Abstract
Background: Lower urinary tract symptoms (LUTS) are commonly experienced among ageing males. The increasing prevalence of late-onset hypogonadism suggests a possible relationship between serum testosterone and severity of LUTS. This study examines the association between serum testosterone and severity of lower urinary tract symptoms among Malaysian men, as reflected by the International Prostate Symptom Score (IPSS). Method: A total of 163 men with LUTS were enrolled in a cross-sectional study in Hospital Canselor Tuanku Mukhriz, Malaysia. Full examination, IPSS, and serum total testosterone (TT) levels were evaluated. Categorical and continuous correlations were analyzed using chi-square test and age-adjusted Pearson’s partial correlation, respectively. Result: Mean age was 66.25 (SD = 7.05), with mean serum TT of 16.74 nmol/L (SD = 6.32). Twenty eight percent (n = 46) had low testosterone levels. Severity of LUTS (mild, moderate, severe) was not found to be dependent on TT status (normal, low, severely low), (χ2 [4, N = 163] = 4.24, p = 0.37). Weak negative correlations between total IPSS and IPSS storage sub-score with serum TT levels were exhibited respectively (r = −0.17, p < 0.05; r = −0.17, p < 0.05). Conclusion: Among elderly Malaysian men, severity of LUTS and TT status were not found to be associated, despite a weak negative correlation between IPSS and serum testosterone levels. Nonetheless, with a high prevalence of hypogonadal ageing men, further research regarding serum testosterone measurement among this population may be valuable as part of a multimodal approach to treatment.
Introduction
Lower urinary tract symptoms (LUTS) are common among men above 50 years of age in Malaysia, with prevalence increasing at the rate of 8% per decade, so that up to 65.4% of men above 70 years of age are affected[1]. An age-related reduction in serum total testosterone is also frequently seen in this population and is viewed as one of the more important endocrine-related changes seen in ageing men[2,3].
While the relationship between ageing and serum testosterone and ageing with LUTS have been widely established, studies that looked into the relationship between serum testosterone and LUTS have not been consistently reported[4,5,6,7,8].
The importance of testosterone in the general well-being of ageing men is readily acknowledged. Nonetheless, especially among those who are diagnosed with late-onset hypogonadism with concomitant LUTS, limited studies are available that highlight the effects of testosterone in the development of LUTS, and whether it could potentially be a surrogate tool to predict LUTS severity, and thus subsequently assist in the management of LUTS among this population.
In the present study, we aim to elicit the relationship between serum total testosterone levels and severity of LUTS among ageing men with LUTS in Malaysia. We also aim to investigate the prevalence of hypogonadism among the different ethnic groups that make up the South East Asian demographic, thus allowing better insight into the epidemiology and management of the condition among the population within this region.
Materials and Methods
The study involved multi-ethnic men aged 50 to 80 years who presented with urinary tract symptoms to the urology outpatient clinic at a tertiary teaching hospital in Kuala Lumpur between May 2021 and January 2022. Clinical data were obtained, including age, body mass index, waist circumference, and medical history. We excluded from the study men with a history of malignancy, liver cirrhosis, or urinary tract infections, as well as those who were previously diagnosed with hypogonadism, those who were taking hormones, antiandrogen, antifungal medications, or any urological medications (including alpha-blocker, anticholinergics, 5-alpha-reductase inhibitors, and phosphodiesterase-5 inhibitors), those who were previously treated surgically for BPH, and those who were diagnosed with other lower urinary tract problems such as urethral stricture. Men with abnormal digital rectal examination or raised prostate specific antigen levels (> 4ng/mL) were also excluded from the study.
Lower urinary tract symptoms were assessed using the validated International Prostate Symptoms Scoring (IPSS) which comprises an 8-item questionnaire, in which the sum of the first 7 items yields an overall score ranging from 0 to 35. Based on the IPSS, LUTS severity is categorized as mild (0 to 7), moderate (8 to 18), or severe (more than 18) for categorical data analysis. The IPSS storage sub-scores were assessed through items 2 (frequency), 4 (urgency), and 7 (nocturia) in the questionnaire, while IPSS voiding sub-scores were assessed based on summing the outcome from item 1 (incomplete emptying), 3 (intermittency), 5 (weak stream), and 6 (straining).
All participants underwent uroflowmetry testing, with a minimal voiding volume requirement of 150 mL. They also underwent transabdominal ultrasonography to assess prostate size (4Mhz, Canon Aplio). Blood measurement of serum total testosterone (TT) was done between 8:00 a.m. and 10:00 a.m. following an overnight fast to minimize the effects of diurnal variation on hormonal levels. Based on the European Association of Urology (EAU) guideline on diagnosing late-onset hypogonadism, we categorized serum total testosterone status as “severely low” at a level of 8 nmol/L or less, as “low” at between 8 and 12 nmol/L, and as “normal” at more than 12 nmol/L.[9]
Data analyses were done using SPSS software (Version 22.0, Inc. Chicago, US). Continuous variables were presented as the mean ± standard deviation (SD) or median (interquartile range). Categorical data were presented as numbers and percentages. Categorical data on LUTS severity (“mild,” “moderate,” “severe”) and serum total testosterone status (“normal,” “low,” “severely low”) were analyzed using the chi-square test. Statistical comparisons of continuous variables comprising the International Prostate Symptom Score and serum testosterone levels were done using an age-adjusted Pearson partial correlation coefficient to minimize the possible confounding effect of age on the outcome. A P-value of < 0.05 was considered significant.
