Abstract
Background: Micro-ultrasound is a novel, high-resolution imaging modality that aims to improve the accuracy of prostate cancer diagnosis compared with TRUS-guided biopsy. While traditional ultrasound systems operate at 8 to 12 MHz, micro-ultrasound operates at 29 MHz, allowing enhanced recognition of microstructures with 300% higher resolution. Micro-ultrasound can potentially identify and target in real-time suspicious lesions, improving sensitivity and the negative predictive value for clinically significant prostate cancer. It may be a low-cost alternative to multiparametric magnetic resonance imaging (mpMRI) in the detection of prostate cancer. Methods: A systematic review and meta-analysis was performed comparing the diagnostic performance of micro-ultrasound-guided prostate biopsies with mpMRI-targeted prostate biopsies in the detection of clinically significant prostate cancer. PubMed, EMBASE, SCOPUS, and Cochrane CENTRAL databases were searched to identify relevant studies published up to July 2022. Results: A total of 15 studies were included for the systematic review, with 12 of those studies being included for the meta-analysis. The pooled sensitivity and specificity for micro-ultrasound-guided biopsies detecting clinically significant prostate cancer were 89% (95% CI 83 to 93) and 31% (95% CI 23 to 40) respectively (I2 = 0%). In comparison, the pooled sensitivity and specificity for mpMRI-targeted biopsies detecting clinically significant prostate cancer were 86% (95% CI 73 to 93) and 32% (95% CI 18 to 50) respectively (I2 = 16%). There was no statistically significant difference in the sensitivity or specificity between micro-ultrasound and mpMRI. Subgroup analysis found no difference in MRI subgroups based on blinding (P = 0.383). Conclusion: Micro-ultrasound-guided biopsies are comparable to mpMRI targeted biopsies with no difference in the detection of clinically significant prostate cancer between the 2 modalities. Large, multicentre, prospective studies are required to further substantiate the use of micro-ultrasound as an alternative to or in conjunction with mpMRI in the detection of prostate cancer.
Introduction
Prostate cancer (PCa) is the second most common solid tumour in men and the fifth most common cause of cancer mortality[1]. Prostate-specific antigen (PSA) testing and digital rectal examination (DRE) are commonly used to aid in the detection of prostate cancer. Most commonly, pathological diagnosis has been made in recent decades using TRUS (transrectal ultrasound) guided 10 to 12 core systematic biopsy[2]. However, the use of PSA, DRE, and TRUS has limitations. Clinically significant prostate cancer can potentially be missed, a large prostate secondary to benign prostatic hypertrophy can decrease the sampling power of systematic biopsy, and elevated PSA levels are not specific to clinically significant prostate cancer[3].
Multiparametric magnetic resonance imaging (mpMRI)
MRI has been verified in the diagnosis of prostate cancer by studies that showed a high sensitivity and negative predictive value in the detection of clinically significant prostate cancer[4,5]. mpMRI uses 3 multiple imaging sequences; typically, these are T2, dynamic contrast-enhanced T1 images, and diffusion-weighted images[6]. mpMRI before biopsy in biopsy-naïve patients can improve the detection of clinically significant prostate cancer[7]. The Prostate Imaging Reporting and Data System (PI-RADS) was formulated to standardise the interpretation and reporting of mpMRI of the prostate[8]. A high PI-RADS score is an important predictor for clinically significant prostate cancer[9]. There are, however, disadvantages to MRI, including cost, risk of renal injury with use of contrast agents, potential lack of availability and the inability to use MRI on patients with contraindications[10].
Micro-ultrasound imaging
Micro-ultrasound is a novel high-resolution imaging modality developed by Exact imaging (Toronto, Ontario, Canada), which aims to improve the accuracy of prostate cancer diagnosis compared with TRUS-guided biopsy. It was initially assessed on patients awaiting a radical prostatectomy in 2013, which found it to be superior to conventional TRUS[11].
While traditional ultrasound systems operate at 8 to 12 MHz, micro-ultrasound operates at 29 MHz allowing enhanced recognition of microstructures with a resolution to 70 mm, 300% higher than previous[12]. 70 mm is in keeping with the diameter of a prostatic duct enabling identification of ductal anatomy in addition to cellular density[13]. The ability to differentiate varying cellular densities facilitates the detection of tissue arrangements related to prostate cancer[14]. As a result, micro-ultrasound can potentially identify and target in real-time suspicious lesions, thus improving sensitivity and the negative predictive value for clinically significant prostate cancer[13]. It also has the potential to detect clinically significant prostate cancer not identified on mpMRI[15]. Two previous studies found micro-ultrasound to have similar detection rates for prostate cancer to mpMRI[10,12]. Additionally, it offers advantages such as lower cost and a short learning curve[16].
