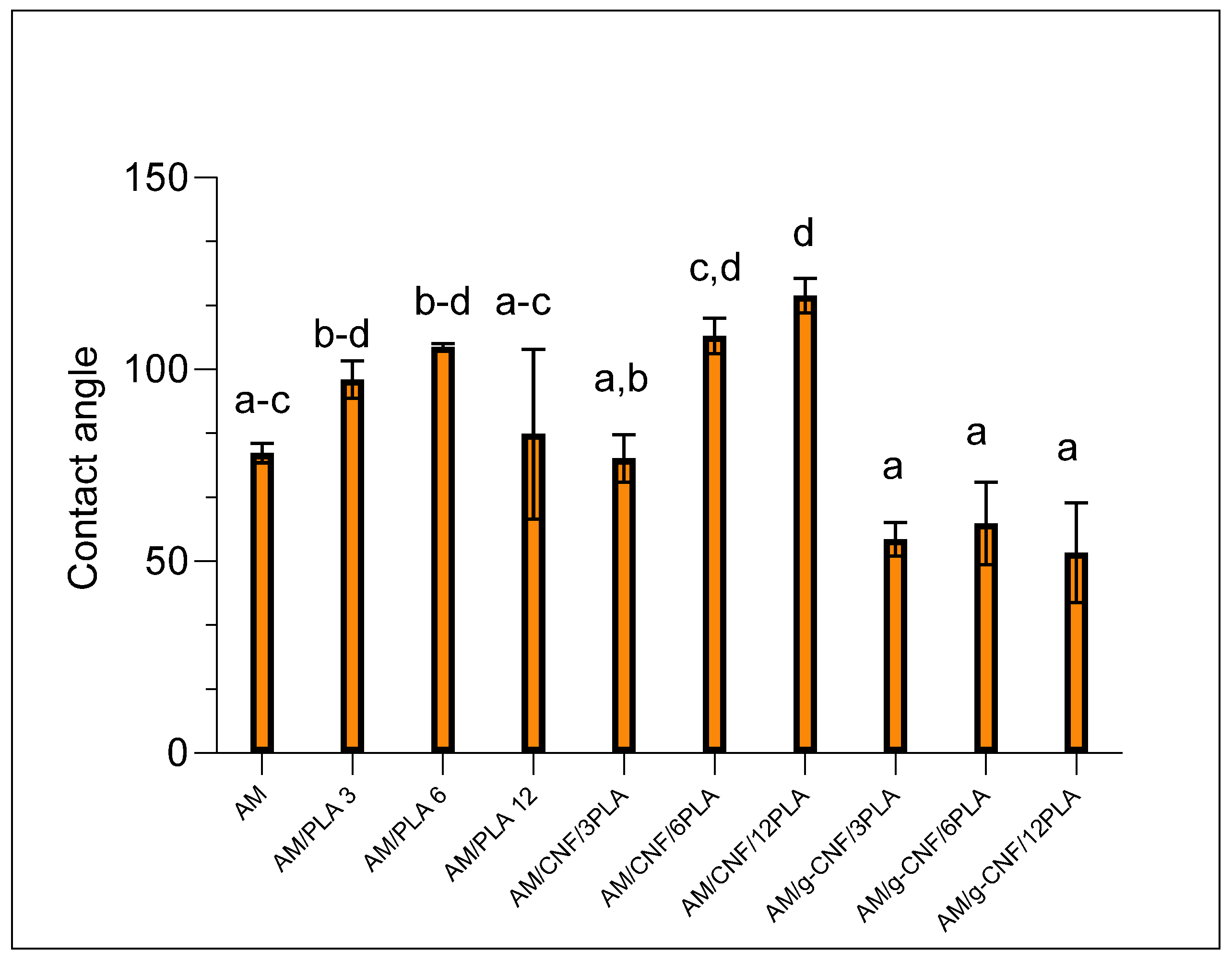
3.8. Wettability by Water Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of Biodegradable Plastics: New Problem or Solution to Solve the Global Plastic Pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Faisal, M.; Kou, T.; Zhong, Y.; Blennow, A. High Amylose-Based Bio Composites: Structures, Functions and Applications. Polymers 2022, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tai, L.; Blennow, A.; Ding, L.; Herburger, K.; Qu, J.; Xin, A.; Guo, D.; Hebelstrup, K.H.; Liu, X. High-Amylose Starch: Structure, Functionality and Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 8568–8590. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hebelstrup, K.H.; Leroy, E.; Rolland-Sabaté, A.; Guilois, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Lourdin, D.; Blennow, A. Plant-Crafted Starches for Bioplastics Production. Carbohydr. Polym. 2016, 152, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buléon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted Suppression of All Starch Branching Enzyme Genes in Barley Produces Amylose-Only Starch Granules. BMC Plant Biol. 2012, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Luyt, A.S.; Malik, S.S. Can Biodegradable Plastics Solve Plastic Solid Waste Accumulation? Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131404. [Google Scholar]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sharafi Zamir, S.; Fathi, B.; Ajji, A.; Robert, M.; Elkoun, S. Crystallinity and Gas Permeability of Poly (Lactic Acid)/Starch Nanocrystal Nanocomposite. Polymers 2022, 14, 2802. [Google Scholar] [CrossRef]
- Pluta, M.; Jeszka, J.K.; Boiteux, G. Polylactide/Montmorillonite Nanocomposites: Structure, Dielectric, Viscoelastic and Thermal Properties. Eur. Polym. J. 2007, 43, 2819–2835. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Zhang, C.; Tang, Z.; Na, H.; Zhu, J. Effect of 1,3,5-Trialkyl-Benzenetricarboxylamide on the Crystallization of Poly(Lactic Acid). J. Appl. Polym. Sci. 2013, 130, 1328–1336. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Osorio, B.H.; Aguirre-Loredo, R.Y.; Calambas, H.L.; Caicedo, C. Miscibility Study of Thermoplastic Starch/Polylactic Acid Blends: Thermal and Superficial Properties. Carbohydr. Polym. 2022, 293, 119744. [Google Scholar] [CrossRef] [PubMed]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. J. Polym. Environ. 2022, 30, 75–91. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Fikriyyah, A.K.; Putri, O.D.; Puspa Asri, P.P. Fabrication and Characterization of Poly Lactic Acid (PLA)-Starch Based Bioplastic Composites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012052. [Google Scholar] [CrossRef]
