Co-Encapsulation of Paclitaxel and Doxorubicin in Liposomes Layer by Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
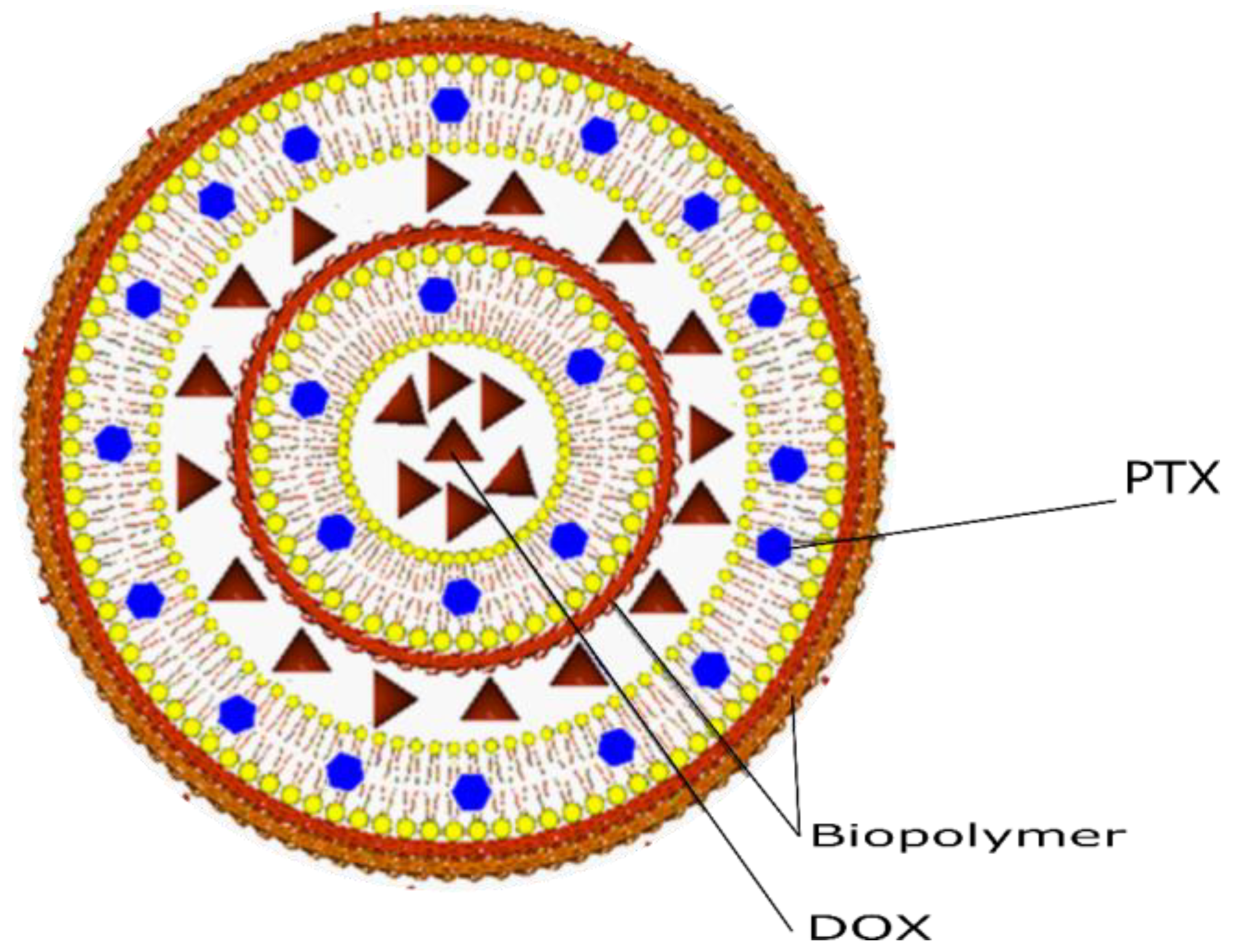
2.2. Preparation of Liposomes
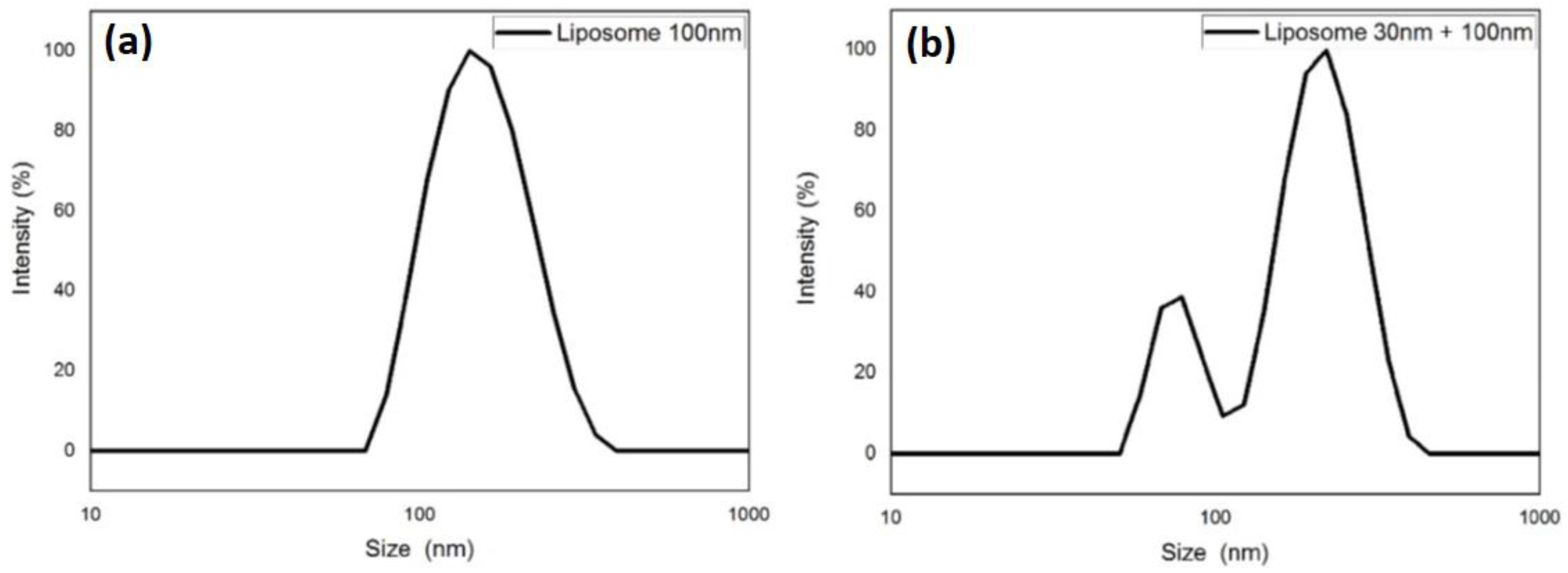
2.3. Size Determination by DLS and Potential ζ
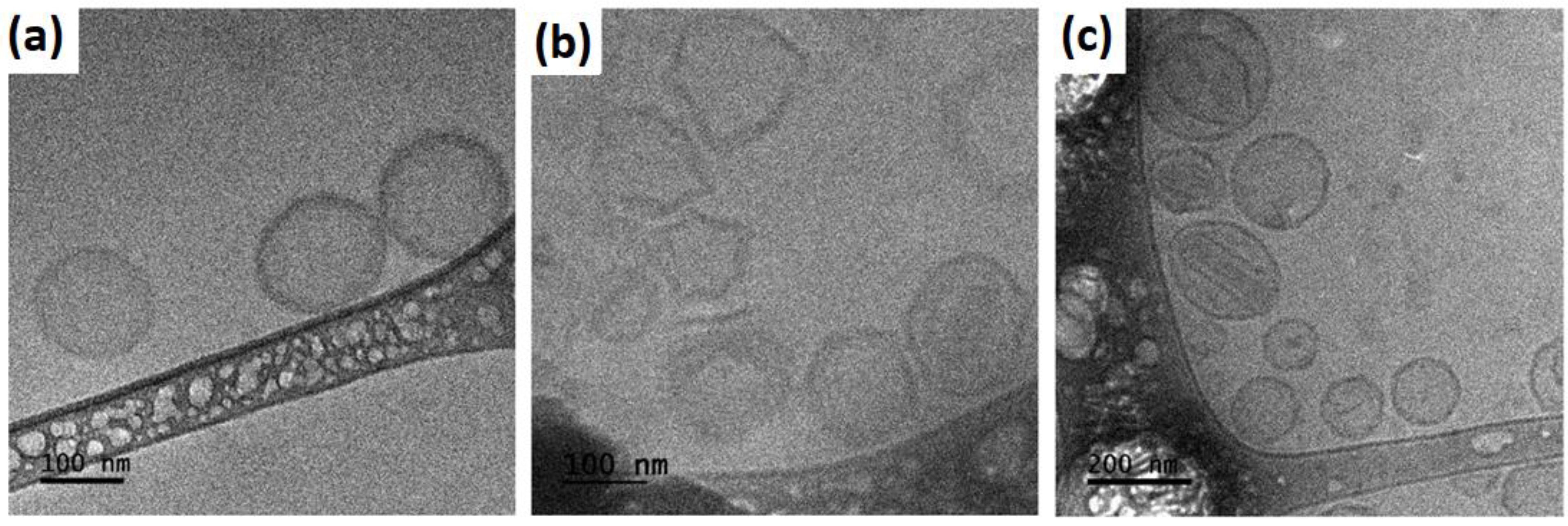
2.4. Morphology by Cryo-TEM
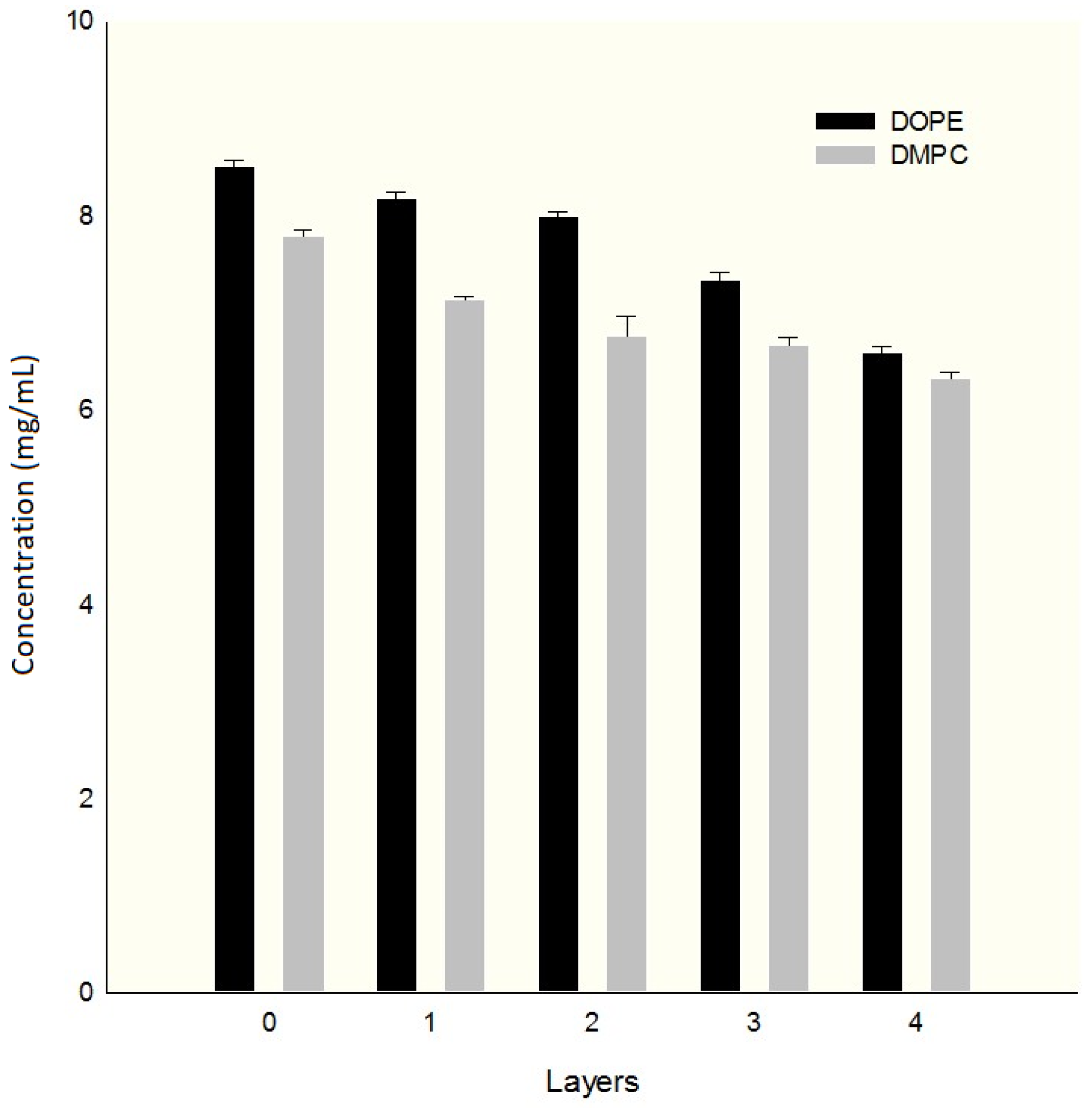
2.5. Determination of Encapsulation Efficiency (%EE)
- DRUGMED—the concentration obtained after the degradation of the nanosystem (final amount).
- DRUGTOT—the concentration of drug used in the elaboration (starting amount).
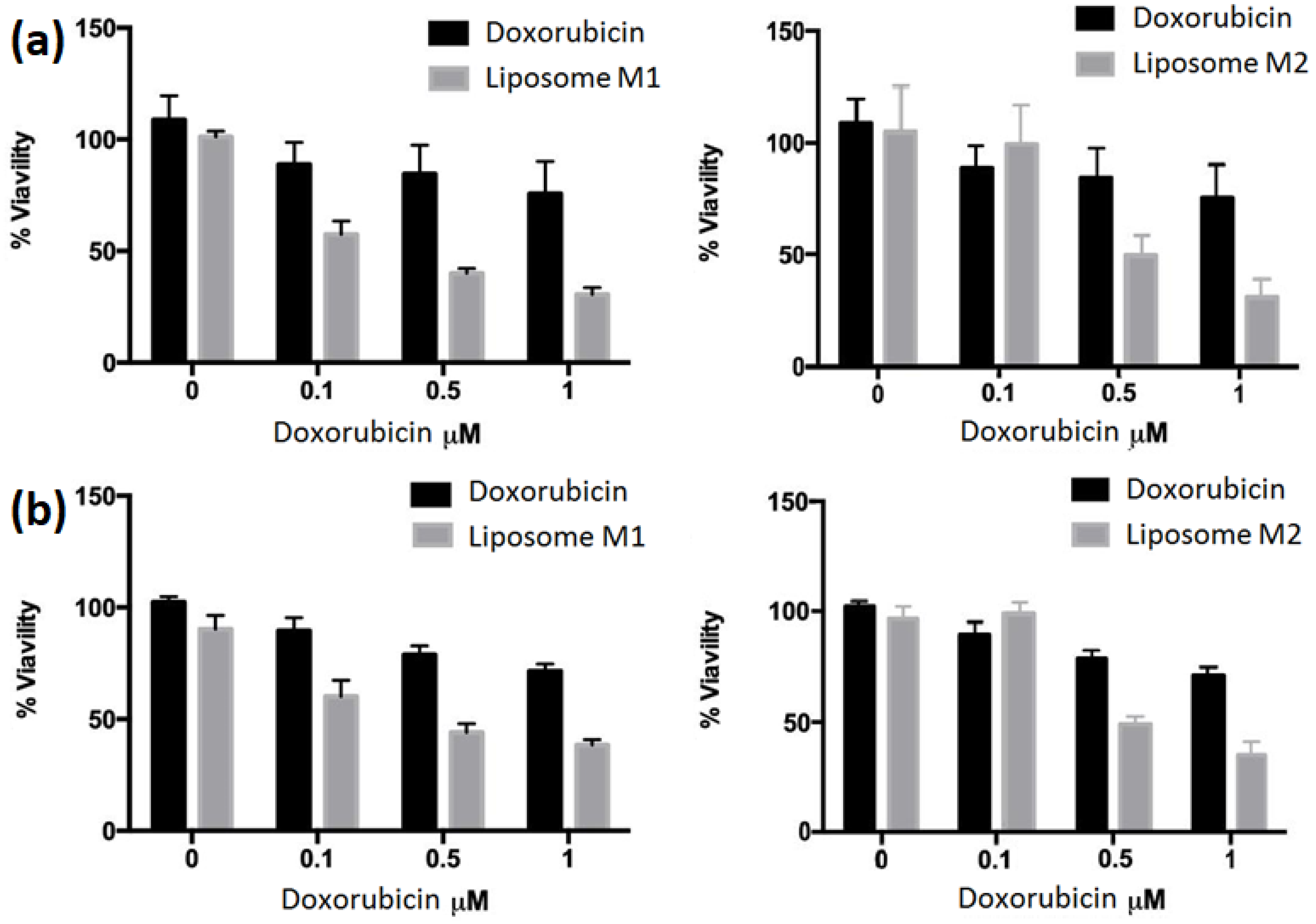
2.6. Cell Viability Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Barenholz, Y. Liposomes: Preparation, characterization, and preservation. Methods Biochem. Anal. 1988, 33, 337–462. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Jash, A.; Krueger, A.; Rizvi, S.S. Venturi-based rapid expansion of supercritical solution (Vent-RESS): Synthesis of liposomes for pH-triggered delivery of hydrophilic and lipophilic bioactives. Green Chem. 2022, 24, 5326–5337. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Karmali, P.P.; Chaudhuri, A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med. Res. Rev. 2007, 27, 696–722. [Google Scholar] [CrossRef]
- Rajappan, K.; Tanis, S.P.; Mukthavaram, R.; Roberts, S.; Nguyen, M.; Tachikawa, K.; Sagi, A.; Sablad, M.; Limphong, P.; Leu, A. Property-driven design and development of lipids for efficient delivery of siRNA. J. Med. Chem. 2020, 63, 12992–13012. [Google Scholar] [CrossRef]
- Olusanya, T.O.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Immordino, M.L.; Brusa, P.; Stella, B.; Arpicco, S.M.; Dosio, F.; Cattel, L. Preparazione e caratterizzazione di liposomi convenzionali ed a lunga circolazione contenenti taxani. Acta Technol. Legis Medicam. 2001, 12, 101–107. [Google Scholar]
