Mixed Adsorption Mono- and Multilayers of ß-Lactoglobulin Fibrils and Sodium Polystyrene Sulfonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
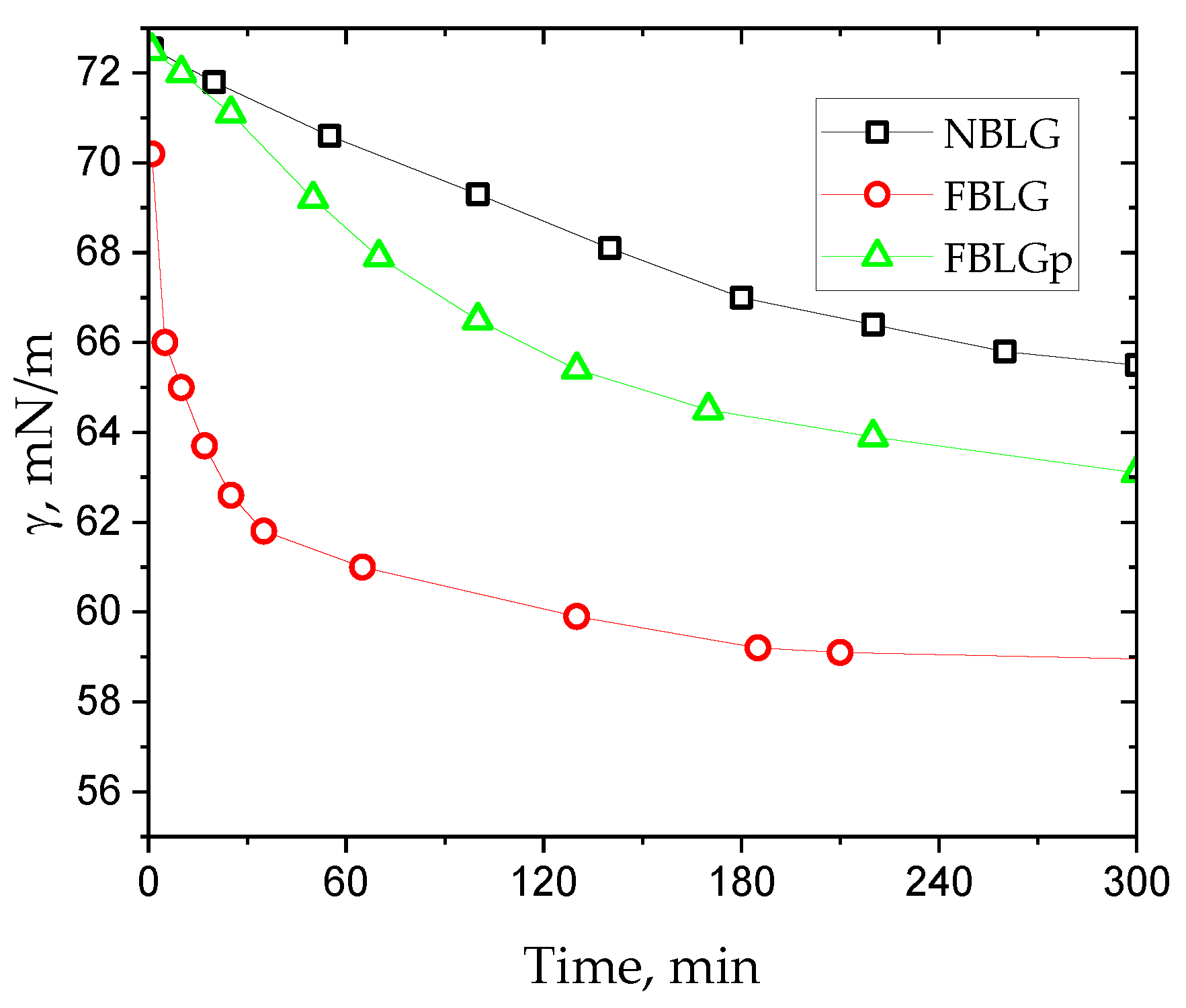
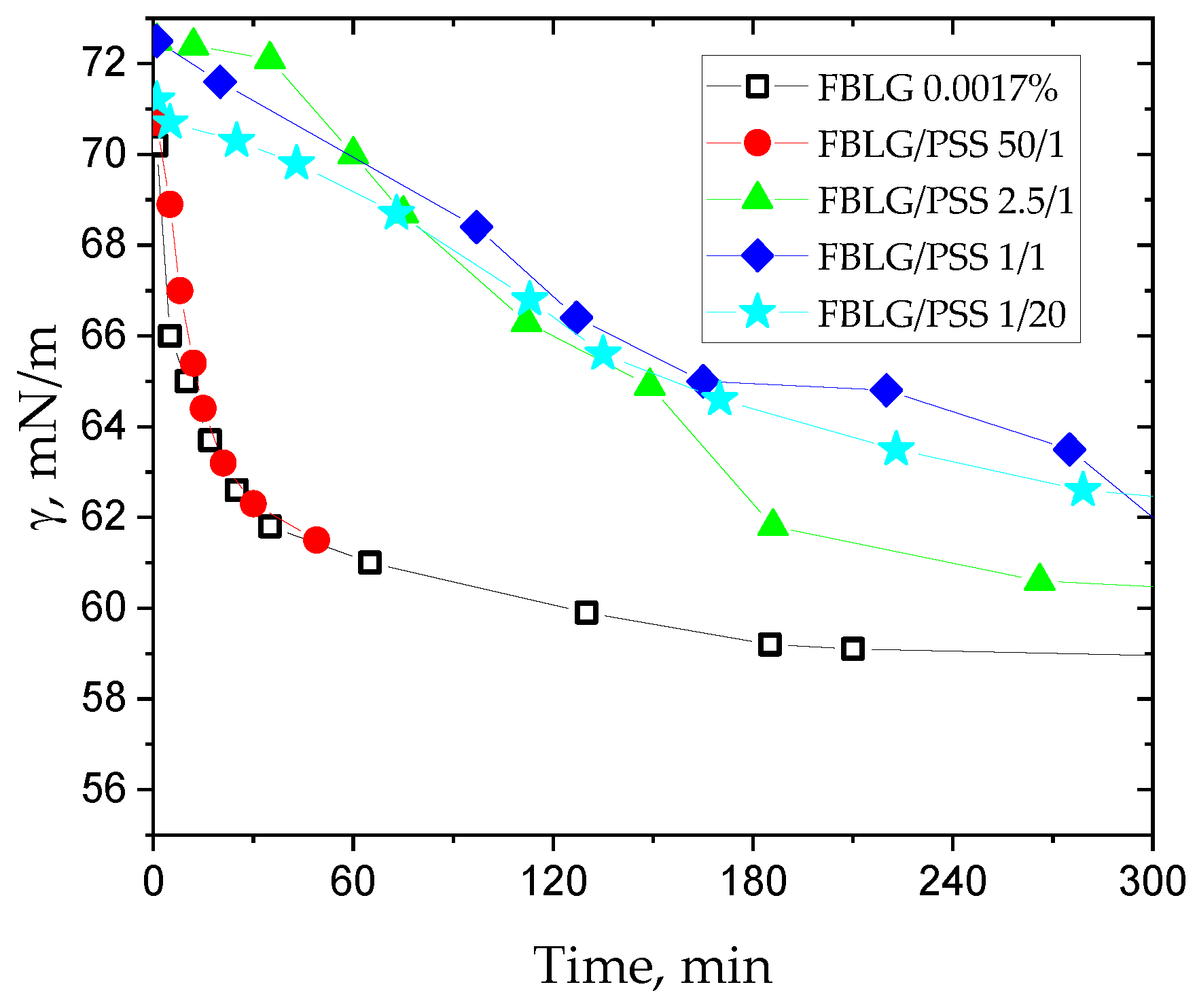
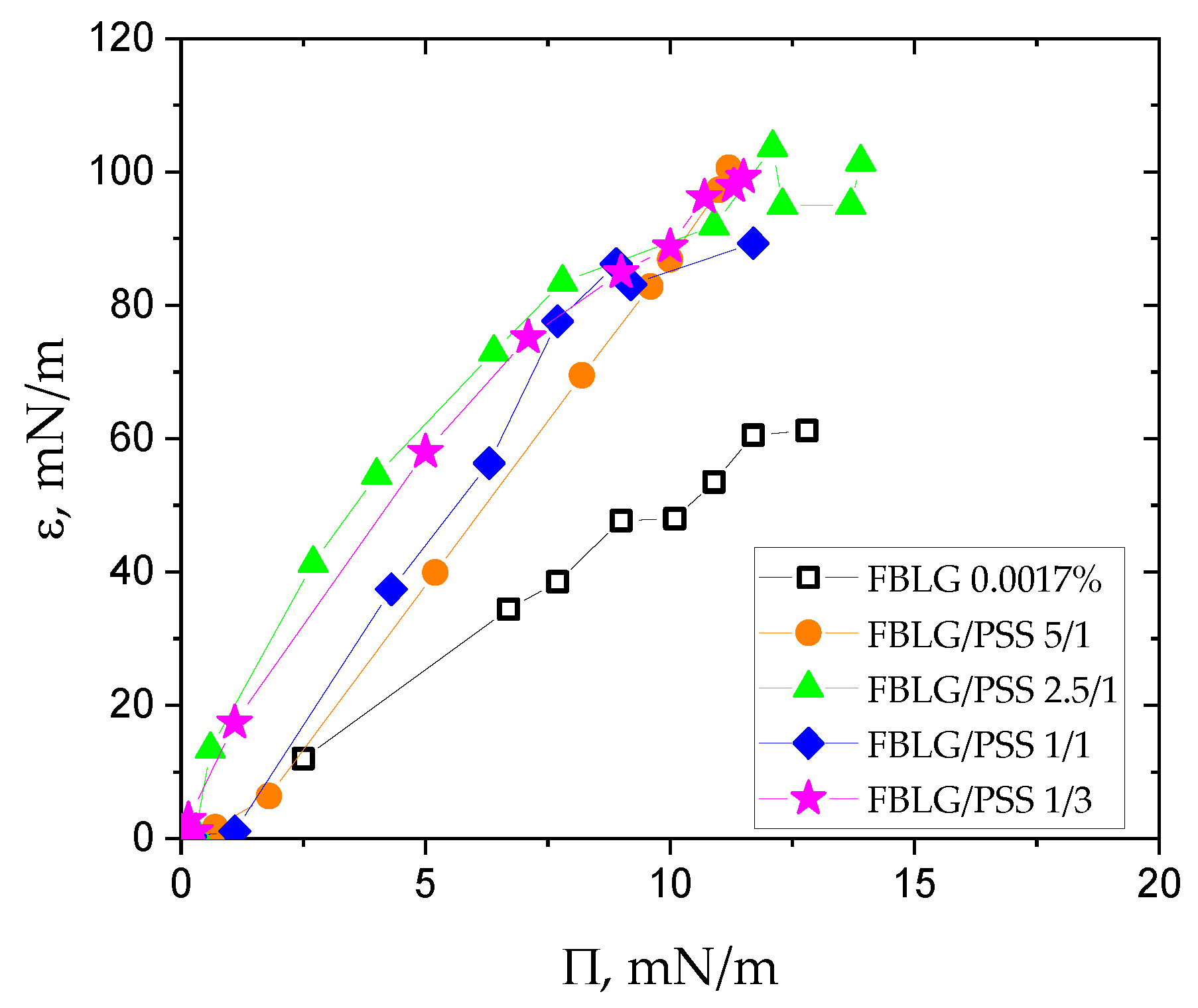
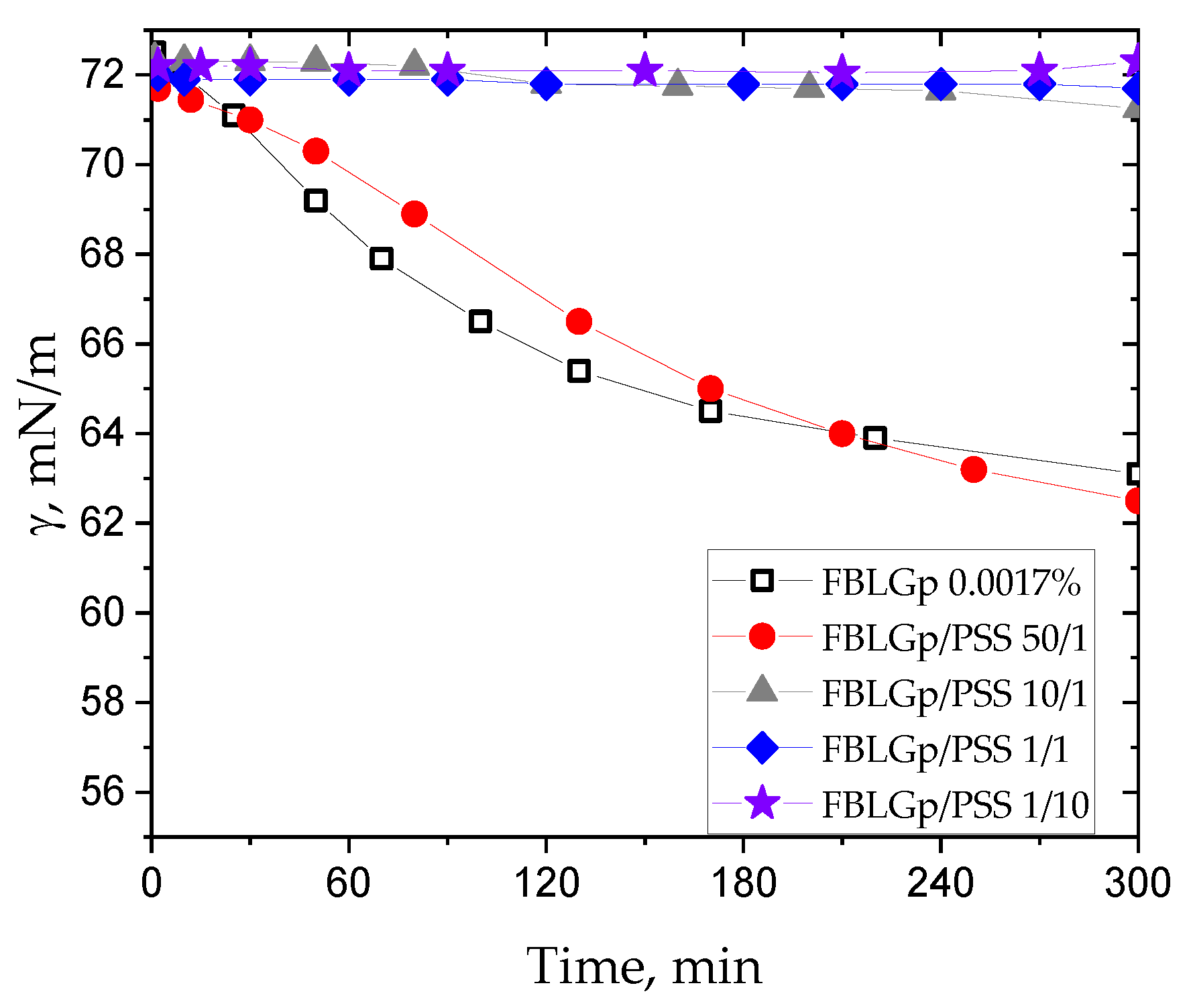
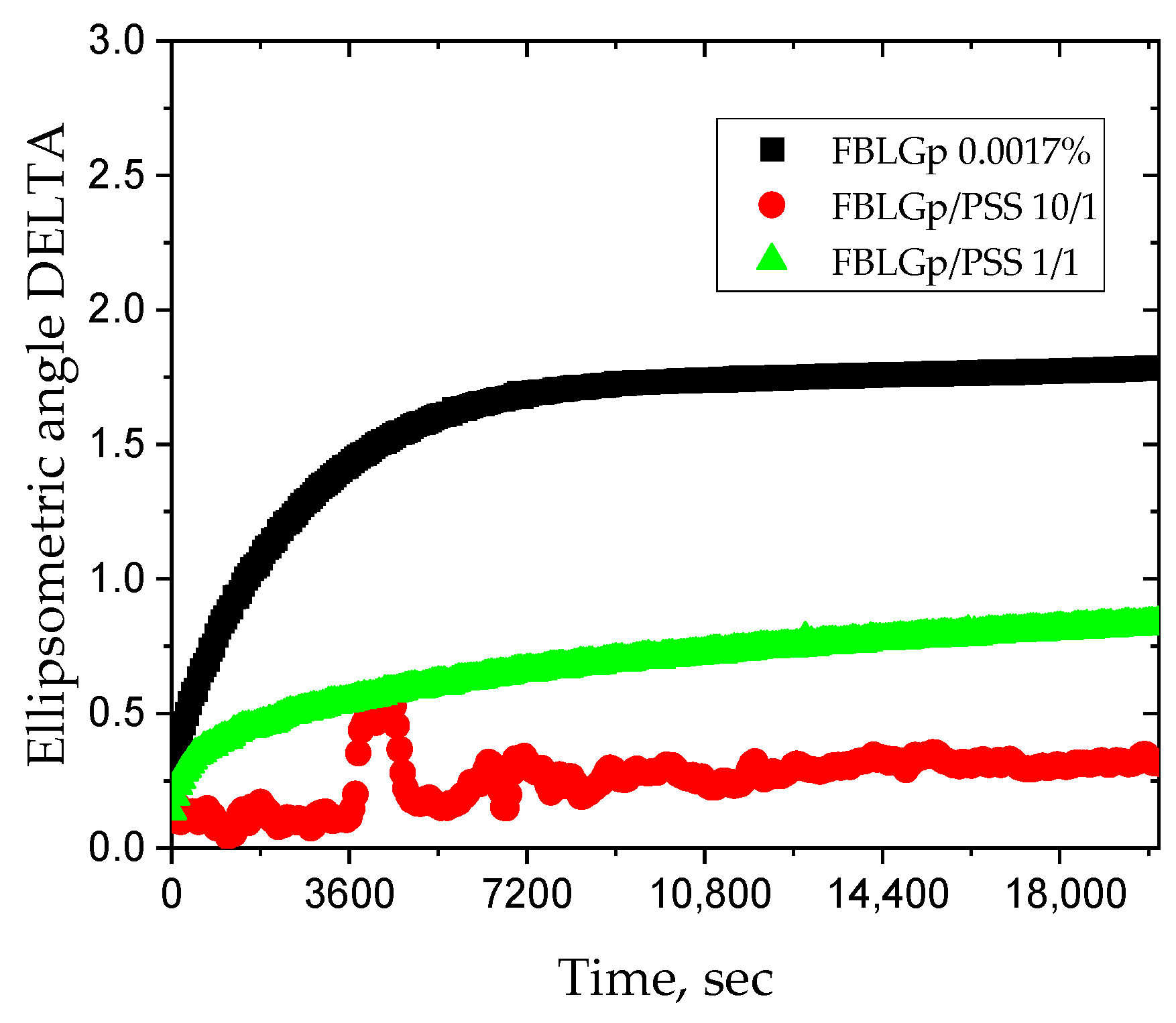
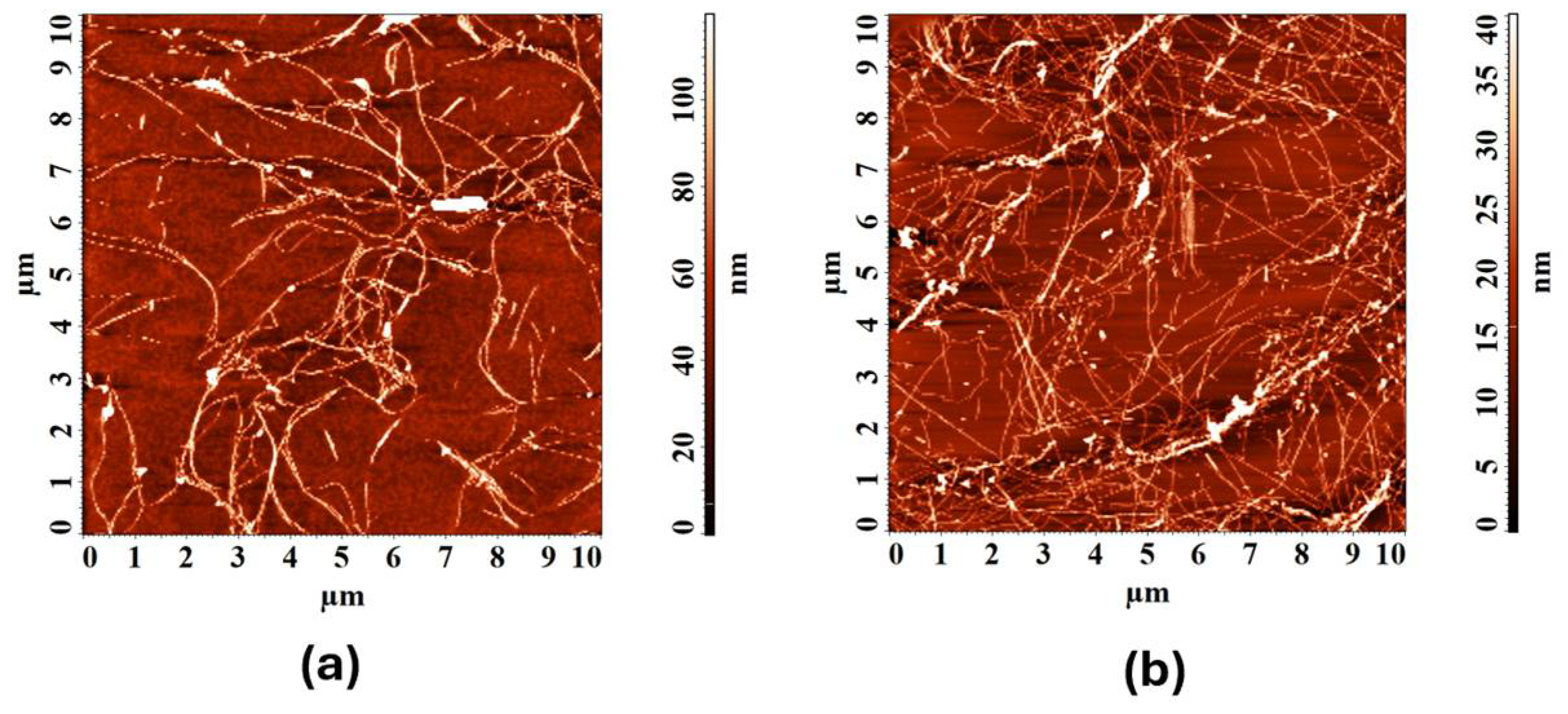
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–Protein Complexes. Curr. Opin. Colloid. Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Schmitt, C.; Turgeon, S.L. Protein/Polysaccharide Complexes and Coacervates in Food Systems. Adv. Colloid. Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and Coacervation of Polyelectrolytes with Oppositely Charged Colloids. Adv. Colloid. Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.; Gummel, J.; Combet, S.; Boué, F. The Model Lysozyme–PSSNa System for Electrostatic Complexation: Similarities and Differences with Complex Coacervation. Adv. Colloid. Interface Sci. 2011, 167, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Kayitmazer, A.B.; Seeman, D.; Minsky, B.B.; Dubin, P.L.; Xu, Y. Protein–Polyelectrolyte Interactions. Soft Matter 2013, 9, 2553. [Google Scholar] [CrossRef]
- Comert, F.; Dubin, P.L. Liquid-Liquid and Liquid-Solid Phase Separation in Protein-Polyelectrolyte Systems. Adv. Colloid. Interface Sci. 2017, 239, 213–217. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex Coacervate-based Materials for Biomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef]
