Transport Behavior of Paranitroaniline through a Flat-Sheet Supported Liquid Membrane Using Tributylphosphate as a Carrier
Abstract
:1. Introduction
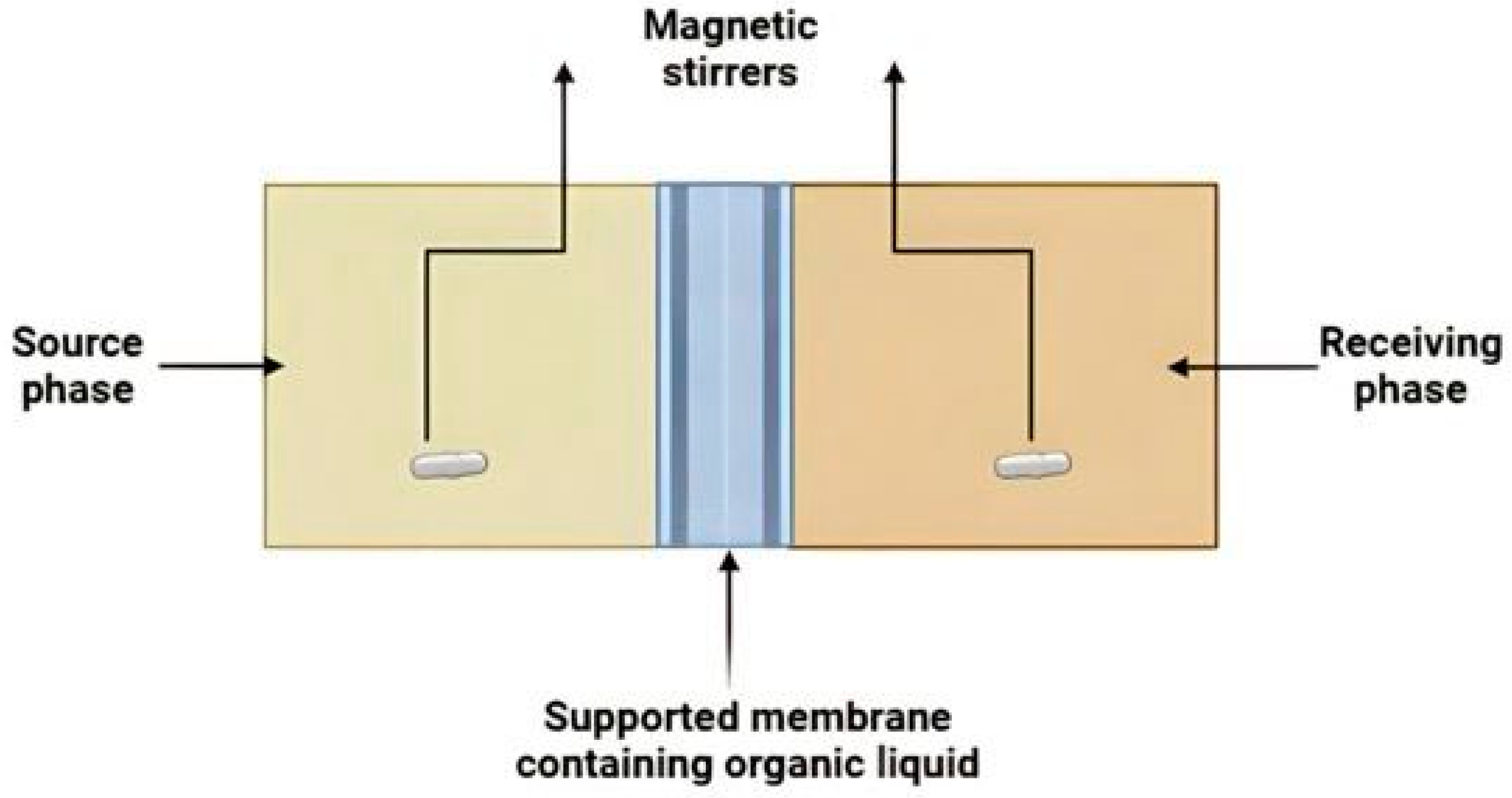
2. Materials and Methods
2.1. Materials
2.1.1. Polymeric Support
2.1.2. Extractant