PRI-MUS protocol
PRI-MUS (Prostate Risk Identification using Micro-Ultrasound) is a protocol and standardised risk identification system used with high resolution (29 MHz) transrectal micro-ultrasound to assess and grade the risk of prostate cancer[17]. It is comparable with PI-RADS for mpMRI and also utilises a scale between 1 and 5 for suspicion of cancer.
Micro-ultrasound-guided prostate biopsy versus mpMRI-guided prostate biopsy
The aim of this project is to compare the diagnostic performance of micro-ultrasound-guided prostate biopsy versus multiparametric magnetic resonance imaging-targeted prostate biopsy in the detection of clinically significant prostate cancer in men with a clinical suspicion of prostate cancer.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and the diagnostic test accuracy studies (PRISMA-DTA) checklist[18,19]. The review was registered in the PROSPERO database (awaiting ID number).
Search strategy
PubMed, Scopus, Embase, and CENTRAL databases were systemically searched from their establishment to July 2022 for studies comparing the diagnostic accuracy of micro-ultrasound-guided prostate biopsies to mpMRI-guided prostate biopsies for clinically significant prostate cancer (Gleason Grade Group ≥ 2)[20]. The systematic search strategy was designed for PubMed and modified accordingly. The comprehensive search syntax and string can be found in Online Supplementary Appendices 1 & 2. Reference lists of all relevant studies and reviews were checked for additional studies.
Inclusion and exclusion criteria
The study was designed in line with the PICO (Population, Intervention, Comparator, Outcome) model[21]. The population being investigated are men with clinical suspicion of prostate cancer based on PSA level and/or an abnormal digital rectal examination. Micro-ultrasound-guided prostate biopsy is the intervention, while the comparator is mpMRI-guided prostate biopsy. Finally, the outcome is clinically significant prostate cancer (Gleason Grade Group ≥ 2).
The following inclusion and exclusion criteria were applied:
We included
- Studies comparing the diagnostic performance of micro-ultrasound-guided biopsy versus mpMRI-guided biopsy.
- Studies published in any language until July 2022.
- Prospective or retrospective studies.
We excluded
- Studies comparing micro-ultrasound-guided biopsy or multiparametric MRI-targeted biopsy versus only systematic ultrasound-guided prostate biopsy.
- Studies including patients undergoing only micro-ultrasound or mpMRI-targeted prostate biopsy.
- Case reports.
- Animal or cadaveric studies.
- Secondary research studies.
- Patients already diagnosed with prostate cancer or on active surveillance protocol.
When records had overlapping patient populations, the most recent study or the study with the most available data was included.
Quality assessment and data extraction
The abstracts of all studies found from the above search strategy were screened to exclude irrelevant studies. The inclusion and exclusion criteria were applied to the remaining full-text articles. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess for risk of bias and applicability concerns in the studies[22].
With regard to data extraction, the number of true and false positives, and true and false negatives from micro-ultrasound-guided and mpMRI-guided biopsy were obtained. When these data were not accessible, they were calculated from the relevant sensitivity, specificity, prevalence, and sample size. If these data were also not available, then the author of the paper was emailed requesting the data.
Data synthesis and statistical analysis
The index test (micro-ultrasound-guided prostate biopsy) provided a binary outcome (presence or absence of prostate cancer) at a patient level. The data was transferred to 2 × 2 matrices in order to calculate the sensitivity and specificity for each included study. Individual study data were presented graphically as forest plots and summary receiver operating characteristics (SROC). The bivariate random-effects model was used to acquire summary estimates of the test sensitivity and specificity. A 95% confidence interval was produced from the bivariate model in addition to a combined estimate according to the evaluation method used. The statistical analysis was undertaken using R version 4.2.1.
The bivariate model with covariates was used to examine heterogeneity. Where the data were available, subgroup analyses were performed to ascertain if individual covariates or patterns of covariates affected heterogeneity. If the data for the outlined subgroups were incomplete, the data were presented as SROC or forest plots to prevent the meta-analysis of low-powered data.