- Laura Immordino, M.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine 2006, 1, 297–315. [Google Scholar]
- Gürsoy, A.; Kut, E.; Özkırımlı, S. Co-encapsulation of isoniazid and rifampicin in liposomes and characterization of liposomes by derivative spectroscopy. Int. J. Pharm. 2004, 271, 115–123. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Franco, M.S.; Oliveira, M.C. Liposomes co-encapsulating anticancer drugs in synergistic ratios as an approach to promote increased efficacy and greater safety. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2019, 19, 17–28. [Google Scholar] [CrossRef]
- Gabizon, A.; Ohana, P.; Amitay, Y.; Gorin, J.; Tzemach, D.; Mak, L.; Shmeeda, H. Liposome co-encapsulation of anti-cancer agents for pharmacological optimization of nanomedicine-based combination chemotherapy. Cancer Drug Resist. 2021, 4, 463. [Google Scholar] [CrossRef]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1845, 84–89. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenetics Genom. 2011, 21, 440. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Yusuf, R.; Duan, Z.; Lamendola, D.; Penson, R.; Seiden, M. Paclitaxel resistance: Molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef]
- Lim, P.T.; Goh, B.H.; Lee, W.-L. Taxol: Mechanisms of action against cancer, an update with current research. In Paclitaxel; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–71. [Google Scholar]
- Jain, S.; Kumar, D.; Swarnakar, N.K.; Thanki, K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 2012, 33, 6758–6768. [Google Scholar] [CrossRef]
- Tan, X.; Fang, Y.; Ren, Y.; Li, Y.; Wu, P.; Yang, X.; Liu, W. D-α-tocopherol polyethylene glycol 1000 succinate-modified liposomes with an siRNA corona confer enhanced cellular uptake and targeted delivery of doxorubicin via tumor priming. Int. J. Nanomed. 2019, 14, 1255–1268. [Google Scholar] [CrossRef]