Results
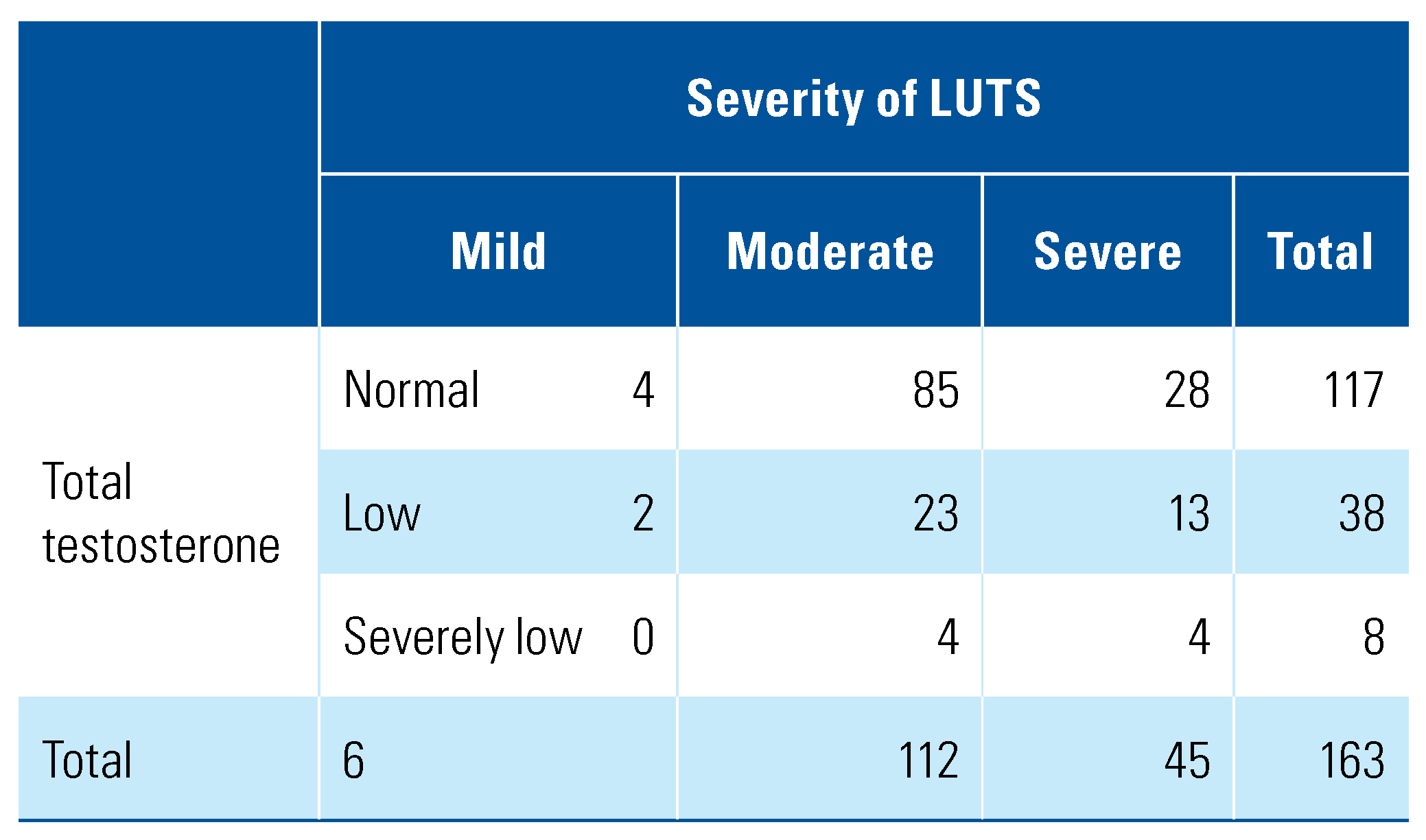
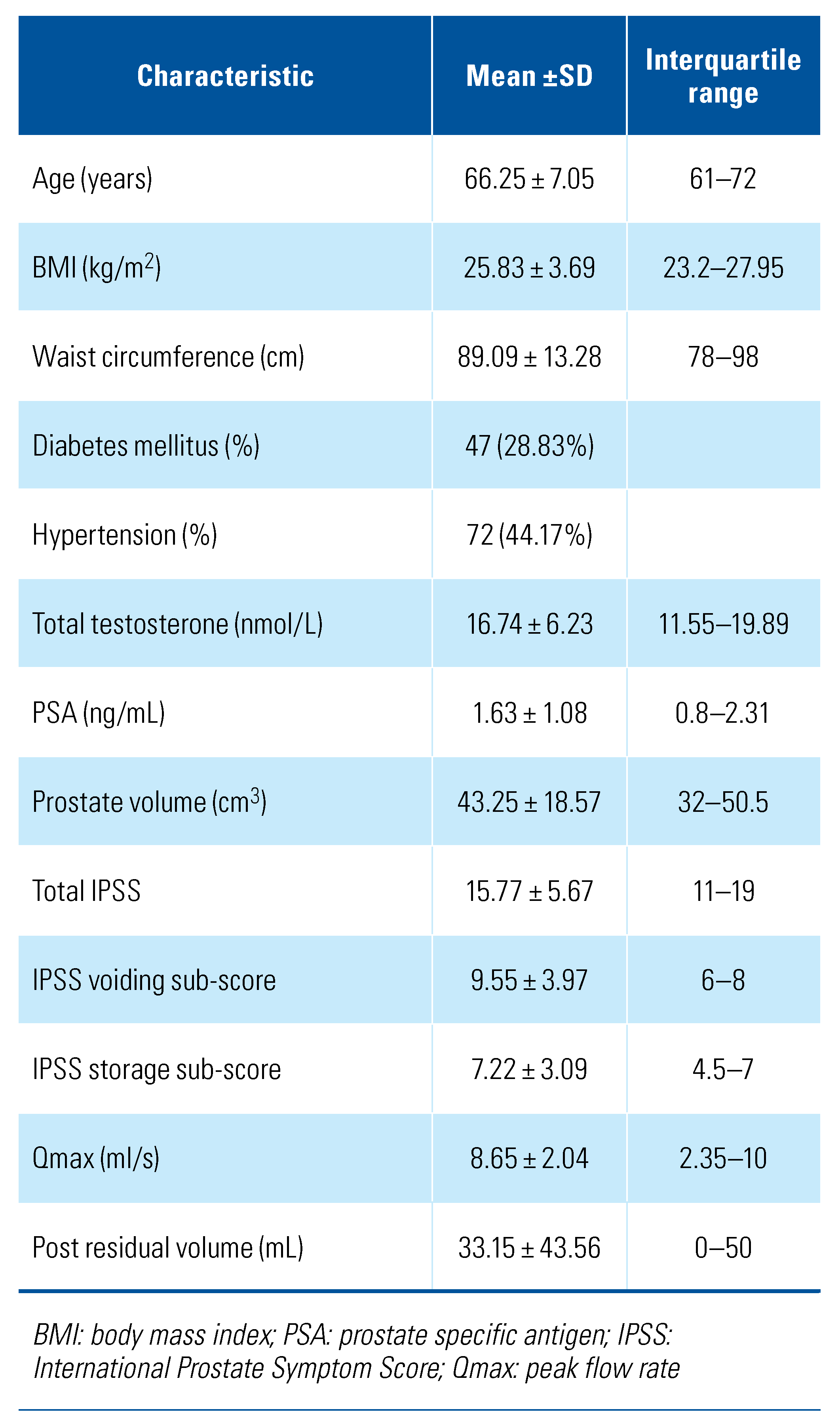
A total of 163 men were enrolled in the study, with a mean age of 66.25 years (SD = 7.05). Of these, 6 (0.04%) had mild LUTS, 112 (68.71%) had moderate LUTS while the remaining 45 (27.6%) had severe LUTS (Table 1). The mean serum TT level was 16.74 nmol/L (SD = 6.32), and mean prostate volume was 43.25 cm3 (SD = 18.57). Further patient characteristics are summarized in Table 2 and Table 3.

Table 1.
Frequency table of severity of LUTS and serum total testosterone status.

Table 2.
Patient characteristics.