- Blocher McTigue, W.C.; Perry, S.L. Protein Encapsulation Using Complex Coacervates: What Nature Has to Teach Us. Small 2020, 16, 1907671. [Google Scholar] [CrossRef]
- Milyaeva, O.Y.; Akentiev, A.V.; Chirkov, N.S.; Lin, S.-Y.; Tseng, W.-C.; Vlasov, P.S.; Miller, R.; Noskov, B.A. Surface Properties of Protein–Polyelectrolyte Solutions. Impact of Polyelectrolyte Hydrophobicity. Langmuir 2023, 39, 8424–8434. [Google Scholar] [CrossRef]
- Anikin, K.; Röcker, C.; Wittemann, A.; Wiedenmann, J.; Ballauff, M.; Nienhaus, G.U. Polyelectrolyte-Mediated Protein Adsorption: Fluorescent Protein Binding to Individual Polyelectrolyte Nanospheres. J. Phys. Chem. B 2005, 109, 5418–5420. [Google Scholar] [CrossRef]
- Yu, A.; Caruso, F. Thin Films of Polyelectrolyte-Encapsulated Catalase Microcrystals for Biosensing. Anal. Chem. 2003, 75, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.K.; Bertoncello, P.; Ding, H.; Paddeu, S.; Nicolini, C. Cholesterol Biosensors Prepared by Layer-by-Layer Technique. Biosens. Bioelectron. 2001, 16, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, M.B.; Muronetz, V.I.; Izumrudov, V.A.; Galaev, I.Y.; Mattiasson, B. Production of Fab Fragments of Monoclonal Antibodies Using Polyelectrolyte Complexes. Anal. Biochem. 2000, 277, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mattison, K.W.; Brittain, I.J.; Dubin, P.L. Protein—Polyelectrolyte Phase Boundaries. Biotechnol. Prog. 1995, 11, 632–637. [Google Scholar] [CrossRef]