- Alavi, S.; Haeri, A.; Dadashzadeh, S. Utilization of chitosan-caged liposomes to push the boundaries of therapeutic delivery. Carbohydr. Polym. 2017, 157, 991–1012. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef]
- Song, M.; Liang, Y.; Li, K.; Zhang, J.; Zhang, N.; Tian, B.; Han, J. Hyaluronic acid modified liposomes for targeted delivery of doxorubicin and paclitaxel to CD44 overexpressing tumor cells with improved dual-drugs synergistic effect. J. Drug Deliv. Sci. Technol. 2019, 53, 101179. [Google Scholar] [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef]
- Hu, M.; Gou, T.; Chen, Y.; Xu, M.; Chen, R.; Zhou, T.; Liu, J.; Peng, C.; Ye, Q. A Novel Drug Delivery System: Hyodeoxycholic Acid-Modified Metformin Liposomes for Type 2 Diabetes Treatment. Molecules 2023, 28, 2471. [Google Scholar] [CrossRef]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.-L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in cancer therapy: How did we start and where are we now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal nanomedicine: Applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Felice, B.; Prabhakaran, M.P.; Rodríguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C 2014, 41, 178–195. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical basis of interactions between engineered nanoparticles and biological systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J. Control. Release 2006, 116, 275–284. [Google Scholar] [CrossRef]
- Rolle, F.; Bincoletto, V.; Gazzano, E.; Rolando, B.; Lollo, G.; Stella, B.; Riganti, C.; Arpicco, S. Coencapsulation of disulfiram and doxorubicin in liposomes strongly reverses multidrug resistance in breast cancer cells. Int. J. Pharm. 2020, 580, 119191. [Google Scholar] [CrossRef]
