Salvage Versus Adjuvant Radiation Therapy Following Radical Prostatectomy in Localised Prostate Cancer: A War Without a Winner
Abstract
:Introduction
Methods
Results
Available evidence
New investigations and findings
 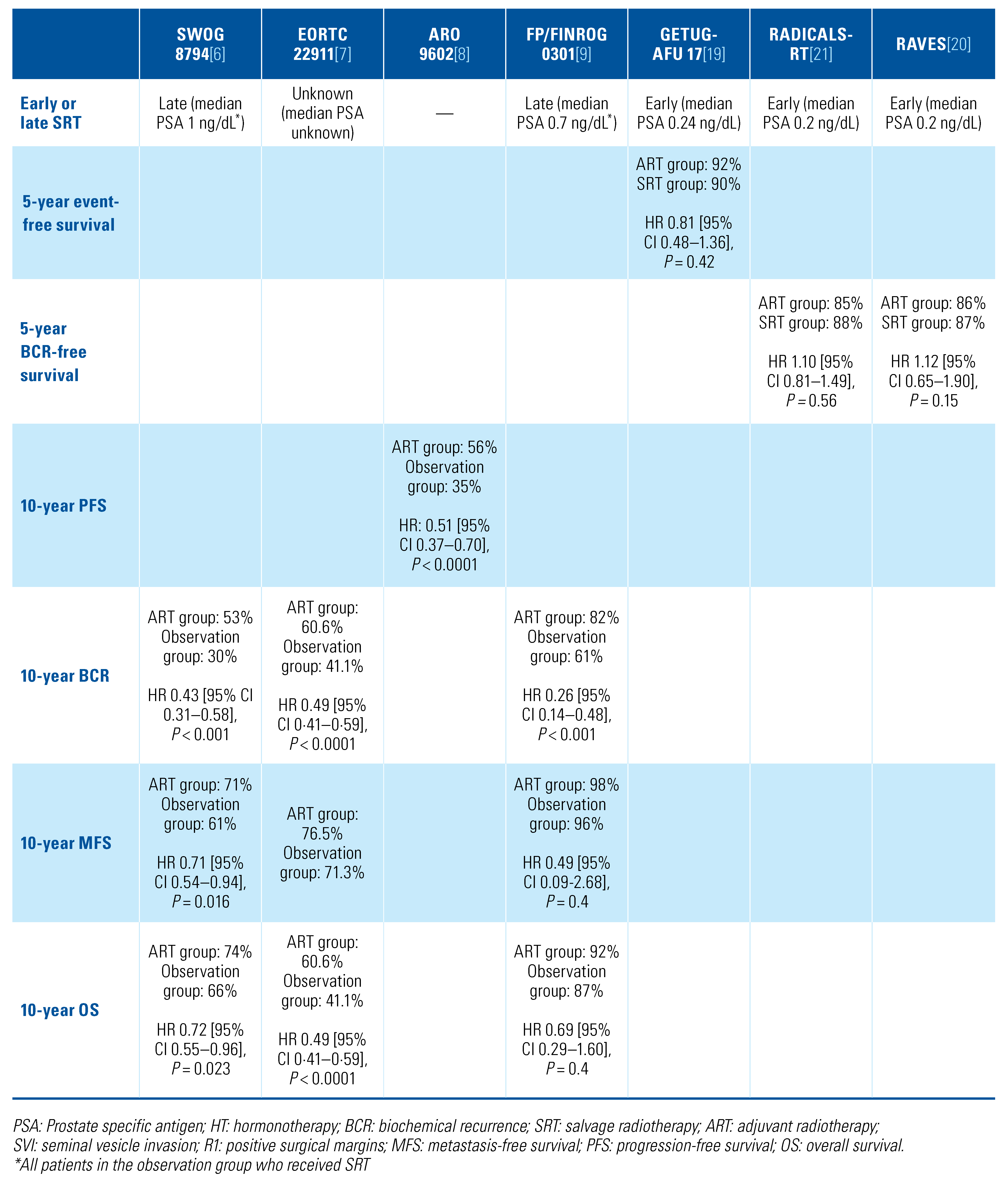 |
Discussion
Accurate risk of recurrence estimation
How should patients with very high-risk features and a combination of high-risk features and undetectable postoperative serum PSA levels be treated?