- Bromberg, L. Temperature-Responsive Gels and Thermogelling Polymer Matrices for Protein and Peptide Delivery. Adv. Drug Deliv. Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.N.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef]
- Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Tightly Bound Polyelectrolytes Enhance Enzyme Proteolysis and Destroy Amyloid Aggregates. Soft Matter 2018, 14, 3768–3773. [Google Scholar] [CrossRef]
- Makshakova, O.; Bogdanova, L.; Faizullin, D.; Khaibrakhmanova, D.; Ziganshina, S.; Ermakova, E.; Zuev, Y.; Sedov, I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics 2023, 15, 624. [Google Scholar] [CrossRef]
- Usuelli, M.; Germerdonk, T.; Cao, Y.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nyström, G.; Mezzenga, R. Polysaccharide-Reinforced Amyloid Fibril Hydrogels and Aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef]
- Zhang, Y.; Nian, Y.; Shi, Q.; Hu, B. Protein Fibrillation and Hybridization with Polysaccharides Enhance Strength, Toughness, and Gas Selectivity of Bioplastic Packaging. J. Mater. Chem. A 2023, 11, 9884–9901. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid. Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Oboroceanu, D.; Wang, L.; Magner, E.; Auty, M.A.E. Fibrillization of Whey Proteins Improves Foaming Capacity and Foam Stability at Low Protein Concentrations. J. Food Eng. 2014, 121, 102–111. [Google Scholar] [CrossRef]