- Lee, W.L.; Guo, W.M.; Ho, V.H.; Saha, A.; Chong, H.C.; Tan, N.S.; Tan, E.Y.; Loo, S.C.J. Delivery of doxorubicin and paclitaxel from double-layered microparticles: The effects of layer thickness and dual-drug vs. single-drug loading. Acta Biomaterialia 2015, 27, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Sarpal, R.S. Binding of paclitaxel to lipid interfaces: Correlations with interface compliance. J. Liposome Res. 1998, 8, 147–163. [Google Scholar] [CrossRef]
- Steffes, V.M.; Zhang, Z.; MacDonald, S.; Crowe, J.; Ewert, K.K.; Carragher, B.; Potter, C.S.; Safinya, C.R. PEGylation of paclitaxel-loaded cationic liposomes drives steric stabilization of bicelles and vesicles thereby enhancing delivery and cytotoxicity to human cancer cells. ACS Appl. Mater. Interfaces 2019, 12, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, Š.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, J.; Kim, Y.-J.; Wong, M.K.; Wang, P. Codelivery of doxorubicin and paclitaxel by cross-linked multilamellar liposome enables synergistic antitumor activity. Mol. Pharm. 2014, 11, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar] [CrossRef] [PubMed]
- Antipov, A.A.; Sukhorukov, G.B. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Adv. Colloid Interface Sci. 2004, 111, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Picart, C. Layer-by-Layer assemblies for cancer treatment and diagnosis. Adv. Mater. 2016, 28, 1295–1301. [Google Scholar] [CrossRef]
- Guenneau, S.; Puvirajesinghe, T. Fick’s second law transformed: One path to cloaking in mass diffusion. J. R. Soc. Interface 2013, 10, 20130106. [Google Scholar] [CrossRef]