- Wokadala, O.C.; Emmambux, N.M.; Ray, S.S. Inducing PLA/Starch Compatibility through Butyl-Etherification of Waxy and High Amylose Starch. Carbohydr. Polym. 2014, 112, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Raghu, N.; Raj, A. Effect of Maleic Anhydride Grafted Polylactic Acid Concentration on Mechanical and Thermal Properties of Thermoplasticized Starch Filled Polylactic Acid Blends. Polym. Polym. Compos. 2021, 29, S400–S410. [Google Scholar] [CrossRef]
- Clasen, S.H.; Müller, C.M.O.; Pires, A.T.N. Maleic Anhydride as a Compatibilizer and Plasticizer in TPS/PLA Blends. J. Braz. Chem. Soc. 2015, 26, 1583–1590. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A New Approach in Compatibilization of the Poly(Lactic Acid)/Thermoplastic Starch (PLA/TPS) Blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef]
- Xu, J.; Sagnelli, D.; Faisal, M.; Perzon, A.; Taresco, V.; Mais, M.; Giosafatto, C.V.L.; Hebelstrup, K.H.; Ulvskov, P.; Jørgensen, B.; et al. Amylose/Cellulose Nanofiber Composites for All-Natural, Fully Biodegradable and Flexible Bioplastics. Carbohydr. Polym. 2021, 253, 117277. [Google Scholar] [CrossRef]
- Perzon, A.; Jørgensen, B.; Ulvskov, P. Sustainable Production of Cellulose Nanofiber Gels and Paper from Sugar Beet Waste Using Enzymatic Pre-Treatment. Carbohydr. Polym. 2020, 230, 115581. [Google Scholar] [CrossRef]
- Goffin, A.L.; Raquez, J.M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From Interfacial Ring-Opening Polymerization to Melt Processing of Cellulose Nanowhisker-Filled Polylactide-Based Nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Qi, R.; Liu, L.; Juan, G.; Huang, S. Synthesis of Poly(L-Lactide) via Solvothermal Method. Int. J. Polym. Sci. 2009, 2009, 929732. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.H.; Park, J.K. Improvement of Processability of Clay/Polylactide Nanocomposites by a Combinational Method: In Situ Polymerization of L-Lactide and Melt Compounding of Polylactide. J. Appl. Polym. Sci. 2006, 101, 1664–1669. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Zhou, L.; Duan, Y.; Zhang, J. Synthesis and Characterization of Cellulose Nanocrystal-Graft-Poly(D-Lactide) and Its Nanocomposite with Poly(L-Lactide). Polymer 2016, 103, 365–375. [Google Scholar] [CrossRef]
- Gauss, C.; Pickering, K.L. A New Method for Producing Polylactic Acid Biocomposites for 3D Printing with Improved Tensile and Thermo-Mechanical Performance Using Grafted Nanofibrillated Cellulose. Addit. Manuf. 2023, 61, 103346. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yu, T.; Wang, N.; Wang, C.; Wang, H. Preparation of Polylactic Acid/TEMPO-Oxidized Bacterial Cellulose Nanocomposites for 3D Printing via Pickering Emulsion Approach. Compos. Commun. 2019, 16, 162–167. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and Characterization of Modified Cellulose Nanofibers Reinforced Polylactic Acid Nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Ansari, F.; Skrifvars, M.; Berglund, L. Nanostructured Biocomposites Based on Unsaturated Polyester Resin and a Cellulose Nanofiber Network. Compos. Sci. Technol. 2015, 117, 298–306. [Google Scholar] [CrossRef]