2.2. Membrane Preparation
2.3. Buffer Solution Preparation
3. Results and Discussion
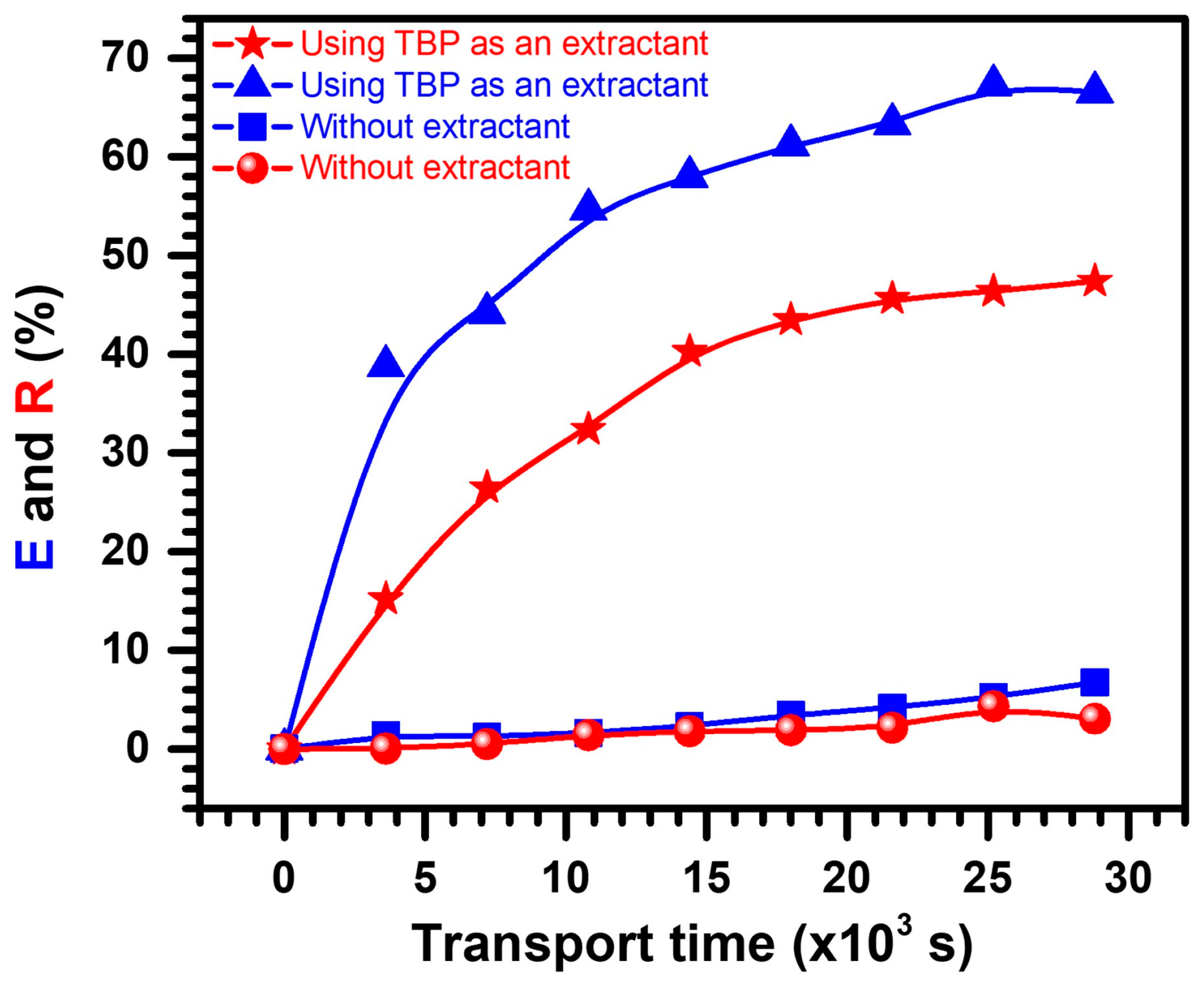
3.1. Transport of PNA with and without Carrier
3.2. Liquid Membrane Loss
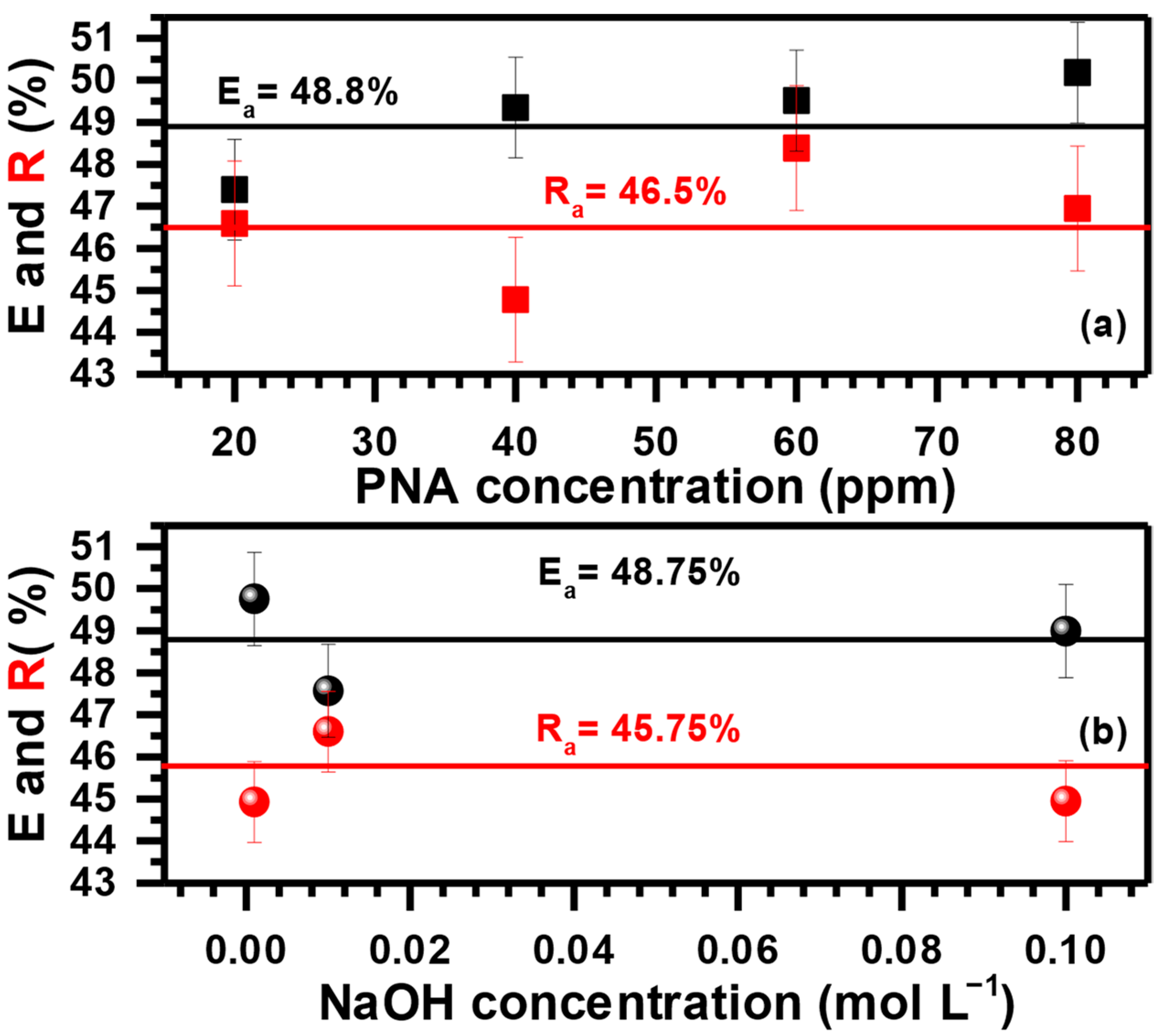