Impact of novel imaging techniques on treatment
RT technique
Timing and duration of ADT with SRT
Benefit of SRT in cases of BCR
Conclusions
Conflicts of Interest
Abbreviations
| ADT | androgen-deprivation therapy |
| ART | adjuvant radiation therapy |
| BCR | biochemical recurrence |
| CT | computed tomography |
| MFS | metastasis-free survival |
| OS | overall survival |
| PCa | prostate cancer |
| PET | positron emission tomography |
| PFS | progression-free survival |
| PSADT | PSA doubling time |
| RCT | randomized controlled trial |
| RP | radical prostatectomy |
| SRT | salvage radiation therapy |
References
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; et al. Management of patients with advanced prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, R.; Takayama, K.; Nakamura, K.; Inoue, T.; Yamasaki, T.; Kobayashi, T.; et al. Ten-year outcomes of high-dose intensity-modulated radiation therapy for nonmetastatic prostate cancer with unfavorable risk: Early initiation of salvage therapy may replace long-term adjuvant androgen deprivation. Int J Clin Oncol. 2019, 24, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Kristiansen, G. The heterogeneity of prostate cancer: A practical approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; D’Amico, A.V. Timing of radiotherapy after radical prostatectomy. Lancet 2020, 396, 1374–1375. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; ARTISTIC Meta-analysis Group; et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Tangen, C.M.; Paradelo, J.; Lucia, M.S.; Miller, G.; Troyer, D.; et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol. 2009, 181, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; van Poppel, H.; Tombal, B.; Vekemans, K.; Da Pozzo, L.; de Reijke, T.M.; et al. European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012, 380, 2018–2027. [Google Scholar] [CrossRef]
- Wiegel, T.; Bartkowiak, D.; Bottke, D.; Bronner, C.; Steiner, U.; Siegmann, A.; et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol. 2014, 66, 243–450. [Google Scholar] [CrossRef] [PubMed]
- Hackman, G.; Taari, K.; Tammela, T.L.; Matikainen, M.; Kouri, M.; Joensuu, T.; FinnProstate Group; et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol. 2019, 76, 586–595. [Google Scholar] [CrossRef]
- Tao, R.; Dai, J.; Bai, Y.; Yang, J.; Sun, G.; Zhang, X.; et al. The prognosis benefits of adjuvant versus salvage radiotherapy for patients after radical prostatectomy with adverse pathological features: A systematic review and meta-analysis. Radiat Oncol. 2019, 14, 197. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Gillesen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. 2022. Available online: https://uroweb.org/eau-guidelines.
- Buscariollo, D.L.; Drumm, M.; Niemierko, A.; Clayman, R.H.; Galland-Girodet, S.; Rodin, D.; et al. Long-term results of adjuvant versus early salvage postprostatectomy radiation: A large single-institutional experience. Pract Radiat Oncol. 2017, 7, e125–e133. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Karnes, R.J.; Boorjian, S.A.; Moschini, M.; Morlacco, A.; Bossi, A.; et al. Long-term impact of adjuvant versus early salvage radiation therapy in pT3N0 prostate cancer patients treated with radical prostatectomy: Results from a multi-institutional series. Eur Urol. 2017, 71, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, A.; Bottke, D.; Faehndrich, J.; Brachert, M.; Lohm, G.; Miller, K.; et al. Salvage radiotherapy after prostatectomy—What is the best time to treat? Radiother Oncol. 2012, 103, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Wiegel, T.; Joniau, S.; Cozzarini, C.; Bianchi, M.; Sun, M.; et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: Results of a match-controlled multi-institutional analysis. Eur Urol. 2012, 62, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Karnes, R.J.; Cozzarini, C.; Fiorino, C.; Gandaglia, G.; Joniau, S.; et al. Assessing the optimal timing for early salvage radiation therapy in patients with prostate-specific antigen rise after radical prostatectomy. Eur Urol. 2016, 69, 728–733. [Google Scholar] [CrossRef]
- Tendulkar, R.D.; Agrawal, S.; Gao, T.; Efstathiou, J.A.; Pisansky, T.M.; Michalski, J.M.; et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016, 34, 3648–3654. [Google Scholar] [CrossRef]