- Bataille, P.; Statioukha, G.; Statioukha, O. Simulation and Optimization of Cellulose and Styrene Graft Copolymerization Process. Can. J. Chem. Eng. 1996, 74, 501–510. [Google Scholar] [CrossRef]
- Faisal, M.; Žmirić, M.; Kim, N.; Bruun, S.; Mariniello, L.; Famiglietti, M.; Bordallo, H.; Kirkensgaard, J.; Jørgensen, B.; Ulvskov, P.; et al. A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics. Coatings 2023, 13, 1573. [Google Scholar] [CrossRef]
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Edible Composite Films of Wheat Gluten and Lipids: Water Vapour Permeability and Other Physical Properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Laing, M. Characterization of Physicochemical Properties of Starches from Improved Cassava Varieties Grown in Zambia. AIMS Agric. Food 2019, 4, 939–966. [Google Scholar]
- Yoksan, R.; Boontanimitr, A.; Klompong, N. Poly (Lactic Acid)/Thermoplastic Cassava Starch Blends Filled with Duckweed Biomass. Int. J. Biol. Macromol. 2022, 203, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W.Y. In-Situ Polymerized Cellulose Nanocrystals (CNC)—Poly(L-Lactide) (PLLA) Nanomaterials and Applications in Nanocomposite Processing. Carbohydr. Polym. 2016, 153, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and Barrier Properties of Starch Blend Films Enhanced with Kaolin for Application in Food Packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO Nanoparticles on the Mechanical, Barrier and Optical Properties of Thermoplastic Cationic Starch/Montmorillonite Biodegradable Films. Int. J. Biol. Macromol. 2019, 124, 519–529. [Google Scholar] [CrossRef]
- Cuevas-Carballo, Z.B.; Duarte-Aranda, S.; Canché-Escamilla, G. Properties and Biodegradation of Thermoplastic Starch Obtained from Grafted Starches with Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 2607–2617. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M. Mechanical, Physical and Thermal Properties of Sugar Palm Nanocellulose Reinforced Thermoplastic Starch (TPS)/Poly (Lactic Acid) (PLA) Blend Bionanocomposites. Polymers 2020, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Chotiprayon, P.; Chaisawad, B.; Yoksan, R. Thermoplastic Cassava Starch/Poly(Lactic Acid) Blend Reinforced with Coir Fibres. Int. J. Biol. Macromol. 2020, 156, 960–968. [Google Scholar] [CrossRef]
- Mohd Nizar, M.; Hamzah, M.S.A.; Abd Razak, S.I.; Mat Nayan, N.H. Thermal Stability and Surface Wettability Studies of Polylactic Acid/Halloysite Nanotube Nanocomposite Scaffold for Tissue Engineering Studies. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012006. [Google Scholar] [CrossRef]
