The Clinical Frailty Scale as a Predictor of Trial of Void Outcomes in Men Undergoing Transurethral Resection of Prostate Surgery
Abstract
:Introduction
Methods
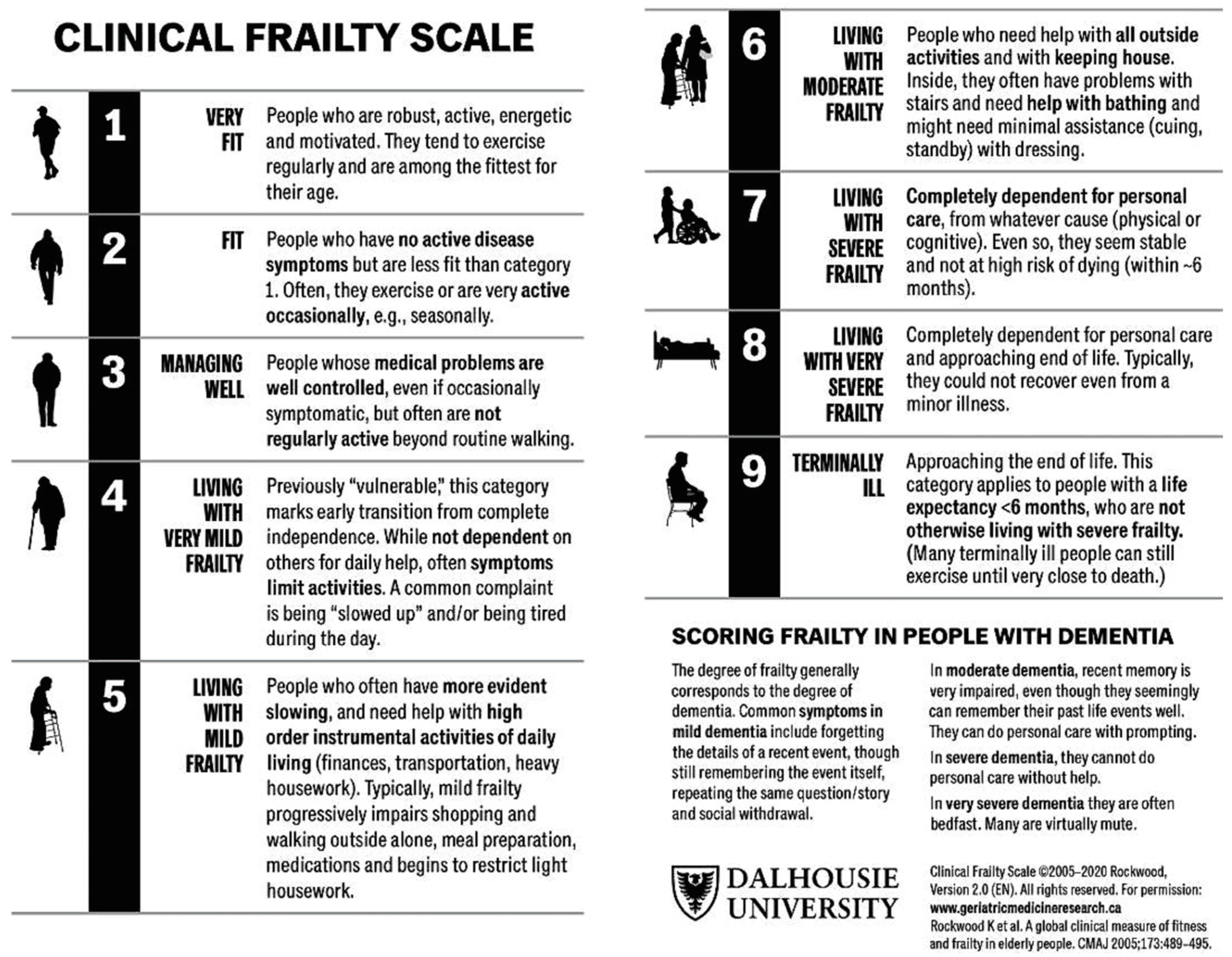
Inclusion criteria
- Adult male patients aged 18 years and above.
- Patients who underwent elective monopolar or bipolar transurethral resection of prostate surgery for bladder outlet obstruction secondary to benign prostatic hyperplasia related lower urinary tract symptoms.
Exclusion criteria
- Patients with a previous diagnosis of neurogenic bladder.
- Patients with a concurrent diagnosis of lower urinary tract pathology, including urethral strictures. Patients who had previous radiotherapy for prostate cancer.
- Patients with a history of previous bladder surgery, including ureteric implantation such as from renal transplants.