3.3. Effect of PNA and NaOH Concentration
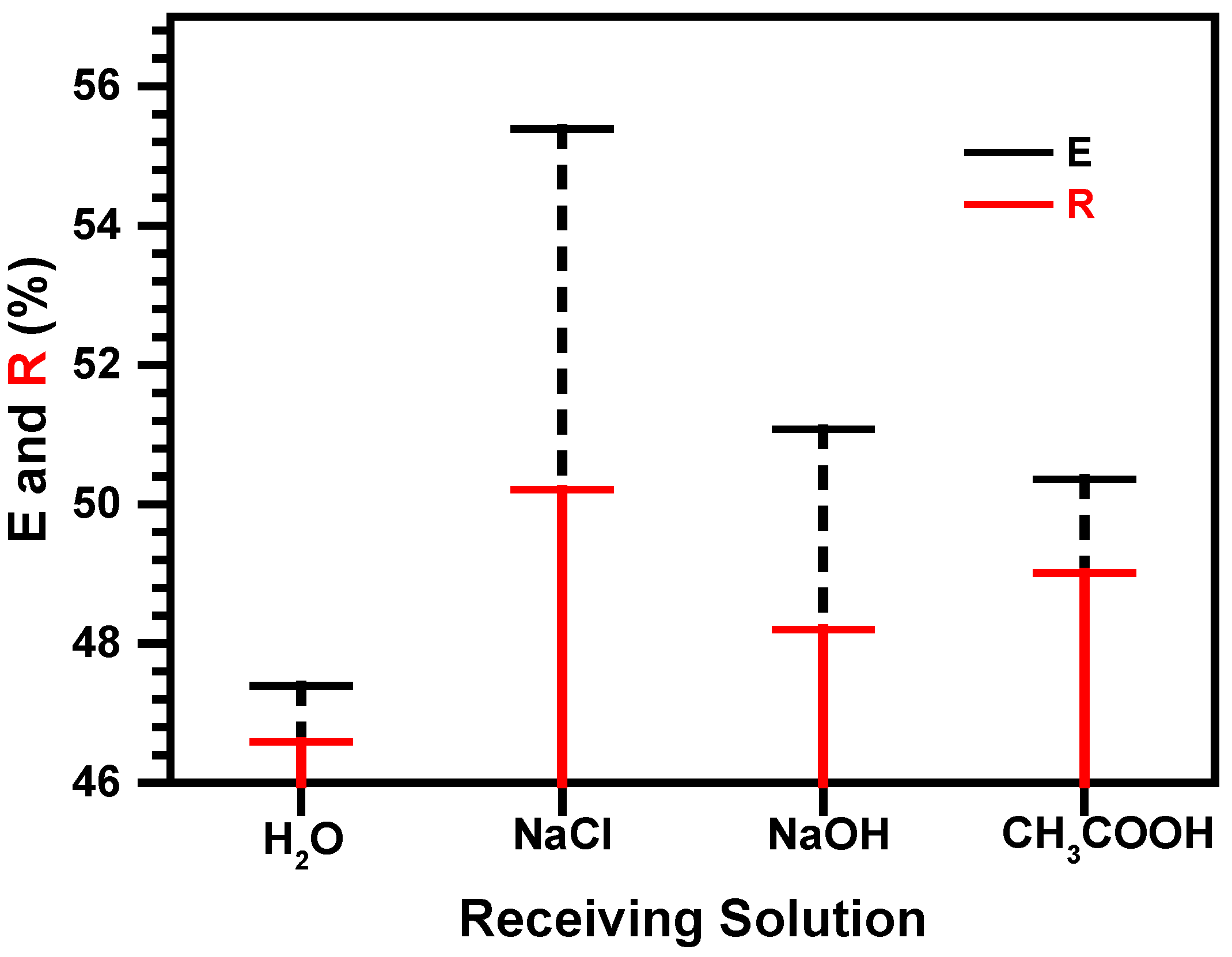
3.4. Selection of Stripping Agents
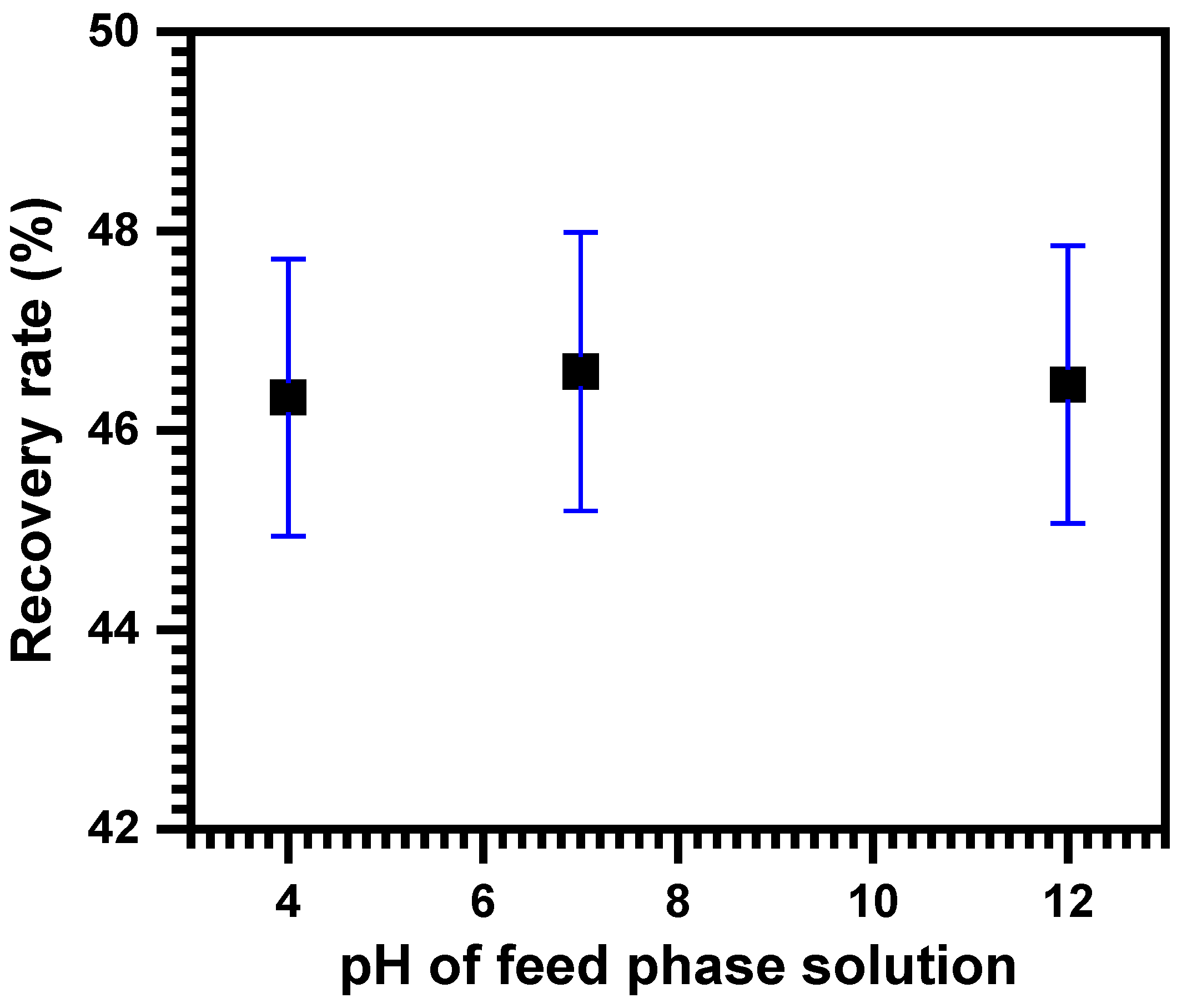
3.5. Effect of the Initial pH of the Source Phase
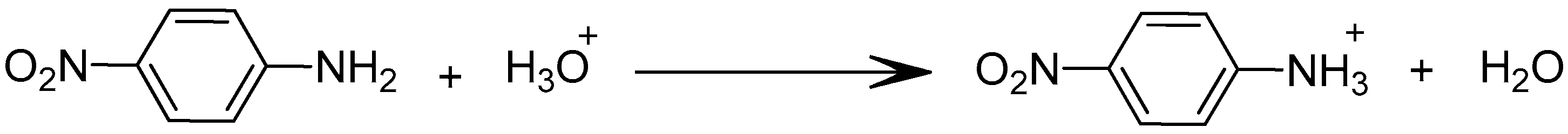
- Step 1: Formation of a complex between PNA and TBP via hydrogen bond at the first interface feed phase-membrane;
- Step 2: Migration of the formed complex through the membrane;
- Step 3: Formation of a complex between water existing in the receiving phase and release of the PNA in the receiving phase.