- Bottke, D.; Bartkowiak, D.; Schrader, M.; Wiegel, T. Radiotherapy after radical prostatectomy: Immediate or early delayed? Strahlenther Onkol. 2012, 188, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Huelster, H.L.; Laviana, A.A.; Joyce, D.D.; Huang, L.-C.; Zhao, Z.; Koyama, T.; Hoffman, K.E.; et al. Radiotherapy after radical prostatectomy: Effect of timing of postprostatectomy radiation on functional outcomes. Urol Oncol. 2020, 38, 930.e23–930.e32. [Google Scholar] [CrossRef] [PubMed]
- Chapin, B.F.; Nguyen, J.N.; Achim, M.F.; Navai, N.; Williams, S.B.; Prokhorova, I.N.; et al. Positive margin length and highest Gleason grade of tumor at the margin predict for biochemical recurrence after radical prostatectomy in patients with organ-confined prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.N.; Shoag, J.E.; Golan, R.; Halpern, J.A.; Schaeffer, E.M.; Hsu, W.C.; et al. Contemporary incidence and outcomes of prostate cancer lymph node metastases. J Urol. 2018, 199, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, P.L.; Shin, H.; Yousefi, K.; Thompson, D.J.; Hornberger, J.; Hyatt, A.S.; et al. Impact of a genomic classifier of metastatic risk on postprostatectomy treatment recommendations by radiation oncologists and urologists. Urology 2015, 86, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Marascio, J.; Spratt, D.E.; Zhang, J.; Trabulsi, E.J.; Le, T.; Sedzorme, W.S.; et al. Prospective study to define the clinical utility and benefit of Decipher testing in men following prostatectomy. Prostate Cancer Prostatic Dis. 2020, 23, 295–302. [Google Scholar] [CrossRef]
- Messina, C.; Cattrini, C.; Soldato, D.; Vallome, G.; Caffo, O.; Castro, E.; et al. BRCA mutations in prostate cancer: Prognostic and predictive implications. J Oncol. 2020, 4986365. [Google Scholar] [CrossRef]
- Giri, V.N.; Knudsen, K.E.; Kelly, W.K.; Cheng, H.H.; Cooney, K.A.; Cookson, M.S.; et al. Implementation of germline testing for prostate cancer: Philadelphia Prostate Cancer Consensus Conference 2019. J Clin Oncol. 2020, 38, 2798–2811. [Google Scholar] [CrossRef]
- Borque-Fernando, A.; Espílez, R.; Miramar, D.; Corbatón, D.; Rodríguez, A.; Castro, E.; et al. Genetic counselling in prostate cancer: How to implement it in daily clinical practice? Asesoramiento genético en cáncer de próstata: ¿cómo implementarlo en la práctica clínica diaria? Actas Urol Esp. 2021, 45, 8–20. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Baras, A.S.; Lotan, T.L.; Antonarakis, E.S. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: Association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis. 2019, 22, 59–65. [Google Scholar] [CrossRef]
- Spratt, D.E.; Yousefi, K.; Deheshi, S.; Ross, A.E.; Den, R.B.; Schaeffer, E.M.; et al. Individual patient-level meta-analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J Clin Oncol. 2017, 35, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, F.; Karnes, R.J.; Suardi, N.; Cozzarini, C.; Gandaglia, G.; Fossati, N.; et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014, 32, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Valerio, M.; Heidegger, I.; Tsaur, I.; Mathieu, R.; Ceci, F.; et al. Management of patients with node-positive prostate cancer at radical prostatectomy and pelvic lymph node dissection: A systematic review. Eur Urol Oncol. 2020, 3, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Preisser, F.; Tennstedt, P.; Tober, P.; Mandel, P.; Schlomm, T.; et al. Adjuvant radiation therapy is associated with better oncological outcome compared with salvage radiation therapy in patients with pN1 prostate cancer treated with radical prostatectomy. BJU Int. 2017, 119, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Chen, M.H.; Wu, J.; Huland, H.; Graefen, M.; D’Amico, A.V. Adjuvant versus early salvage radiation therapy after radical prostatectomy for pN1 prostate cancer and the risk of death. J Clin Oncol. 2022, 40, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.J.; Souvatzoglou, M.; Tuncel, M.; Herrmann, K.; Buck, A.K.; Praus, C.; et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2008, 35, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Pereira Mestre, R.; Ferrari, M.; Bosetti, D.G.; Pascale, M.; et al. Radiolabelled choline versus PSMA PET/CT in prostate cancer restaging: A meta-analysis. Am J Nucl Med Mol Imaging 2019, 9, 127–139. [Google Scholar] [PubMed]
- FDA approves first PSMA-targeted PET imaging drug for men with prostate cancer. News release. US Food and Drug Administration. December 1, 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 1 June 2022).