- Patients with a history of previous TURP surgery.
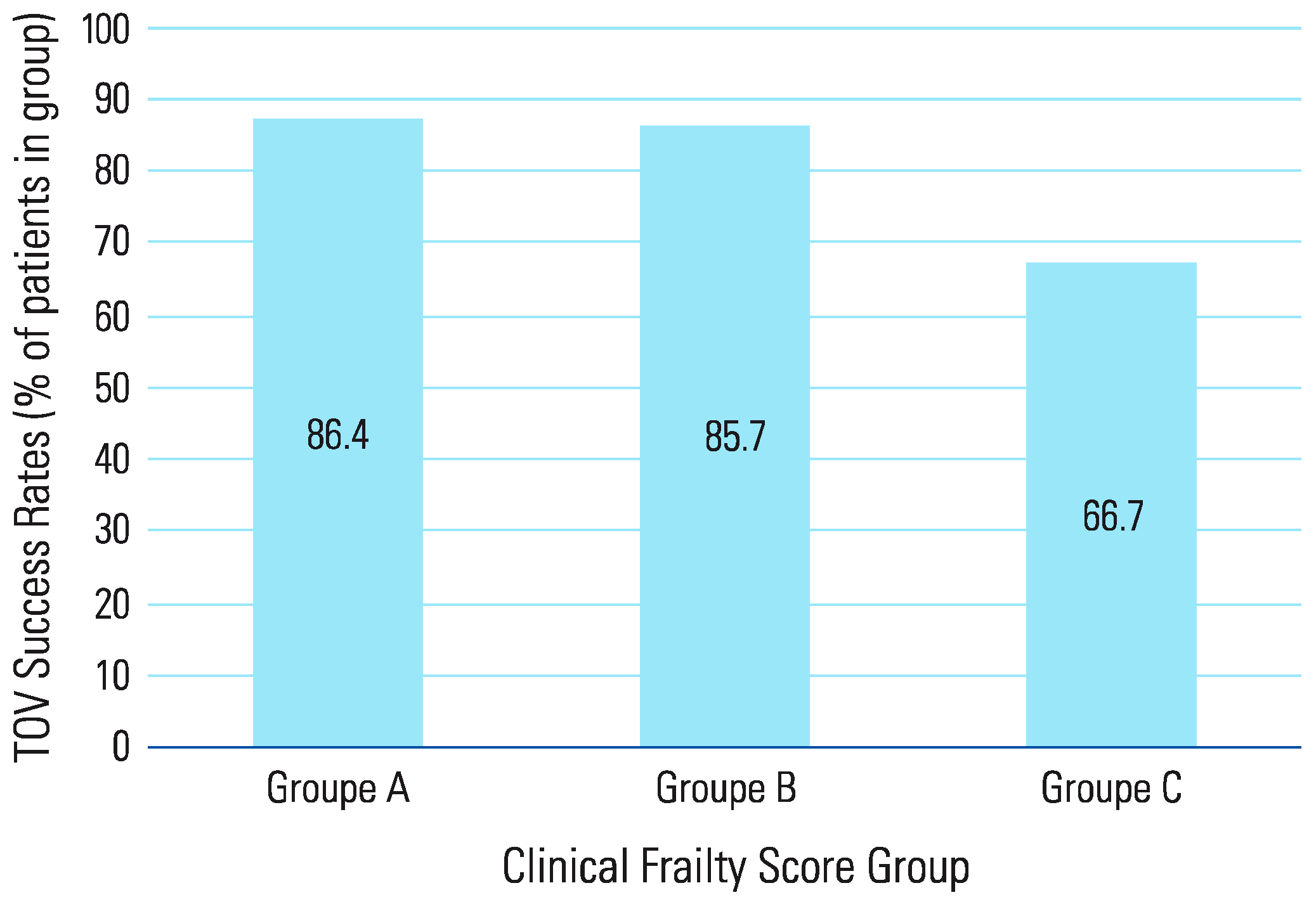
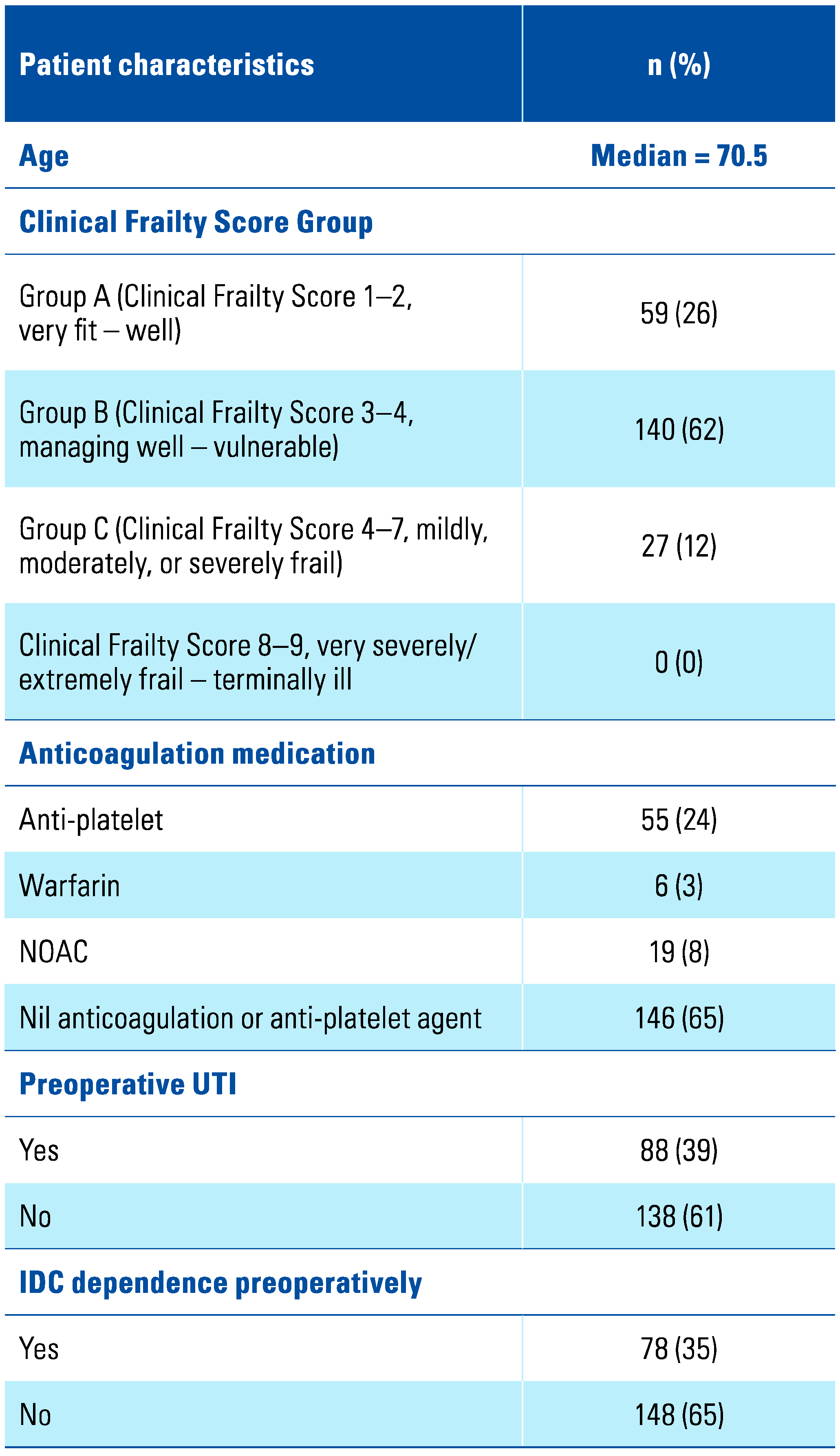
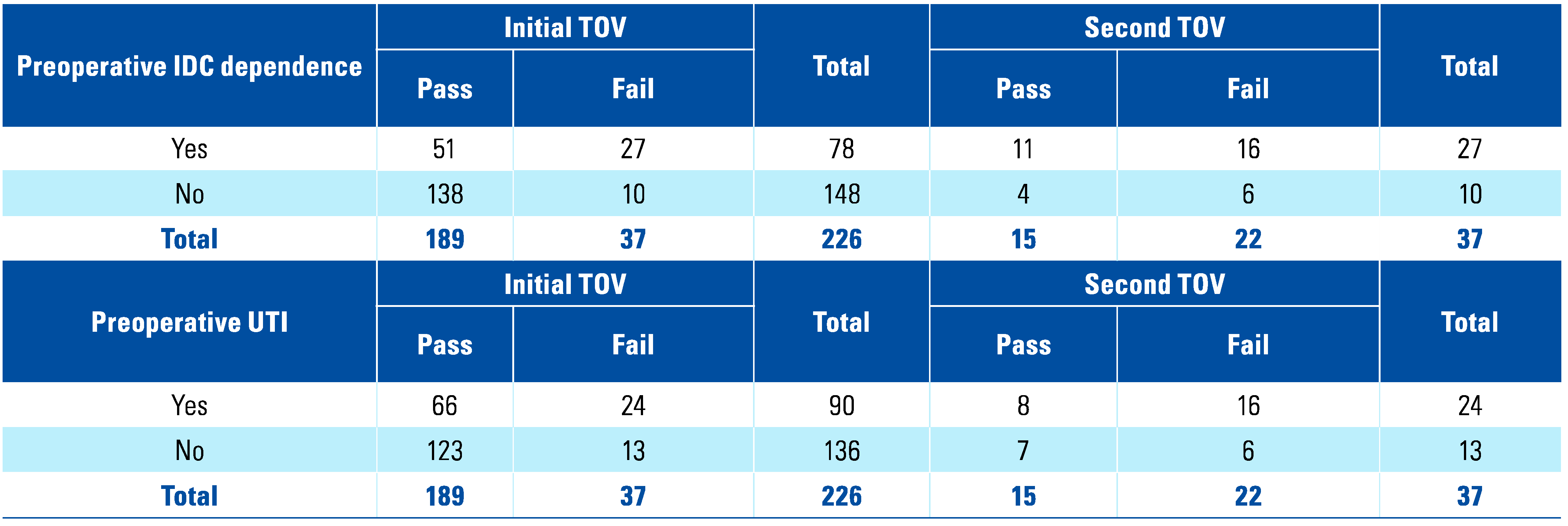
Results
Discussion
Conclusion
Acknowledgments
Abbreviations
| BOO | Bladder outlet obstruction |
| CFS | Clinical Frailty Scale |
| IDC | Indwelling catheter |
| IPSS | International Prostate Symptom Score |
| LUTS | Lower urinary tract symptoms |
| PVR | Post-void residual |
| TOV | Trial of void |
| TURP | Transurethral resection of prostate |
References
- Lang, P.-O.; Michel, J.-P.; Zekry, D. Frailty syndrome: A transitional state in a dynamic process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Rolfson, D.B.; Stolee, P.; Borrie, M.J.; Speechley, M. Frailty is associated with postoperative complications in older adults with medical problems. Arch. Gerontol. Geriatr. 