- Peng, J.; Simon, J.R.; Venema, P.; Van Der Linden, E. Protein Fibrils Induce Emulsion Stabilization. Langmuir 2016, 32, 2164–2174. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Nonlinear Surface Dilatational Rheology and Foaming Behavior of Protein and Protein Fibrillar Aggregates in the Presence of Natural Surfactant. Langmuir 2016, 32, 3679–3690. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Contribution of Long Fibrils and Peptides to Surface and Foaming Behavior of Soy Protein Fibril System. Langmuir 2016, 32, 8092–8101. [Google Scholar] [CrossRef]
- Loveday, S.M.; Anema, S.G.; Singh, H. β-Lactoglobulin Nanofibrils: The Long and the Short of It. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of pH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Mantovani, R.A.; De Figueiredo Furtado, G.; Netto, F.M.; Cunha, R.L. Assessing the Potential of Whey Protein Fibril as Emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Xu, Y.; Zhang, K.; Nishinari, K.; Phillips, G.O.; Fang, Y. Comparative Study on Foaming and Emulsifying Properties of Different Beta-Lactoglobulin Aggregates. Food Funct. 2019, 10, 5922–5930. [Google Scholar] [CrossRef]
- Murray, B.S. Recent Developments in Food Foams. Curr. Opin. Colloid. Interface Sci. 2020, 50, 101394. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, Y.; Peng, D.; Huang, W.; Shen, W.; Jin, W.; Huang, Q. Tunable Self-Assemblies of Whey Protein Isolate Fibrils for Pickering Emulsions Structure Regulation. Food Hydrocoll. 2022, 124, 107264. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Karrar, E.; Qi, X.; Zhang, H.; Wu, G. Pickering Foams Stabilized by Protein-Based Particles: A Review of Characterization, Stabilization, and Application. Trends Food Sci. Technol. 2023, 133, 148–159. [Google Scholar] [CrossRef]
- Peydayesh, M.; Kistler, S.; Zhou, J.; Lutz-Bueno, V.; Victorelli, F.D.; Meneguin, A.B.; Spósito, L.; Bauab, T.M.; Chorilli, M.; Mezzenga, R. Amyloid-Polysaccharide Interfacial Coacervates as Therapeutic Materials. Nat. Commun. 2023, 14, 1848. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.W.; Farkas, B.E.; Jones, O.G. Dynamic and Viscoelastic Interfacial Behavior of β-Lactoglobulin Microgels of Varying Sizes at Fluid Interfaces. J. Colloid. Interface Sci. 2016, 466, 12–19. [Google Scholar] [CrossRef]
- Dombrowski, J.; Johler, F.; Warncke, M.; Kulozik, U. Correlation between Bulk Characteristics of Aggregated β-Lactoglobulin and Its Surface and Foaming Properties. Food Hydrocoll. 2016, 61, 318–328. [Google Scholar] [CrossRef]