- Han, S.; Woo, S.; Kim, Y.J.; Suh, C.H. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: A systematic review and meta-analysis. Eur Urol. 2018, 74, 179–190. [Google Scholar] [CrossRef]
- Armstrong, W.R.; Kishan, A.U.; Booker KMFendler, W.P.; Hope, T.A.; Nickols, N.G.; et al. Impact of PSMA PET/CT on prostate cancer salvage radiotherapy management: Results from the prospective randomized phase 3 trial [PSMA SRT NCT03582774]. J Clin Oncol. 2022, 40 (Suppl. 16), 5028. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). (2007, December). Prostate radiation therapy or short-term androgen deprivation therapy and pelvic lymph node radiation therapy with or without prostate radiation therapy in treating patients with a rising prostate specific antigen (PSA) after surgery for prostate cancer. Identifier NCT00567580. Available online: https://clinicaltrials.gov/ct2/show/NCT00567580.
- Sachdev, S.; Carroll, P.; Sandler, H.; Nguyen, P.N.; Wafford, E.; Auffenberg, G.; et al. Assessment of postprostatectomy radiotherapy as adjuvant or salvage therapy in patients with prostate cancer: A systematic review. JAMA Oncol. 2020, 6, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Ghadjar, P.; Hayoz, S.; Bernhard, J.; Zwhalen, D.R.; Hölscher, T.; Gut, P.; et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: First results of the randomized trial SAKK 09/10. J Clin Oncol. 2015, 33, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer Version 4.2022 — May 10, 2022. Available online: http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf.
- Guckenberger, M.; Andratschke, N.; Alheit, H.; Holy, R.; Noustakis, C.; Nestle, U.; Deutschen Gesellschaft für Radioonkologie (DEGRO); et al. Definition of stereotactic body radiotherapy: Principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014, 190, 26–33. [Google Scholar] [CrossRef]
- Francolini, G.; Jereczek-Fossa, B.A.; Di Cataldo, V.; Simontacchi, G.; Marvaso, G.; Zerella, M.A.; et al. Stereotactic radiotherapy for prostate bed recurrence after prostatectomy, a multicentric series. BJU Int. 2020, 125, 417–425. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; NRG Oncology RTOG; et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017, 376, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Magné, N.; Burban-Provost, P.; Sargos, P.; Latorzeff, I.; Lagrange, J.-L.; et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019, 20, 1740–1749. [Google Scholar] [CrossRef]
- Fossati, N.; Robesti, D.; Karnes, R.J.; Soligo, M.; Boorjian, S.A.; Bossi, A.; et al. Assessing the role and optimal duration of hormonal treatment in association with salvage radiation therapy after radical prostatectomy: Results from a multi-institutional study. Eur Urol. 2019, 76, 443–449. [Google Scholar] [CrossRef]
- Kapoor, R.; Deek, M.P.; McIntyre, R.; Raman, N.; Kummerlowe, M.; Chen, I.; et al. A phase II randomized placebo-controlled double-blind study of salvage radiation therapy plus placebo versus SRT plus enzalutamide with high-risk PSA-recurrent prostate cancer after radical prostatectomy (SALV-ENZA). BMC Cancer 2019, 19, 572. [Google Scholar] [CrossRef]
- Posadas, E.M.; Gay, H.A.; Pugh, S.L.; Morgan, T.M.; Yu, J.B.; Lechpammer, S.; et al. RTOG 3506 (STEEL): A study of salvage radiotherapy with or without enzalutamide in recurrent prostate cancer following surgery. J Clin Oncol. 2020. [CrossRef]
- National Library of Medicine (U.S.). (2019, November). Combined apalutamide, radiotherapy, and LHRH agonist in prostate cancer patients after prostatectomy (CARLHA-2). Identifier NCT04181203. Available online: https://clinicaltrials.gov/ct2/show/NCT04181203.