3.6. Membrane Stability
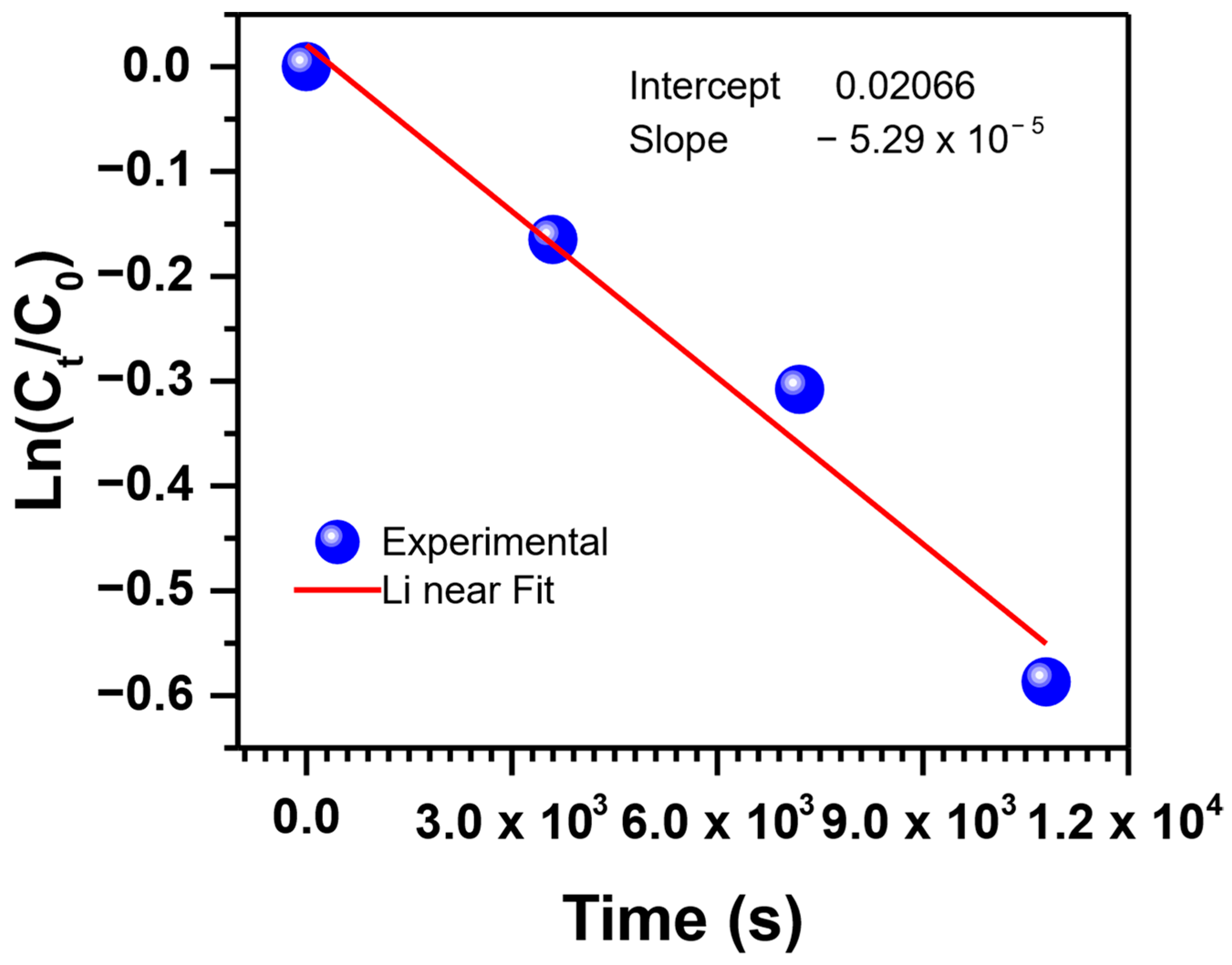
3.7. Extraction Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Impact of biofield treatment on spectroscopic and physicochemical properties of p-nitroaniline. Insights Anal. Electrochem. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Jayachandrabal, B.; Tikker, P.; Preis, S. Oxidation of aqueous p-Nitroaniline by pulsed corona discharge. Sep. Purif. Technol. 2022, 297, 121473. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdou, A.E.; Shehata, A.K.; Header, H.M.; Hamed, E.A. Behavior of γ-Al2O3-bonded-3-chloropropyltrimethoxysilane nanosorbent toward potential binding and removal of 4-nitroaniline and 2-amino-3-nitro-pyridine from water. J. Mol. Liq. 2016, 224, 1358–1369. [Google Scholar] [CrossRef]
- Malakootian, M.; Gharaghani, M.A.; Dehdarirad, A.; Khatami, M.; Ahmadian, M.; Heidari, M.R.; Mahdizadeh, H. ZnO nanoparticles immobilized on the surface of stones to study the removal efficiency of 4-nitroaniline by the hybrid advanced oxidation process (UV/ZnO/O3). J. Mol. Struct. 2018, 1176, 766–776. [Google Scholar] [CrossRef]
- Liou, T.-H.; Huang, J.-J. Efficient Removal of Hazardous P Nitroaniline from Wastewater by Using Surface-Activated and Modified Multiwalled Carbon Nanotubes with Mesostructure. Toxics 2024, 12, 88. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Li, K. Optimization of preparation of microporous activated carbon with high surface area from Spartina alterniflora and its p-nitroaniline adsorption characteristics. J. Environ. Chem. Eng. 2013, 1, 389–397. [Google Scholar] [CrossRef]
- Ali, N.S.; Harharah, H.N.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S.; Albayati, T.M. Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 2023, 13, 9837. [Google Scholar] [CrossRef]
- Bao, T.; Chen, T.; Wille, M.-L.; Ahmadi, N.E.; Rathnayake, S.I.; Chen, D.; Frost, R. Synthesis, application and evaluation of non-sintered zeolite porous filter (ZPF) as novel filter media in biological aerated filters (BAFs). J. Environ. Chem. Eng. 2016, 4, 3374–3384. [Google Scholar] [CrossRef]
- Farabegoli, G.; Chiavola, A.; Rolle, E. The Biological Aerated Filter (BAF) as alternative treatment for domestic sewage. Optimization of plant performance. J. Hazard. Mater. 2009, 171, 1126–1132. [Google Scholar] [CrossRef]
- Mei, X.; Ding, Y.; Wang, Y.; Yang, Y.; Xu, L.; Wang, Y.; Shen, W.; Zhang, Z.; Ma, M.; Guo, Z.; et al. A novel membrane-aerated biofilter for the enhanced treatment of nitroaniline wastewater: Nitroaniline biodegradation performance and its influencing factors. Bioresour. Technol. 2020, 307, 123241. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Pournamdari, M.; Asadipour, A.; Mahdizadeh, H. Degradation and removal of p-nitroaniline from aqueous solutions using a novel semi-fluid Fe/charcoal micro-electrolysis reactor. Korean J. Chem. Eng. 2019, 36, 217–225. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Fan, M.-H.; Guo, H.-Q.; Qiao, L.-P.; Sun, R.-X. A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J. Hazard. Mater. 2007, 148, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Aazam, E.S. Photocatalytic conversion of 4-nitroaniline to p-phenylenediamine using Ni/ZnSn(OH)6 nanoparticles. J. Ind. Eng. Chem. 2014, 20, 3329–3334. [Google Scholar] [CrossRef]