- Paradisi, P.; Cesari, R.; Mainardi, F.; Tampieri, F. The fractional Fick’s law for non-local transport processes. Phys. A Stat. Mech. Its Appl. 2001, 293, 130–142. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Piludu, M.; Miguel, M.G.; Lindman, B.; Ceglie, A. Release of small hydrophilic molecules from polyelectrolyte capsules: Effect of the wall thickness. J. Colloid Interface Sci. 2015, 447, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.E.; Cerqueira, M.A.; Cook, M.T.; Bourbon, A.I.; Khutoryanskiy, V.V.; Charalampoulos, D.; Teixeira, J.A.; Vicente, A.A. Development of an immobilization system for in situ micronutrients release. Food Res. Int. 2016, 90, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ansarifar, E.; Mohebbi, M.; Shahidi, F.; Koocheki, A.; Ramezanian, N. Novel multilayer microcapsules based on soy protein isolate fibrils and high methoxyl pectin: Production, characterization and release modeling. Int. J. Biol. Macromol. 2017, 97, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Yang, L.; Wei, W.; Sun, B.; Na, K.; Song, Y.; Zhang, H.; He, Z.; Sun, J. Redox dual-responsive paclitaxel-doxorubicin heterodimeric prodrug self-delivery nanoaggregates for more effective breast cancer synergistic combination chemotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102066. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Zhou, S.; Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel–doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Niidome, T.; Katoh, E.; Xiang, H.; Han, L.; Mori, T.; Katayama, Y. Polydispersity as a parameter for indicating the thermal stability of proteins by dynamic light scattering. Anal. Sci. 2010, 26, 659–663. [Google Scholar] [CrossRef]