- National Library of Medicine (U.S.). (2017 May). Randomized phase II study of salvage XRT + ADT +/- abiraterone and apalutamide for rising PSA after RP (FORMULA-509). Identifier NCT03141671. Available online: https://clinicaltrials.gov/ct2/show/NCT03141671.
- Pak, S.; You, D.; Jeong, I.G.; Kim, Y.S.; Hong, J.H.; Kim, C.-S.; et al. Time to biochemical relapse after radical prostatectomy and efficacy of salvage radiotherapy in patients with prostate cancer. Int J Clin Oncol. 2019, 24, 1238–1246. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Arfi, N.; Gross, T.; Moris, L.; Briers, E.; et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: A systematic review. Eur Urol. 2019, 75, 967–987. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Preisser, F.; Graefen, M.; Huland, H.; Pompe, R.S. External validation of the European Association of Urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur Urol. 2019, 75, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Bowman, L.; Landray, M.; Peto, R. The magic of randomization versus the myth of real-world evidence. New Engl J Med. 2020, 382, 674–678. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Valicenti, R.K.; Hanlon, A.L.; Pisansky, T.M.; Sandler, H.M.; Kuban, D.A.; et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3–4N0 prostate cancer. Urology. 2008, 72, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Rodriguez-Sanchez, L.; Macek, P.; Lanz, C.; Mandoorah, Q.; Dias, N.; Colandrea, G.; Secin, F.P.; Arora, A.M.; Sanchez-Salas, R.; Cathelineau, X. Salvage Versus Adjuvant Radiation Therapy Following Radical Prostatectomy in Localised Prostate Cancer: A War Without a Winner. Soc. Int. Urol. J. 2023, 4, 40-50. https://doi.org/10.48083/MSVK1934
Rodriguez-Sanchez L, Macek P, Lanz C, Mandoorah Q, Dias N, Colandrea G, Secin FP, Arora AM, Sanchez-Salas R, Cathelineau X. Salvage Versus Adjuvant Radiation Therapy Following Radical Prostatectomy in Localised Prostate Cancer: A War Without a Winner. Société Internationale d’Urologie Journal. 2023; 4(1):40-50. https://doi.org/10.48083/MSVK1934
Chicago/Turabian StyleRodriguez-Sanchez, Lara, Petr Macek, Camille Lanz, Qusay Mandoorah, Nuno Dias, Gianmarco Colandrea, Fernando P. Secin, Amandeep M. Arora, Rafael Sanchez-Salas, and Xavier Cathelineau. 2023. "Salvage Versus Adjuvant Radiation Therapy Following Radical Prostatectomy in Localised Prostate Cancer: A War Without a Winner" Société Internationale d’Urologie Journal 4, no. 1: 40-50. https://doi.org/10.48083/MSVK1934
APA StyleRodriguez-Sanchez, L., Macek, P., Lanz, C., Mandoorah, Q., Dias, N., Colandrea, G., Secin, F. P., Arora, A. M., Sanchez-Salas, R., & Cathelineau, X. (2023). Salvage Versus Adjuvant Radiation Therapy Following Radical Prostatectomy in Localised Prostate Cancer: A War Without a Winner. Société Internationale d’Urologie Journal, 4(1), 40-50. https://doi.org/10.48083/MSVK1934