2009, 48, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Theou, O. Using the clinical frailty scale in allocating scarce health care resources. Can. Geriatr. J. 2020, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Watts, J.; Peel, N.; Hubbard, R. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Ravindrarajah, R.; Lee, D.M.; Pye, S.R.; Gielen, E.; Boonen, S.; Vanderschueren, D.; et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS). Arch. Gerontol. Geriatr. 2013, 57, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jeong, B.C.; Jeon, S.S.; Lee, S.W.; Lee, K.-S. Comparison of efficacy of different surgical techniques for benign prostatic obstruction. Int. Neurourol. J. 2021, 25, 252. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Eredics, K.; Meyer, C.; Gschliesser, T.; Lodeta, B.; Heissler, O.; Kunit, T.; et al. Can a simple geriatric assessment predict the outcome of TURP? Urol. Int. 2020, 104, 367–372. [Google Scholar] [CrossRef]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef]
- Suskind, A.M.; Walter, L.C.; Zhao, S.; Finlayson, E. Functional outcomes after transurethral resection of the prostate in nursing home residents. J. Am. Geriatr. Soc. 2017, 65, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- NSW Agency for Clinical Innovation. Clinical Guidelines, Urology, Trial of Void - Hospital - Clinical Guideline: NSW Government; 2010. Available online: https://aci.health.nsw.gov.au/__data/assets/pdf_file/0007/191068/ACI_TOV_Hospital_Jan13.pdf (accessed on 10 August 2023).