- Eljaddi, T.; Laurent, L.; Miloudi, H. Review on mechanism of facilitated transport on liquid membranes. J. Membr. Sci. Res. 2017, 3, 199–208. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy Metals”—A Meaningless Term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Goh, S.S.; Rafatullah, M.; Ismail, N.; Alam, M.; Siddiqui, M.R.; Seow, E.-K. Separation of chromium (VI), copper and zinc: Chemistry of transport of metal ions across supported liquid membrane. Membranes 2022, 12, 685. [Google Scholar] [CrossRef]
- Sarfraz, S.; Abid, A.J.; Javed, M.; Iqbal, S.; Aljazzar, S.O.; Zahra, M.; Alrbyawi, H.; Elkaeed, E.B.; Somaily, H.H.; Pashameah, R.A.; et al. Chromium (III) Ions Were Extracted from Wastewater Effluent Using a Synergistic Green Membrane with a BinaryCombination of D2EHPA and Kerosene. Catalysts 2022, 12, 1220. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A. Separation iron (III)-manganese (II) via supported liquid membrane technology in the treatment of spent alkaline batteries. Membranes 2021, 11, 991. [Google Scholar] [CrossRef]
- Pont, N.; Salvadó, V.; Fontàs, C. Applicability of a supported liquid membrane in the enrichment and determination of cadmium from complex aqueous samples. Membranes 2018, 8, 21. [Google Scholar] [CrossRef]
- León, G.; Hidalgo, A.M.; Gómez, M.; Gómez, E.; Miguel, B. Efficiency, Kinetics and Mechanism of 4-Nitroaniline Removal from Aqueous Solutions by Emulsion Liquid Membranes Using Type 1 Facilitated Transport. Membranes 2024, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.; Giralt, J.; Stüber, F.; Font, J.; Fabregat, A.; Fortuny, A. Supported liquid membranes for the removal of pharmaceuticals from aqueous solutions. J. Water Process. Eng. 2022, 49, 103170. [Google Scholar] [CrossRef]
- Ghoshal, A.K.; Saha, P. Liquid–membrane filters. In Progress in Filtration and Separation; Academic Press: Cambridge, MA, USA, 2015; pp. 155–205. [Google Scholar] [CrossRef]
- Sastre, A.M.; Kumar, A.; Shukla, J.P.; Singh, R.K. Improved techniques in liquid membrane separations: An overview. Sep. Purif. Methods 1998, 27, 213–298. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Liquid membranes for separation of metal ions from wastewaters. Phys. Sci. Rev. 2021, 8, 937–982. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, J.; Liu, X.; Hu, Y.; Wei, X.; Hu, Y.; Wang, W.; Ren, Z. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent. Hydrometallurgy 2020, 191, 105244. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, T.; Lv, L.; Chen, Y.; Zhong, B.; Tang, S. Extraction performance and mechanism of TBP in the separation of Fe3+ from wet-processing phosphoric acid. Sep. Purif. Technol. 2021, 272, 118822. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Qin, Z.; Lu, L.; Wang, L.; Sun, L.; Gu, Y.; Zhang, Y. Synergism induced intensification for the recovery of phenolic compounds using polymer inclusion membranes containing binary mixed carriers. J. Environ. Chem. Eng. 2024, 12, 112981. [Google Scholar] [CrossRef]