- Yeap, S.P.; Lim, J.; Ngang, H.P.; Ooi, B.S.; Ahmad, A.L. Role of particle–particle interaction towards effective interpretation of Z-average and particle size distributions from dynamic light scattering (DLS) analysis. J. Nanosci. Nanotechnol. 2018, 18, 6957–6964. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Yuan, M.; Qiu, Y.; Zhang, L.; Gao, H.; He, Q. Targeted delivery of transferrin and TAT co-modified liposomes encapsulating both paclitaxel and doxorubicin for melanoma. Drug Deliv. 2016, 23, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Geraldes, D.; da Silva, C.; Oliveira, M.; Nascimento, L. Long-Circulating and Fusogenic Liposomes Loaded with Paclitaxel and Doxorubicin: Effect of Excipient, Freezing, and Freeze-Drying on Quality Attributes. Pharmaceutics 2022, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wu, S.; Hu, C.; Chen, Z.; Wang, H.; Fan, F.; Qin, Y.; Wang, C.; Sun, H.; Leng, X. Folate-targeted polymersomes loaded with both paclitaxel and doxorubicin for the combination chemotherapy of hepatocellular carcinoma. Acta Biomater. 2017, 58, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Guzelsu, N.; Wienstien, C.; Kotha, S. A new streaming potential chamber for zeta potential measurements of particulates. Rev. Sci. Instrum. 2010, 81, 015106. [Google Scholar] [CrossRef] [PubMed]
- Mehn, D.; Iavicoli, P.; Cabaleiro, N.; Borgos, S.E.; Caputo, F.; Geiss, O.; Calzolai, L.; Rossi, F.; Gilliland, D. Analytical ultracentrifugation for analysis of doxorubicin loaded liposomes. Int. J. Pharm. 2017, 523, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, M.; Berkó, S.; Budai-Szűcs, M.; Kovács, A.; Csányi, E. DSC for evaluating the encapsulation efficiency of lidocaine-loaded liposomes compared to the ultracentrifugation method. J. Therm. Anal. Calorim. 2017, 130, 1619–1625. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Cavallo, J.A.; Chuliá-Jordán, R.; Gómez, C.; Strumia, M.C.; Ortega, F.; Rubio, R.G. pH-induced changes in the fabrication of multilayers of poly (acrylic acid) and chitosan: Fabrication, properties, and tests as a drug storage and delivery system. Langmuir 2011, 27, 6836–6845. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, J.; Wang, Z.; Yin, J.; Zheng, Y.; Chen, X. Layer-by-layer assembly of poly (L-glutamic acid)/chitosan microcapsules for high loading and sustained release of 5-fluorouracil. Eur. J. Pharm. Biopharm. 2011, 78, 336–345. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Lopez, F.; Miguel, M.G.; Lindman, B.R. Vesicle-templated layer-by-layer assembly for the production of nanocapsules. Langmuir 2010, 26, 10555–10560. [Google Scholar] [CrossRef] [PubMed]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Kursch, B.; Lüllmann, H.; Mohr, K. Influence of various cationic amphiphilic drugs on the phase-transition temperature of phosphatidylcholine liposomes. Biochem. Pharmacol. 1983, 32, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Pandey, B.; Lakhera, P.; Singh, K. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int. J. Nanomed. 2010, 5, 101–108. [Google Scholar] [CrossRef]
- Gubernator, J.; Chwastek, G.; Korycińska, M.; Stasiuk, M.; Grynkiewicz, G.; Lewrick, F.; Süss, R.; Kozubek, A. The encapsulation of idarubicin within liposomes using the novel EDTA ion gradient method ensures improved drug retention in vitro and in vivo. J. Control. Release 2010, 146, 68–75. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef]
- Li, T.; Nowell, C.J.; Cipolla, D.; Rades, T.; Boyd, B.J. Direct comparison of standard transmission electron microscopy and cryogenic-TEM in imaging nanocrystals inside liposomes. Mol. Pharm. 2019, 16, 1775–1781. [Google Scholar] [CrossRef]

| RH (nm) | ζ (mV) | PDI | |
|---|---|---|---|
| blank | 94.0850 ± 0.600 | −26.610 ± 0.850 | 0.00500 ± 0.0004 |
| Layer 1 | 101.190 ± 0.247 | +18.705 ± 0.441 | 0.0130 ± 0.001 |
| Layer 2 | 131.283 ± 0.344 | −14.193 ± 0.214 | 0.209 ± 0.006 |