For studies with both a low risk of bias and low concerns regarding applicability for the reference standard (domain 3 of the QUADAS-2 tool outlined in the appendix), sensitivity analyses were performed.
Results
Our search identified 2967 studies, and 2 studies were found from references. After the titles and abstracts of the studies were screened and duplicates removed, 2904 studies were excluded. The remaining 65 studies were reviewed in full text. A further 50 studies were excluded with the application of the inclusion and exclusion criteria (Online Appendix 3). The flow diagram for the study selection process can be found in Online Appendix 4. We then also randomly screened 5% of the total studies identified.
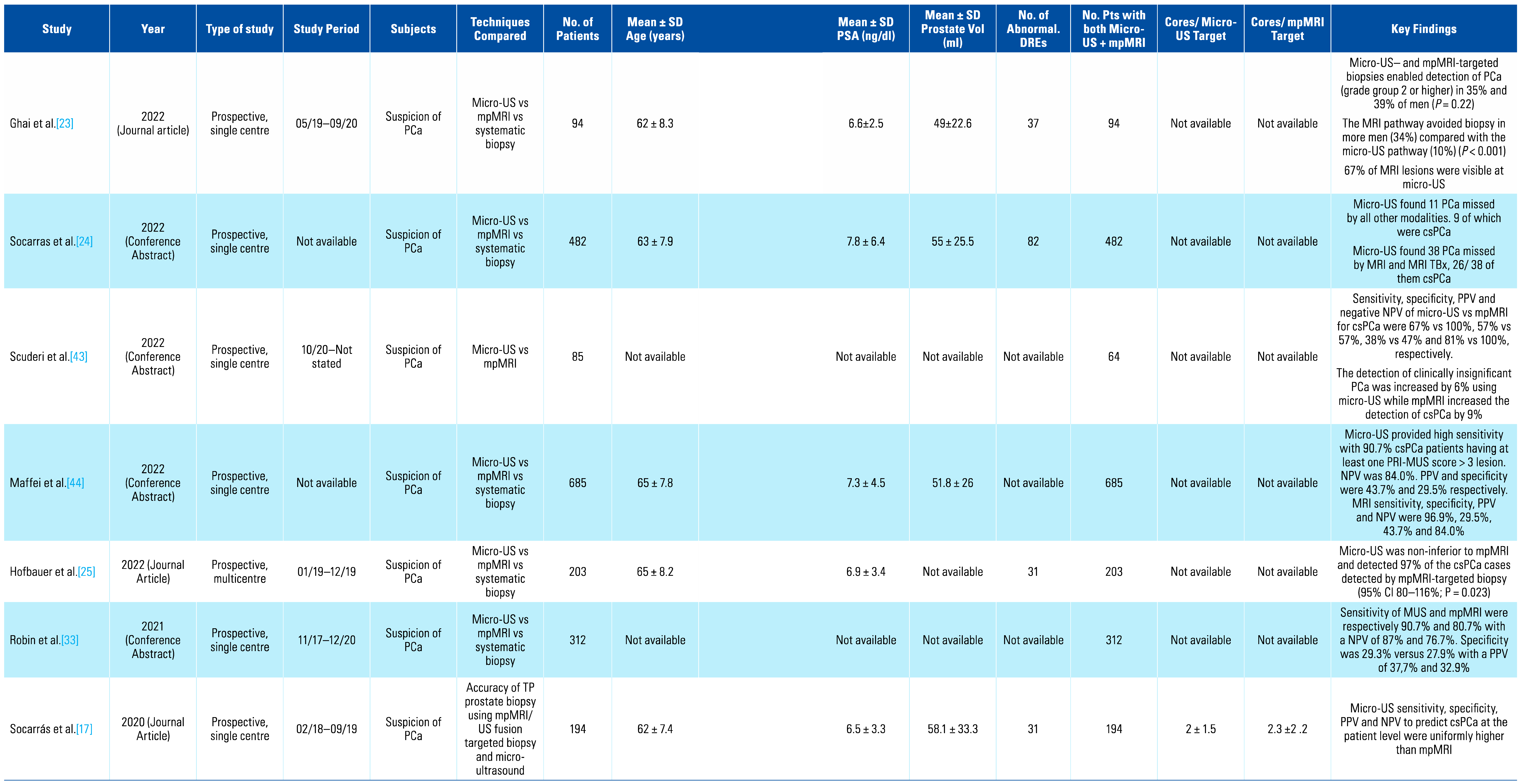
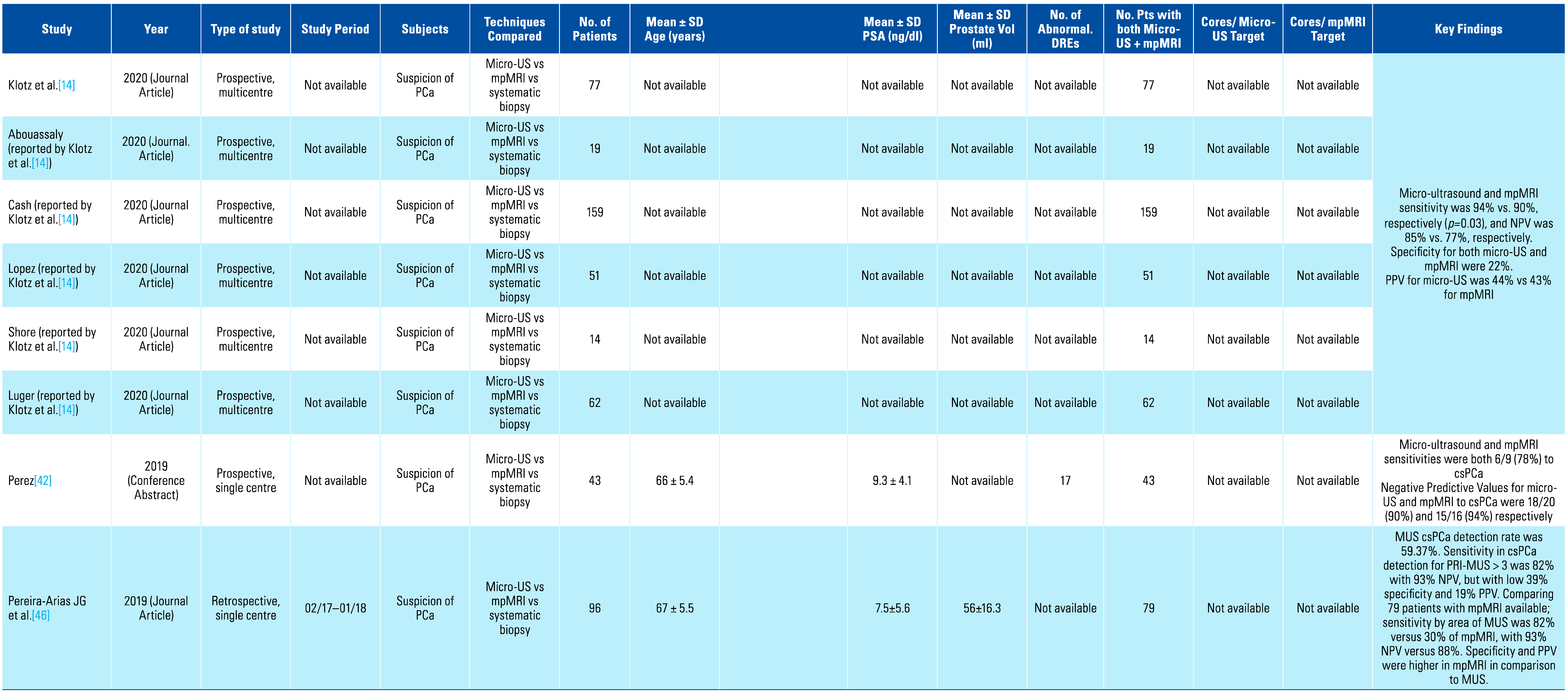
A total of 15 studies were included in the systematic review, with 12 of those being included in the meta-analysis, while the other 3 had insufficient data[23,24,25]. Table 1 shows the characteristics and key findings of the included studies. The total number of patients included in the meta-analysis was 1759.

Table 1.
Summary of studies included in systematic review.