- Campderrós, M.E.; Marchese, J. Facilitated transport of niobium(V) and tantalum (V) with supported liquid membrane using TBP as carrier. J. Memb. Sci. 2000, 164, 205–210. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Othman, N.; Jusoh, N.; Noah, N.F.M.; Rashid, R.; Saufi, S.M. Intensification reactive recovery of tetravalent platinum from spent catalyst via synergism of TBP/Cyanex 302 system. Chem. Eng. Process. 2021, 168, 108581. [Google Scholar] [CrossRef]
- Danesi, P.R.; Reichley, L.; Rickert, P.G. Lifetime of supported liquid membranes: The influence of interfacial properties, chemical composition and water transport on the long term stability of the membranes. J. Membr. Sci. 1987, 31, 117–145. [Google Scholar] [CrossRef]
- Chiarizia, R. Stability of SLMs containing long-chain aliphatic amines as carriers. J. Membr. Sci. 1991, 55, 65–77. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, B.; Wu, Y.; Ren, Q. Instability Mechanisms of Supported Liquid Membrane for Phenol Transport. Chin. J. Chem. Eng. 2009, 17, 750–755. [Google Scholar] [CrossRef]
- Stolwijk, T.B.; Sudhoelter, E.J.R.; Reinhoudt, D.N. Crown Ether Mediated Transport: A Kinetic Study of Potassium Perchlorate Transport through a Supported Liquid Membrane Containing Dibenzo-18-Crown-6. J. Am. Chem. Soc. 1987, 109, 7042–7047. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Jusoh, N.; Othman, N.; Noah, N.F.M.; Rosly, M.B.; Rahman, H.A. Supported liquid membrane extraction of nickel using stable composite SPEEK/PVDF support impregnated with a sustainable liquid membrane. J. Hazard. Mater. 2019, 380, 120895. [Google Scholar] [CrossRef]
- Zhao, W.; He, G.; Nie, F.; Zhang, L.; Feng, H.; Liu, H. Membrane liquid loss mechanism of Supported ionic liquid membrane for gas separation. J. Membr. Sci. 2012, 411–412, 73–80. [Google Scholar] [CrossRef]
- Mahdavi, H.R.; Arzani, M.; Isanejad, M.; Mohammadi, T. Effect of hydrophobic and hydrophilic nanoparticles loaded in D2EHPA/M2EHPA—PTFE supported liquid membrane for simultaneous cationic dyes pertraction. J. Environ. Manag. 2018, 213, 288–296. [Google Scholar] [CrossRef]
- Kazemi, P.; Peydayesh, M.; Bandegi, A.; Mohammadi, T.; Bakhtiari, O. Pertraction of methylene blue using a mixture of D2EHPA/M2EHPA and sesame oil as a liquid membrane. Chem. Pap. 2013, 67, 722–729. [Google Scholar] [CrossRef]
- Madaeni, S.; Jamali, Z.; Islami, N. Highly efficient and selective transport of methylene blue through a bulk liquid membrane containing Cyanex 301 as carrier. Sep. Purif. Technol. 2011, 81, 116–123. [Google Scholar] [CrossRef]
- Muthuraman, G.; Palanivelu, K. Transport of textile dye in vegetable oils based supported liquid membrane. Dye Pigment. 2006, 70, 99–104. [Google Scholar] [CrossRef]
- Pilli, S.R.; Banerjee, T.; Mohanty, K. 1-Butyl-2,3-dimethylimidazolium hexafluorophosphate as a green solvent for the extraction of endosulfan from aqueous solution using supported liquid membrane. Chem. Eng. J. 2014, 257, 56–65. [Google Scholar] [CrossRef]
- Bhosale, V.K.; Chana, H.K.; Kamble, S.P.; Kulkarni, P.S. Separation of nitroaromatics from wastewater by using supported ionic liquid membranes. J. Water Process. Eng. 2019, 32, 100925. [Google Scholar] [CrossRef]