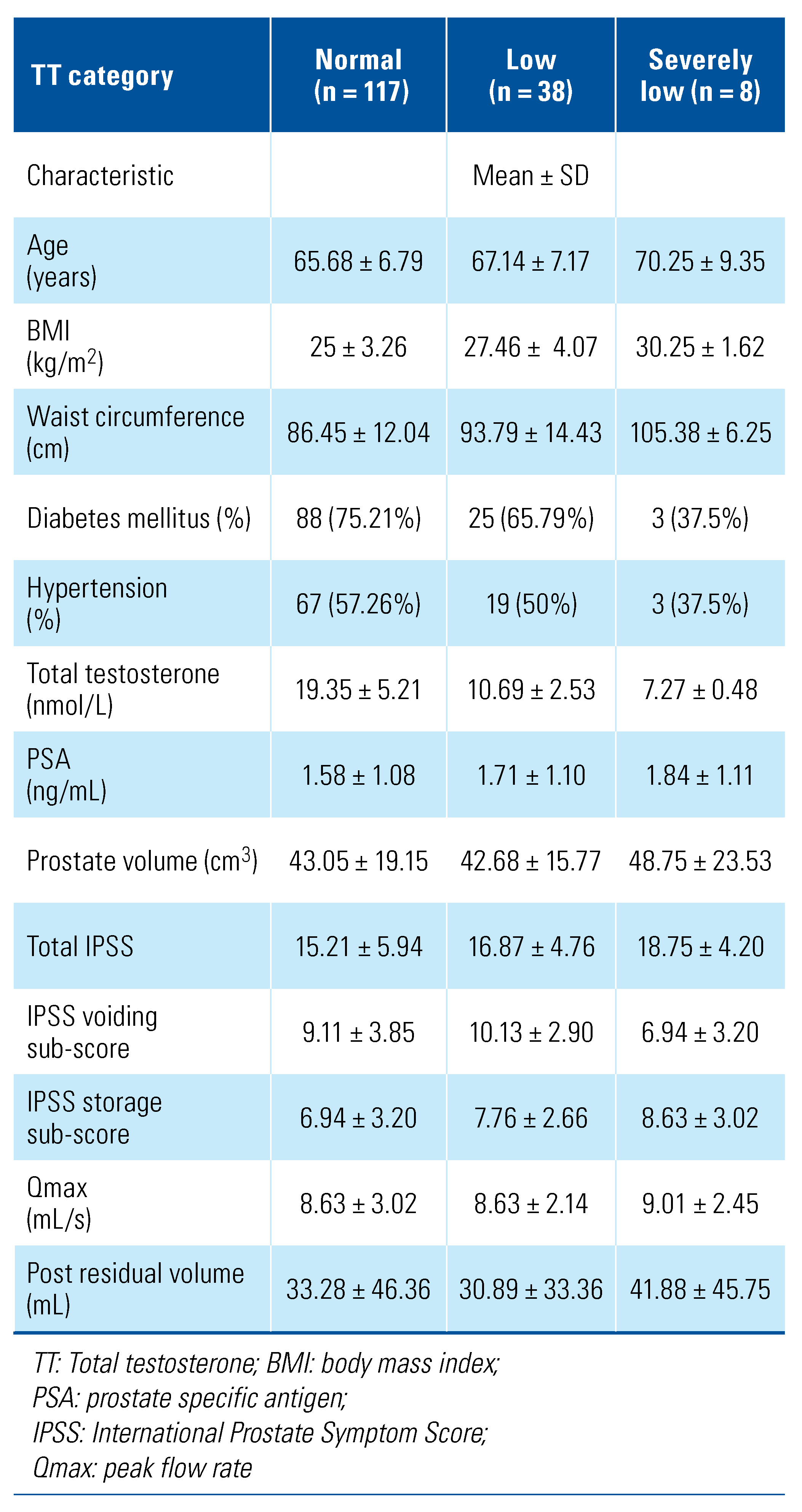
Table 3.
Patient characteristics based on serum total testosterone status.
Based on serum total testosterone level, 4.91% of men (n = 8) were found to have severely low testosterone, 23.31% (n = 38) had low testosterone, and 71.78% (n = 117) had normal testosterone. Of men from Indian ethnicity (n = 14), 35.72% (n = 5) had a testosterone level of less than 12nmol/L. Among Chinese men, 29.76% (25/84) had low to severely low testosterone, and among Malay men, 24.62% (16/65).
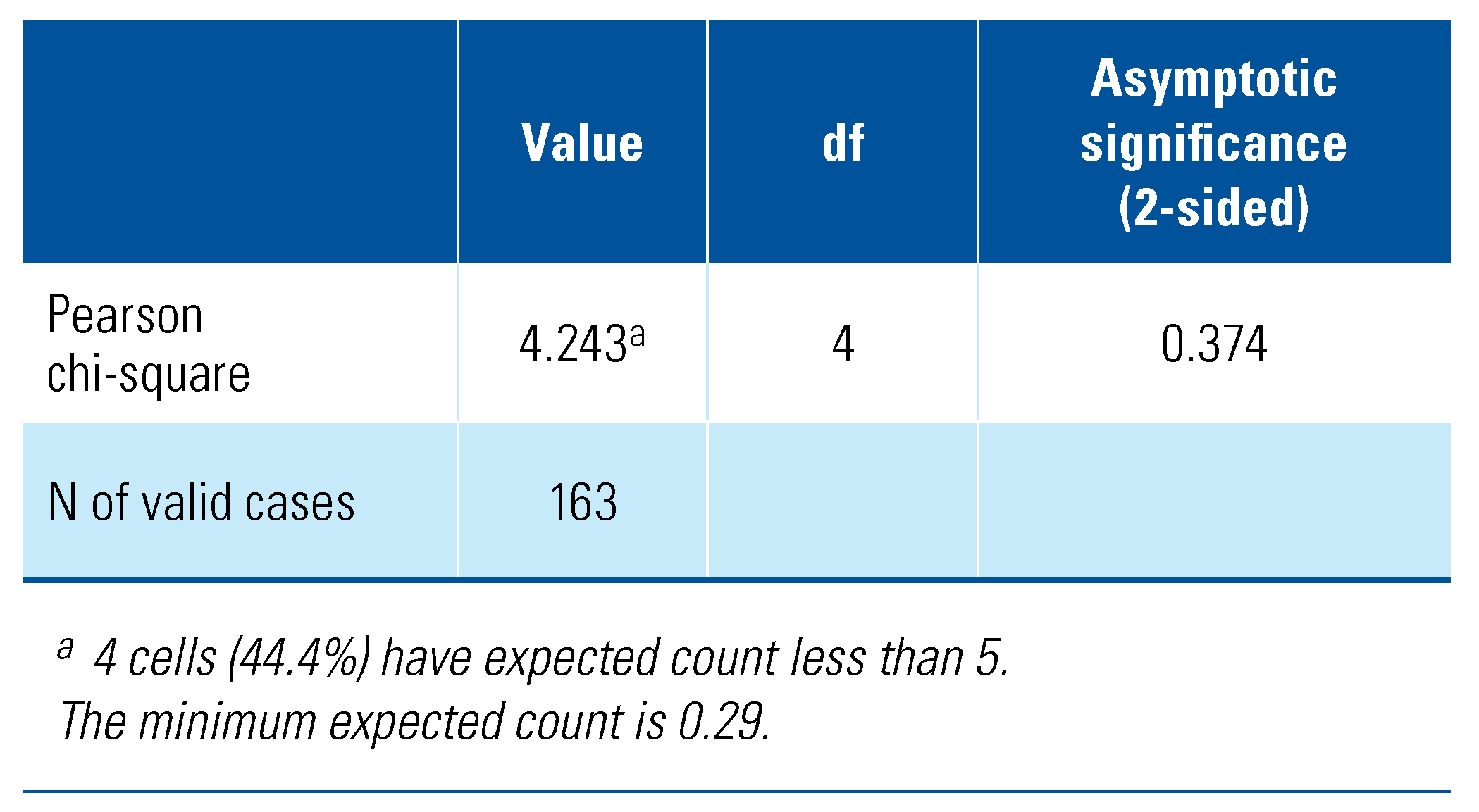
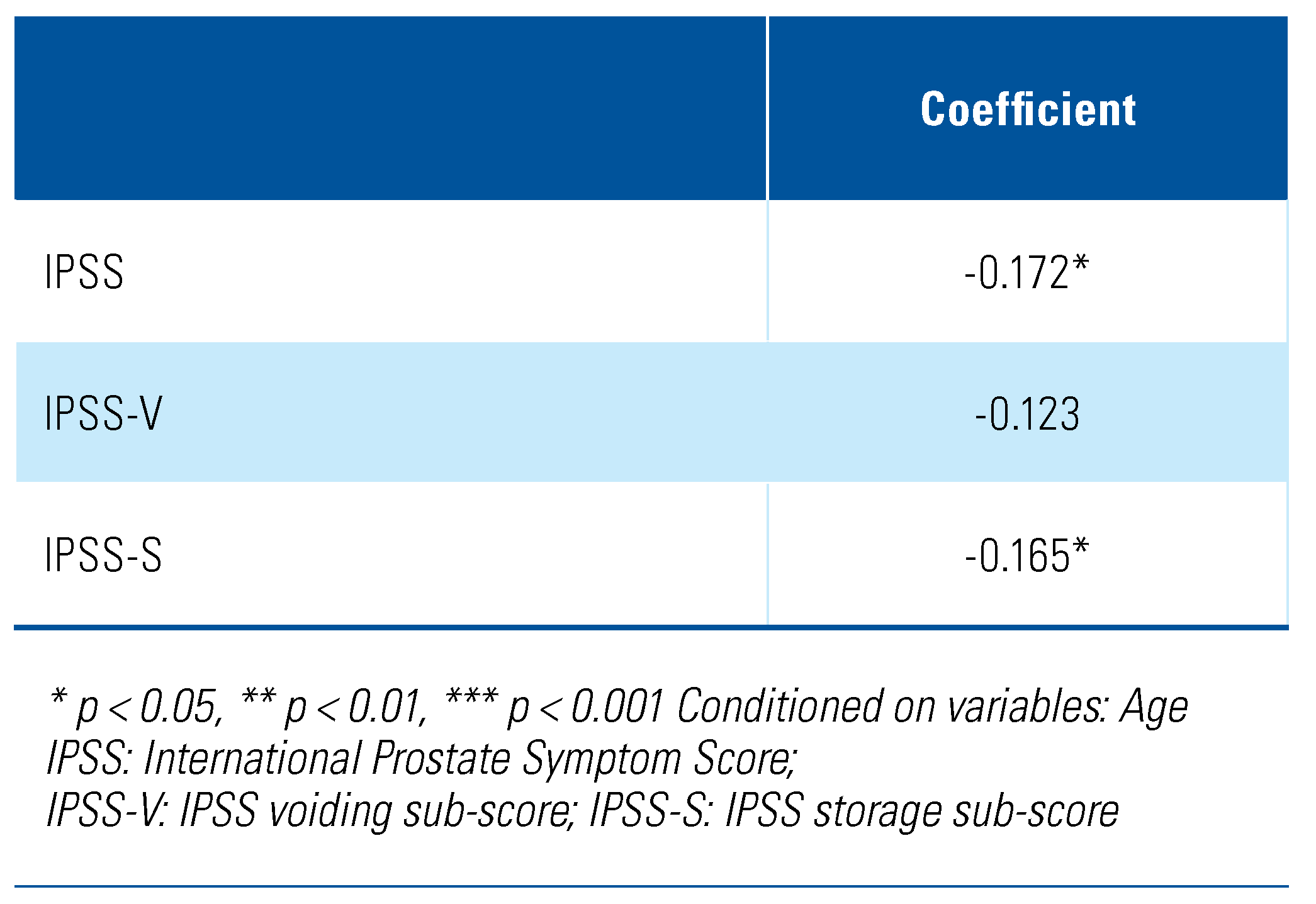
As shown in Table 4, categorical data analysis using the chi-square test revealed that severity of lower urinary tract symptoms is independent of the serum total testosterone status, χ2 (4, N = 163) = 4.24, P = 0.37). Subsequent analysis based on the continuous variables using the Pearson’s partial correlation following age-adjustment showed a negative correlation between total IPSS and serum total testosterone level, although only a weak association was seen (r = −0.17, P < 0.05) (Table 5). Similarly, for the IPSS storage sub-score, a weak correlation with serum total testosterone level was exhibited (r = −0.17, P < 0.05). Contrastingly, for the IPSS voiding sub-score, no significant correlation was seen with the serum total testosterone level (r = −0.12, P = 0.07).