| Code | AM (wt.%) | PLA (wt.%) | Glycerol (wt.%) | CNF (wt.%) | g-CNF (wt.%) |
|---|---|---|---|---|---|
| AM/PLA blends | |||||
| AM | 77 | 0 | 23 | 0 | 0 |
| AM/3PLA | 67 | 3 | 23 | 0 | 0 |
| AM/6PLA | 64 | 6 | 23 | 0 | 0 |
| AM/12PLA | 58 | 12 | 23 | 0 | 0 |
| AM/CNF/PLA blends | |||||
| AM/CNF/3PLA | 59 | 3 | 23 | 15 | 0 |
| AM/CNF/6PLA | 57 | 6 | 23 | 14 | 0 |
| AM/CNF/12PLA | 52 | 12 | 23 | 13 | 0 |
| AM/g-CNF/PLA blends | |||||
| AM/g- CNF/3PLA | 59 | 3 | 23 | 0 | 15 |
| AM/g- CNF/6PLA | 57 | 6 | 23 | 0 | 14 |
| AM/g-CNF/12PLA | 52 | 12 | 23 | 0 | 13 |
| Code | Tensile Strength (MPa) | EAB (%) | Relative Crystallinity (%) |
|---|---|---|---|
| AM | 11.4 ± 0.4 b | 28.2 ± 1.7 e | 21.9 ± 0.1 a |
| AM/PLA 3 | 7.7 ± 3.5 a,b | 19.0 ± 5.2 c,d | 24 ± 1.4 a,b |
| AM/PLA 6 | 5.9 ± 0.1 a,b | 16.6 ± 4.5 d | 29.5 ± 2.9 a,b |
| AM/PLA 12 | 3.5 ± 0.4 a | 8.9 ± 1.0 b,c | 31.2 ± 3.7 a,b |
| AM/CNF/3PLA | 21.3 ± 2.3 c | 3.0 ± 1.3 a,b | 32.6 ± 1.8 a,b |
| AM/CNF/6PLA | 17.1 ± 2.6 c | 8.7 ± 3.9 b,c | 34.5 ± 1.5 a,b |
| AM/CNF/12PLA | 2.8 ± 0.5 a | 1.1 ± 0.2 a | 38 ± 4.0 a |
| AM/g-CNF/3PLA | 18.4 ± 4.8 c | 6.0 ± 2.9 a,b | 33.5 ± 0.7 a,b |
| AM/g-CNF/6PLA | 20.2 ± 4.6 c | 4.5 ± 1.9 a,b | 32.6 ± 1.9 a,b |
| AM/g-CNF/12PLA | 8.8 ± 4.5 a,b | 4.4 ± 2.0 a,b | 37.1 ± 3 a,b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Kirkensgaard, J.J.K.; Jørgensen, B.; Ulvskov, P.; Rée, M.; Kang, S.; Andersson, N.; Jørgensen, M.; Simonsen, J.; Hebelstrup, K.H.; et al. Biocomposite Films of Amylose Reinforced with Polylactic Acid by Solvent Casting Method Using a Pickering Emulsion Approach. Colloids Interfaces 2024, 8, 37. https://doi.org/10.3390/colloids8030037
Faisal M, Kirkensgaard JJK, Jørgensen B, Ulvskov P, Rée M, Kang S, Andersson N, Jørgensen M, Simonsen J, Hebelstrup KH, et al. Biocomposite Films of Amylose Reinforced with Polylactic Acid by Solvent Casting Method Using a Pickering Emulsion Approach. Colloids and Interfaces. 2024; 8(3):37. https://doi.org/10.3390/colloids8030037
Chicago/Turabian StyleFaisal, Marwa, Jacob Judas Kain Kirkensgaard, Bodil Jørgensen, Peter Ulvskov, Max Rée, Sue Kang, Nikolai Andersson, Mikkel Jørgensen, Jonas Simonsen, Kim H. Hebelstrup, and et al. 2024. "Biocomposite Films of Amylose Reinforced with Polylactic Acid by Solvent Casting Method Using a Pickering Emulsion Approach" Colloids and Interfaces 8, no. 3: 37. https://doi.org/10.3390/colloids8030037
APA StyleFaisal, M., Kirkensgaard, J. J. K., Jørgensen, B., Ulvskov, P., Rée, M., Kang, S., Andersson, N., Jørgensen, M., Simonsen, J., Hebelstrup, K. H., & Blennow, A. (2024). Biocomposite Films of Amylose Reinforced with Polylactic Acid by Solvent Casting Method Using a Pickering Emulsion Approach. Colloids and Interfaces, 8(3), 37. https://doi.org/10.3390/colloids8030037