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Black, B.; English, S. Is TURP safe with room temperature irrigation? A randomised control trial. J. Urol. 2019, 201 (Suppl 4), e643. [Google Scholar] [CrossRef]
- Culkin, D.J.; Exaire, E.J.; Green, D.; Soloway, M.S.; Gross, A.J.; Desai, M.R.; et al. Anticoagulation and antiplatelet therapy in urological practice: ICUD/AUA review paper. J. Urol. 2014, 192, 1026–34. [Google Scholar] [CrossRef] [PubMed]
- Srinivasrao, P.; Shashidhar, M. Bipolar versus monopolar transurethral resection for benign prostatic hypertrophy: A prospective comparative study. Int. Surg. J. 2022, 9, 1016–1018. [Google Scholar] [CrossRef]
- Tamalunas, A.; Westhofen, T.; Schott, M.; Keller, P.; Atzler, M.; Stief, C.G.; et al. The impact of preoperative lower urinary tract symptoms medication on the functional performance of holmium laser enucleation of the prostate. Cent. European J. Urol. 2021, 74, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Kikuchi, D.; Tateoka, J.; Abe, N.; Banjo, H.; Tsuchida, M.; et al. Long-term symptomatic outcome after transurethral resection of the prostate: A urodynamics-based assessment. Int. J. Urol. 2019, 26, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.J.; Frizelle, F.A.; Geddes, J.A.; Eglinton, T.W.; Hampton, M.B. Frailty in surgical patients. Int. J. Colorectal Dis. 2018, 33, 1657–1666. [Google Scholar] [CrossRef]
- Oqab, Z.; Pournazari, P.; Sheldon, R.S. What is the impact of frailty on prescription of anticoagulation in elderly patients with atrial fibrillation? A systematic review and meta-analysis. J. Atr. Fibrillation 2018, 10, 1870. [Google Scholar] [CrossRef]
- Chao, C.-T.; Lee, S.-Y.; Wang, J.; Chien, K.-L.; Huang, J.-W. Frailty increases the risk for developing urinary tract infection among 79,887 patients with diabetic mellitus and chronic kidney disease. BMC Geriatr. 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Kostakopoulos, N.A.; Karakousis, N.D.; Moschotzopoulos, D. Frailty associated urinary tract infections (FaUTIs). J. Frailty Sarcopenia Falls 2021, 6, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.N.; Alabastro, C.G.; Anger, J.T. Prevalence and cost of catheters to manage neurogenic bladder. Curr. Bladder Dysfunct. Rep. 2018, 13, 215–223. [Google Scholar] [CrossRef]
- Roth, J.D.; Pariser, J.J.; Stoffel, J.T.; Lenherr, S.M.; Myers, J.B.; Welk, B.; et al. Patient subjective assessment of urinary tract infection frequency and severity is associated with bladder management method in spinal cord injury. Spinal Cord. 2019, 57, 700–707. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.; Higgins, A.; Lopez, J.; Chaboyer, W. Predictors of acute urinary retention after transurethral resection of the prostate: A retrospective chart audit. Urol. Nurs. 2011, 31, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, C.Z.; Li, G.Y.; Lin, C.M.; Zhang, X.M.; Gu, H.B.; et al. Causes of and risk factors for failure in catheter removal after transurethral resection of the prostate [Article in Chinese]. Zhonghua Nan Ke Xue 2020, 26, 250–253. [Google Scholar]
- Bierle, D.M.; Raslau, D.; Regan, D.W.; Sundsted, K.K.; Mauck, K.F. Preoperative evaluation before noncardiac surgery. Mayo Clin. Proc. 2020, 95, 807–822. [Google Scholar] [CrossRef] [PubMed]
- Dhesi, J.; Swart, M. Specialist pre-operative assessment clinics. Anaesthesia 2016, 71, 3–8. [Google Scholar] [CrossRef]
- Elmansy, H.; Shabana, W.; Ahmad, A.; Hodhod, A.; Hadi, R.A.; Tablowski, T.; et al. Factors Predicting Successful Same-Day Trial of Void (TOV) After Laser Vaporization of the Prostate. Urology 2022. [Google Scholar] [CrossRef]

This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Zhuo, K.Y.; Quah, S.L.W.; Garcia, C.; Leung, K.Y.; Chung, A. The Clinical Frailty Scale as a Predictor of Trial of Void Outcomes in Men Undergoing Transurethral Resection of Prostate Surgery. Soc. Int. Urol. J. 2023, 4, 378-384. https://doi.org/10.48083/XVWY4791
Zhuo KY, Quah SLW, Garcia C, Leung KY, Chung A. The Clinical Frailty Scale as a Predictor of Trial of Void Outcomes in Men Undergoing Transurethral Resection of Prostate Surgery. Société Internationale d’Urologie Journal. 2023; 4(5):378-384. https://doi.org/10.48083/XVWY4791
Chicago/Turabian StyleZhuo, Kevin Yinkit, Samantha Li Wen Quah, Cindy Garcia, Ki Ying Leung, and Amanda Chung. 2023. "The Clinical Frailty Scale as a Predictor of Trial of Void Outcomes in Men Undergoing Transurethral Resection of Prostate Surgery" Société Internationale d’Urologie Journal 4, no. 5: 378-384. https://doi.org/10.48083/XVWY4791
APA StyleZhuo, K. Y., Quah, S. L. W., Garcia, C., Leung, K. Y., & Chung, A. (2023). The Clinical Frailty Scale as a Predictor of Trial of Void Outcomes in Men Undergoing Transurethral Resection of Prostate Surgery. Société Internationale d’Urologie Journal, 4(5), 378-384. https://doi.org/10.48083/XVWY4791