- Dombrowski, J.; Gschwendtner, M.; Kulozik, U. Evaluation of Structural Characteristics Determining Surface and Foaming Properties of β-Lactoglobulin Aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 286–295. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Bykov, A.G.; Loglio, G.; Miller, R.; Milyaeva, O.Y. Spread and Adsorbed Layers of Protein Fibrils at Water –Air Interface. Colloids Surf. B Biointerfaces 2022, 220, 112942. [Google Scholar] [CrossRef]
- Noskov, B.; Loglio, G.; Miller, R.; Milyaeva, O.; Panaeva, M.; Bykov, A. Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions. Polymers 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Milyaeva, O.Y.; Akentiev, A.V.; Bykov, A.G.; Loglio, G.; Miller, R.; Portnaya, I.; Rafikova, A.R.; Noskov, B.A. Dynamic Properties of Adsorption Layers of κ-Casein Fibrils. Langmuir 2023, 39, 15268–15274. [Google Scholar] [CrossRef]
- Ansarifar, E.; Mohebbi, M.; Shahidi, F.; Koocheki, A.; Ramezanian, N. Novel Multilayer Microcapsules Based on Soy Protein Isolate Fibrils and High Methoxyl Pectin: Production, Characterization and Release Modeling. Inter. J. Biol. Macromol. 2017, 97, 761–769. [Google Scholar] [CrossRef]
- De Moraes, M.A.; Crouzier, T.; Rubner, M.; Beppu, M.M. Factors Controlling the Deposition of Silk Fibroin Nanofibrils during Layer-by-Layer Assembly. Biomacromolecules 2015, 16, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Yanez Arteta, M.; Angus-Smyth, A.; Nylander, T.; Noskov, B.A.; Varga, I. Direct Impact of Nonequilibrium Aggregates on the Structure and Morphology of Pdadmac/SDS Layers at the Air/Water Interface. Langmuir 2014, 30, 8664–8674. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-M.; Gunes, D.Z.; Mezzenga, R. Interfacial Activity and Interfacial Shear Rheology of Native β-Lactoglobulin Monomers and Their Heat-Induced Fibers. Langmuir 2010, 26, 15366–15375. [Google Scholar] [CrossRef] [PubMed]
- Safouane, M.; Miller, R.; Möhwald, H. Surface Viscoelastic Properties of Floating Polyelectrolyte Multilayers Films: A Capillary Wave Study. J. Colloid. Interface Sci. 2005, 292, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.D.; Dong, W.-F.; Benbow, N.L.; Webber, J.L.; Krasowska, M.; Beattie, D.A.; Ferri, J.K. The Influence of Polyanion Molecular Weight on Polyelectrolyte Multilayers at Surfaces: Elasticity and Susceptibility to Saloplasticity of Strongly Dissociated Synthetic Polymers at Fluid–Fluid Interfaces. Phys. Chem. Chem. Phys. 2017, 19, 23781–23789. [Google Scholar] [CrossRef] [PubMed]
- Pivard, S.; Jacomine, L.; Kratz, F.S.; Foussat, C.; Lamps, J.-P.; Legros, M.; Boulmedais, F.; Kierfeld, J.; Schosseler, F.; Drenckhan, W. Interfacial Rheology of Linearly Growing Polyelectrolyte Multilayers at the Water–Air Interface: From Liquid to Solid Viscoelasticity. Soft Matter 2024, 20, 1347–1360. [Google Scholar] [CrossRef]
- Noskov, B.A.; Nuzhnov, S.N.; Loglio, G.; Miller, R. Dynamic Surface Properties of Sodium Poly(Styrenesulfonate) Solutions. Macromolecules 2004, 37, 2519–2526. [Google Scholar] [CrossRef]