Table 4.
Chi-square test of LUTS severity with serum total testosterone.

Table 5.
Age-adjusted Pearson partial correlations between IPSS (total and sub-scores) and serum total testosterone level.
Discussion
Lower urinary tract symptoms (LUTS) are commonly seen among ageing males and largely attributed to benign prostatic hyperplasia (BPH). The main etiologic factors that have been accepted to play a role in the pathogenesis of the condition are ageing and alterations in androgen levels[10,11]. Serum testosterone levels gradually decrease after the age of 40, and it is during this period that the onset of BPH usually occurs[3]. Although it has been well established that serum testosterone and its metabolite, 5-alpha-dihydrotestosterone, is vital for prostate growth, the mechanism of increasing LUTS occurrence in the later stages of life despite the declining androgen levels remains unclear. Xia et al. analyzed the correlations between prostate volume and serum testosterone level over a 4-year period and found a linear increase in prostate volume with the decrease in serum testosterone level, suggesting that prostate volume is not dependent on serum testosterone in the ageing male population[12]. Until now, the persistence of conflicting views on the effect of prostate volume on LUTS shown in previous studies highlights the complex nature of BPH and factors that may contribute to it[13,14].
Our study found that the severity of LUTS, as categorized according to the IPSS, and the serum TT status are independent of each other, which is consistent with previous studies reported by Crawford et al. and Liu et al.[4,6]. Similarly, a cross-sectional study by Schaztl et al. of 312 men above 40 years of age with untreated LUTS showed that hypogonadism had no impact on LUTS status and its clinical parameters, including IPSS and uroflowmetry[15]. This finding is in keeping with the saturation hypothesis, which surmises that incremental increase in testosterone level does not cause further androgen-stimulated prostate tissue proliferation due to the saturation of the available androgen receptors on the gland[16].
Interestingly, based on the continuous variable data analysis, we found negative correlations between serum total testosterone levels with total IPSS and IPSS storage sub-score, although only weak correlations were demonstrated. Several possible mechanisms may explain the inverse association between testosterone and LUTS. The urethral and bladder epithelial cells have been found to contain a large amount of androgen receptors. One animal study showed that testosterone may have an effect in maintaining the pelvic reflex activity of the autonomic nervous system, including the suppression of detrusor activity, which may play an important role in determining the severity of LUTS[17]. Other postulation includes the effect of testosterone on nitric-oxide-mediated smooth muscle relaxation, which may result in the reduction of LUTS severity among those with a higher testosterone level[18]. Additionally, testosterone may also have a physiologic role in the maintenance of the vasopressin level, thus affecting the ability of the kidneys to concentrate urine, thereby improving storage symptoms such as nocturia[19]. Furthermore, testosterone has been demonstrated to enhance the anti-inflammatory effect in prostate, thus dampening the effect of chronic inflammatory process that has been postulated to be one of the major etiologies of LUTS/BPH development[20]. Similarly, the presence of metabolic syndrome, which is commonly seen among men with lower testosterone levels, may perpetuate this chronic inflammatory process, therefore further propagating the development of LUTS/BPH among men in this population[20,21].