| Layer 3 | 164.518 ± 0.331 | +12.276 ± 0.217 | 0.258 ± 0.008 |
| Layer 4 | 204.287 ± 0.513 | −8.9980 ± 0.343 | 0.448 ± 0.007 |
| RH initial (nm) | PDIinitial | RH final (nm) | PDIfinal | ζ (mV) | %EE DOX-PTX | |
|---|---|---|---|---|---|---|
| M1 | 122.613 ± 0.756 | 0.137 ± 0.001 | 144.188 ± 0.473 | 0.232± 0.007 | −26.842 ± 0.278 | 84.4 ± 2.10–73.6 ± 6.23 |
| M2 | 103.073 ± 0.128 63.4820 ± 0.605 | 0.0430 ± 0.005 0.312 ± 0.005 | 193.007 ± 0.533 86.6810 ± 0.279 | 0.795 ± 0.011 | −9.9820 ± 0.0390 | 80.2 ± 1.65–60.4 ± 5.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota Díaz, I.I.; Douda, J.; García López, P.; Cabrera Becerra, S.E.; Gómez Álvarez, M.Á.; Jiménez Rodríguez, R.; Jurado León, R.; López Sánchez, P. Co-Encapsulation of Paclitaxel and Doxorubicin in Liposomes Layer by Layer. Colloids Interfaces 2024, 8, 42. https://doi.org/10.3390/colloids8040042
Mota Díaz II, Douda J, García López P, Cabrera Becerra SE, Gómez Álvarez MÁ, Jiménez Rodríguez R, Jurado León R, López Sánchez P. Co-Encapsulation of Paclitaxel and Doxorubicin in Liposomes Layer by Layer. Colloids and Interfaces. 2024; 8(4):42. https://doi.org/10.3390/colloids8040042
Chicago/Turabian StyleMota Díaz, Isaac Izcoatl, Janna Douda, Patricia García López, Sandra Edith Cabrera Becerra, Miguel Ángel Gómez Álvarez, Rebeca Jiménez Rodríguez, Rafael Jurado León, and Pedro López Sánchez. 2024. "Co-Encapsulation of Paclitaxel and Doxorubicin in Liposomes Layer by Layer" Colloids and Interfaces 8, no. 4: 42. https://doi.org/10.3390/colloids8040042
APA StyleMota Díaz, I. I., Douda, J., García López, P., Cabrera Becerra, S. E., Gómez Álvarez, M. Á., Jiménez Rodríguez, R., Jurado León, R., & López Sánchez, P. (2024). Co-Encapsulation of Paclitaxel and Doxorubicin in Liposomes Layer by Layer. Colloids and Interfaces, 8(4), 42. https://doi.org/10.3390/colloids8040042