- Kumar, A.; Manna, M.S.; Ghoshal, A.K.; Saha, P. Study of the supported liquid membrane for the estimation of the synergistic e_ects of inuential parameters on its stability. J. Environ. Chem. Eng. 2016, 4, 943–949. [Google Scholar] [CrossRef]
- Naganawa, H.; Tachimori, S. Highly Hydrated Associate of Tributyl Phosphate in Dodecane. Anal. Sci. 1994, 10, 607–613. [Google Scholar] [CrossRef]
- Srirachat, W.; Usapein, P.; Kheawhom, S.; Pancharoen, U. Selective separation of trace nickel(II) and gold(I) ions via hollow fiber supported liquid membrane enhanced by synergistic extractants D2EHPA/TBP. Arab. J. Chem. 2021, 14, 103427. [Google Scholar] [CrossRef]
- Parham, H.; Saeed, S. Ultrasound-assisted solid phase extraction of nitro- and chloro-(phenols) using magnetic iron oxide nanoparticles and Aliquat 336 ionic liquid. J. Chromatogr. A 2014, 1336, 34–42. [Google Scholar] [CrossRef]
- Kazemi, P.; Peydayesh, M.; Bandegi, A.; Mohammadi, T.; Bakhtiari, O. Stability and extraction study of phenolic wastewater treatment by supported liquid membrane using tributyl phosphate and sesame oil as liquid membrane. Chem. Eng. Res. Des. 2014, 92, 375–383. [Google Scholar] [CrossRef]


| 4-Nitroaniline | (MERCK-Schuchardt) |
|---|---|
| Chemical formula | C6H6N2O2 |
| Molecular weight | 138.12 g mol−1 |
| Chemical structure | 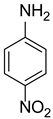 |
| Thickness d0 (μm) | Pore Diameter d (μm) | Porosity ɛ (%) | Tortuosity (τ = 1 − lnε) |
|---|---|---|---|
| 25 | 0.064 | 55 | 1.598 |
| Tributyl Phosphate | (Fluka Chemika, 99%) |
|---|---|
| Chemical formula | (C4H9)3PO4 |
| Molecular weight | 266.32 g mol−1 |
| Chemical structure |  |
| Buffer pH = 4 | Buffer pH = 12 |
|---|---|
| The buffer solution (pH = 4) is prepared by mixing a solution of succinic acid 0.2 mol L−1 (V = 25 mL) and a solution of NaOH 0.2 mol L−1 (10 mL). Ultrapure water was added to the mixture to obtain a 100 mL solution. | The buffer solution (pH = 12) is prepared by mixing a solution of glycine 0.2 mol L−1 (V = 25 mL) and a solution of NaOH 0.2 mol L−1 (23.35 mL). Ultrapure water was added to the mixture to obtain a 100 mL solution. |
| Time of Irradiation (min) | R (%) |
|---|---|
| 0 | 46.59 |
| 15 | 47.72 |
| 180 | 44.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algreiby, A.; Alharbi, L.; Kouki, N.; Tar, H.; Alnafisah, A.; Béji, L. Transport Behavior of Paranitroaniline through a Flat-Sheet Supported Liquid Membrane Using Tributylphosphate as a Carrier. Colloids Interfaces 2024, 8, 49. https://doi.org/10.3390/colloids8050049
Algreiby A, Alharbi L, Kouki N, Tar H, Alnafisah A, Béji L. Transport Behavior of Paranitroaniline through a Flat-Sheet Supported Liquid Membrane Using Tributylphosphate as a Carrier. Colloids and Interfaces. 2024; 8(5):49. https://doi.org/10.3390/colloids8050049
Chicago/Turabian StyleAlgreiby, Azizah, Lama Alharbi, Noura Kouki, Haja Tar, Abrar Alnafisah, and Lotfi Béji. 2024. "Transport Behavior of Paranitroaniline through a Flat-Sheet Supported Liquid Membrane Using Tributylphosphate as a Carrier" Colloids and Interfaces 8, no. 5: 49. https://doi.org/10.3390/colloids8050049