Apart from lower urinary tract symptoms, the effects of low testosterone are diverse, encompassing mental, physical, and sexual functions. The prevalence of male hypogonadism in Malaysia has been reported as being between 17.5 and 18.5% among the general population[22,23]. Nonetheless, there is still a paucity of data available locally with regards to late-onset hypogonadism among men with LUTS/BPH. In our cohort, the prevalence of hypogonadism among ageing men in Malaysia with LUTS was relatively high, accounting about 28%, with almost 5% having a severely low testosterone level. Disparities were also seen in the distribution among the multi-ethnic communities, and men of Indian ethnicity showed the highest preponderance of hypogonadism. Plausible causes of the disparity would include differences in genetic and epigenetic makeup and in socioeconomic background, which require further investigation. Its high prevalence indicates that thorough evaluation of ageing men presenting with LUTS, with or without late-onset hypogonadism syndrome, is vital as these symptoms are closely associated, regardless of testosterone level[24].
As the elderly population increases in Malaysia, the incidence of lower urinary tract symptoms with concurrent hypogonadism needs to be adequately addressed by clinicians, and consideration should be given to testosterone replacement therapy (TRT). In general, most guidelines still include precautions regarding TRT in men with BPH because of the belief it may aggravate the increase in prostate volume and thus hasten the progression of BPH, despite studies showing that exogenous testosterone has no effect on prostate volume or prostate specific antigen in older hypogonadal men[25]. In a systematic review by Cui et al. on 16 randomized controlled trials involving 1030 men, it was found that neither short-term nor long-term TRT increased the risk of prostate growth among hypogonadal men, thereby suggesting its safety among men with LUTS/BPH[26]. A 5-year prospective study by Yassin et al. that examined 261 hypogonadal men with LUTS receiving TRT found that TRT was associated with a significant LUTS improvement of 13.4% in hypogonadal men, suggesting a potential beneficial effect of TRT among this cohort[27]. Kohn et al., in their meta-analysis of 14 clinical trials involving 2029 men receiving TRT for late-onset hypogonadism, reported that IPSS changes were similar among men with mild to moderate LUTS receiving TRT versus placebo, indicating that TRT treatment does not worsen LUTS among men with late-onset hypogonadism[28]. More recently, a systematic review by Lee et. al of 12 clinical trials to investigate the relationship between TRT and LUTS found that there was no significant worsening of LUTS following TRT and concluded there was not sufficient evidence to support warnings that TRT may worsen LUTS among men with hypogonadism[29].
The outcomes from our study highlighted the complexity of the relationship between testosterone and the development of LUTS/BPH. More extensive research is required to improve the management of LUTS among ageing men.
Several limitations to our study were noted. A correlation between the IPSS and serum total testosterone levels was found, but there were only weak associations, and a larger sample size would strengthen the outcome of the study. As compared to a longitudinal study, the cross-sectional study design prevents the ability to make causal inferences from the observed association. Our study included only one sampling of serum total testosterone per participant, which may provide an imperfect evaluation that may be influenced by individual and analytical errors, and the study did not include free testosterone measurement.
Conclusion
Our study found no significant relationship between the severity of LUTS with total testosterone status, although a higher IPSS, particularly those related to storage symptoms, was found to have a weak association with lower levels of serum total testosterone. Among Malaysian men with lower urinary tract symptoms, up to 28% may have concurrent testosterone deficiency, with males of Indian ethnicity having the highest preponderance of hypogonadism. As a high prevalence of low testosterone is seen among this population, more research is needed to elucidate the role of serum testosterone measurement among ageing patients with LUTS and its implication to the clinical practice, so as to better optimize the multimodal approach to its management. Further prospective research could include evaluating measurement of serum testosterone levels as possible biochemical predictor for LUTS progression and response to BPH therapy.
Acknowledgments
The study was performed in accordance with the ethical standards based in the 1964 Declaration of Helsinki and was approved by the local institutional ethics board (UKM FF-2021-082). Special thanks to Associate Professor Dr Rozita Hod for statistical consultation and Consultant Endocrinologist, Associate Professor Dr Norlaila Mustafa for research input.
Competing Interests
None declared.