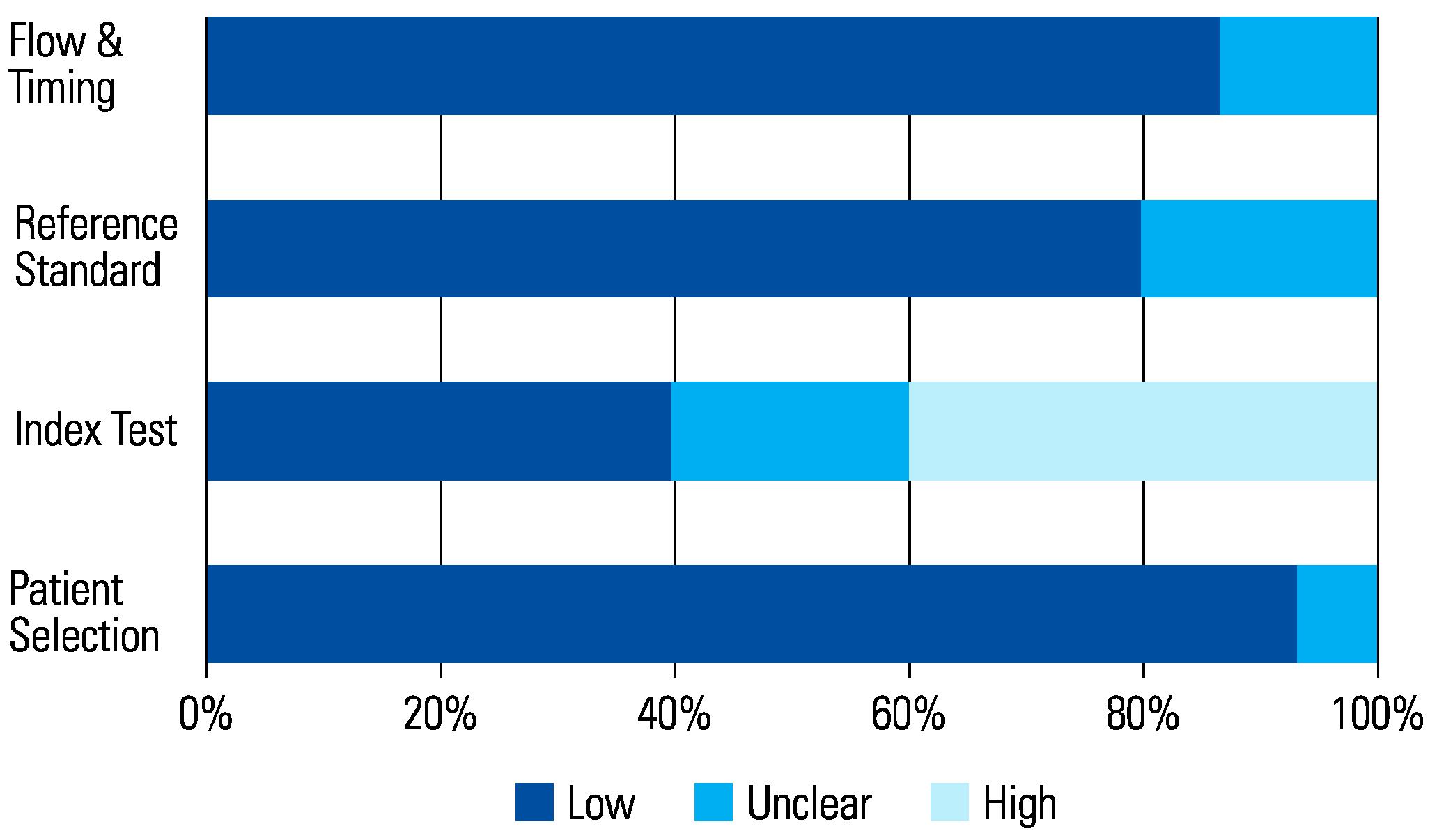
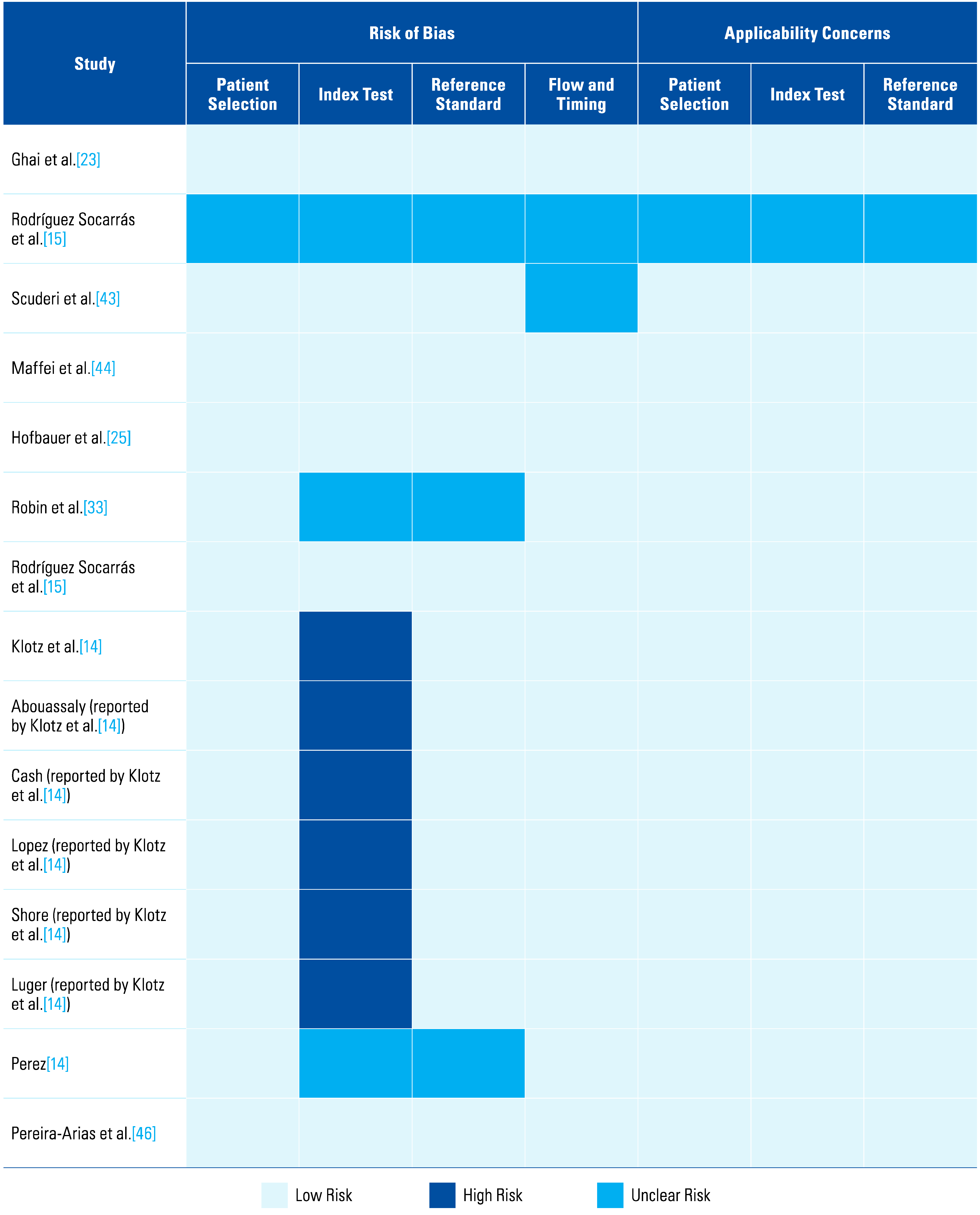
The assessment of each study using the QUADAS-2 tool is shown in Table 2 The risk of bias was determined to be high in 6 studies that scored a high risk in the index test (Figure 1 and Figure 2). This was because in these studies, urologists