- Bykov, A.G.; Liggieri, L.; Noskov, B.A.; Pandolfini, P.; Ravera, F.; Loglio, G. Surface dilational rheological properties in the nonlinear domain. Adv. Colloid. Interface Sci. 2015, 222, 110–118. [Google Scholar] [CrossRef]
- Yu, W.; Wang, P.; Zhou, C. General stress decomposition in nonlinear oscillatory shear flow. J. Rheol. 2009, 53, 215–238. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykov, A.G.; Loglio, G.; Miller, R.; Tsyganov, E.A.; Wan, Z.; Noskov, B.A. Mixed Adsorption Mono- and Multilayers of ß-Lactoglobulin Fibrils and Sodium Polystyrene Sulfonate. Colloids Interfaces 2024, 8, 61. https://doi.org/10.3390/colloids8060061
Bykov AG, Loglio G, Miller R, Tsyganov EA, Wan Z, Noskov BA. Mixed Adsorption Mono- and Multilayers of ß-Lactoglobulin Fibrils and Sodium Polystyrene Sulfonate. Colloids and Interfaces. 2024; 8(6):61. https://doi.org/10.3390/colloids8060061
Chicago/Turabian StyleBykov, A. G., G. Loglio, R. Miller, E. A. Tsyganov, Z. Wan, and B. A. Noskov. 2024. "Mixed Adsorption Mono- and Multilayers of ß-Lactoglobulin Fibrils and Sodium Polystyrene Sulfonate" Colloids and Interfaces 8, no. 6: 61. https://doi.org/10.3390/colloids8060061
APA StyleBykov, A. G., Loglio, G., Miller, R., Tsyganov, E. A., Wan, Z., & Noskov, B. A. (2024). Mixed Adsorption Mono- and Multilayers of ß-Lactoglobulin Fibrils and Sodium Polystyrene Sulfonate. Colloids and Interfaces, 8(6), 61. https://doi.org/10.3390/colloids8060061