Abbreviations
| BPH | benign prostatic hyperplasia |
| IPSS | International Prostate Symptoms Scoring |
| LUTS | lower urinary tract symptoms |
| TRT | testosterone replacement therapy |
| TT | total testosterone |
References
- Teh, G.C.; Sahabudin, R.M.; Lim, T.C.; Chong, W.L.; Woo, S.; Mohan, M.; et al. Prevalence of symptomatic BPE among Malaysian men aged 50 and above attending screening during prostate health awareness campaign. Med. J. Malaysia 2001, 56, 186–195. [Google Scholar]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; et al. Age Trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.W.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Poage, W.; Nyhuis, A.; Price, D.A.; Dowsett, S.A.; Muram, D. Effects of testosterone level on lower urinary tract symptoms. Am. J. Men’s Health 2016, 10, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Chiang, H.S.; Yu, H.J. Serum testosterone levels significantly correlate with nocturia in men aged 40–79 years. Urology 2011, 78, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Huang, S.P.; Li, W.M.; Wang, C.J.; Chou, Y.H.; Li, C.C.; et al. Relationship between serum testosterone and measures of benign prostatic hyperplasia in aging men. Urology 2007, 70, 677–680. [Google Scholar] [CrossRef]
- Martin, S.A.; Haren, M.T.; Marshall, V.R.; Lange, K.; Wittert, G.A. Members of the Florey Adelaide Male Ageing Study. Prevalence and factors associated with uncomplicated storage and voiding lower urinary tract symptoms in community-dwelling Australian men. World J. Urol. 2011, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, J.H.; Yoon, Y.S.; Choi, H.; Park, J.Y.; Bae, J.H. Serum testosterone levels are negatively correlated with international prostate symptom score and transitional prostate volume. Low. Urin. Tract. Symptoms 2018, 10, 143–147. [Google Scholar] [CrossRef]
- EAU guideline. EAU Male Sexual and Reproductive health Guidelines. Edn. presented at the EAU Annual Congress Amsterdam; 2022; ISBN 978-94-92671-16-5. [Google Scholar]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- Partin, A.W.; Oesterling, J.E.; Epstein, J.I.; Horton, R.; Walsh, P.C. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J. Urol. 1991, 145, 405–409. [Google Scholar] [CrossRef]
- Xia, B.W.; Zhao, S.C.; Chen, Z.P.; Chen, C.; Liu, T.S.; Yang, F.; et al. Relationship between serum total testosterone and prostate volume in aging men. Sci. Rep. 2021, 11, 14122. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.S.; Chalise, P.R.; Bhandari, B.B. Correlation of prostate volume with international prostate symptom score and quality of life in men with benign prostatic hyperplasia. Nepal. Med. Coll. J. 2008, 10, 104–107. [Google Scholar]
- Bosch, J.L.; Hop, W.C.; Kirkels, W.J.; Schröder, F.H. The International Prostate Symptom Score in a community-based sample of men between 55 and 74 years of age: prevalence and correlation of symptoms with age, prostate volume, flow rate and residual urine volume. Br. J. Urol. 1995, 75, 622–630. [Google Scholar] [CrossRef]
- Schatzl, G.; Brössner, C.; Schmid, S.; Kugler, W.; Roehrich, M.; Treu, T.; et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology 2000, 55, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Khera, M.; Crawford, D.; Morales, A.; Salonia, A.; Morgentaler, A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur. Urol. 2014, 65, 115–123. [Google Scholar] [CrossRef]
- Yassin, A.A.; El-Sakka, A.I.; Saad, F.; Gooren, L.J.G. Lower urinary-tract symptoms and testosterone in elderly men. World J. Urol. 2008, 26, 359–364. [Google Scholar] [CrossRef]
- Smet, P.J.; Jonavicius, J.; Marshall, V.R.; de Vente, J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience 1996, 71, 337–348. [Google Scholar] [CrossRef]
- Shigehara, K.; Sugimoto, K.; Konaka, H.; Iijima, M.; Fukushima, M.; Maeda, Y.; et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male 2011, 14, 53–58. [Google Scholar] [CrossRef]
- Rastrelli, G.; Vignozzi, L.; Corona, G.; Maggi, M. Testosterone and benign prostatic hyperplasia. Sex. Med. Rev. 2019, 7, 259–271. [Google Scholar] [CrossRef]
- Tsujimura, A.; Miyagawa, Y.; Takezawa, K.; Okuda, H.; Fukuhara, S.; Kiuchi, H.; et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology 2013, 82, 814–819. [Google Scholar] [CrossRef]
- Kang, W.H.; Siruhan, M.; Shree, V.N.; Karupiah, M.; Sukor, N.; Kamaruddin, N.A. Prevalence of hypogonadism among male Type 2 diabetes mellitus patients in Pusat Perubatan Universiti Kebangsaan Malaysia. J. ASEAN Fed. Endocr. Soc. 2021, 36, 12. [Google Scholar] [CrossRef]
- Ho, C.C.; Singam, P.; Hong, G.E.; Zainuddin, Z.M. Male sexual dysfunction in Asia. Asian J. Androl. 2011, 13, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, T.; Tsujimura, A.; Mizushima, K.; Kurosawa, M.; Kure, A.; Uesaka, Y.; et al. International Prostate Symptom Score and quality of life index for lower urinary tract symptoms are associated with aging males symptoms rating scale for late-onset hypogonadism symptoms. World J. Mens. Health 2022, 41, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, J.P.; Gardette, J.; Rollet, J.; Legros, J.J. Prostate-specific antigen (PSA) concentrations in hypogonadal men during 6 years of transdermal testosterone treatment. BJU Int. 2013, 111, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Y. The effect of androgen-replacement therapy on prostate growth: a systematic review and meta-analysis. Eur. Urol. 2013, 64, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Yassin, D.J.; El Douaihy, Y.; Yassin, A.A.; Kashanian, J.; Shabsigh, R.; Hammerer, P.G. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J. Urol. 2014, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.P.; Mata, D.A.; Ramasamy, R.; Lipshultz, L.I. Effects of testosterone replacement therapy on lower urinary tract symptoms: A systematic review and meta-analysis. Eur. Urol. 2016, 69, 1083–1090. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, Y.S.; Kam, S.C. Correlation between testosterone replacement treatment and lower urinary tract symptoms. Int. Neurourol. J. 2021, 25, 12–22. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.