performed the micro-ultrasound biopsies unblinded to the mpMRI results. The operators in 3 of the studies were blinded to the mpMRI results while in the other 3, it is unclear.

Table 2.
QUADAS-2 Tool.
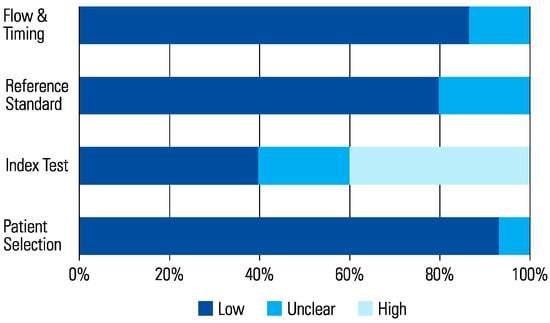
Figure 1.
Risk of Bias.
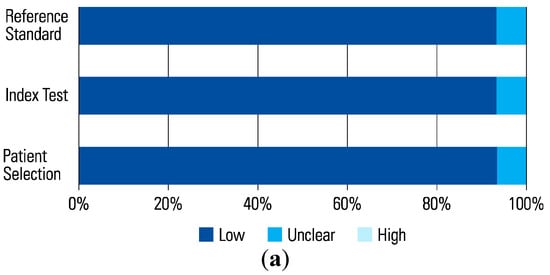
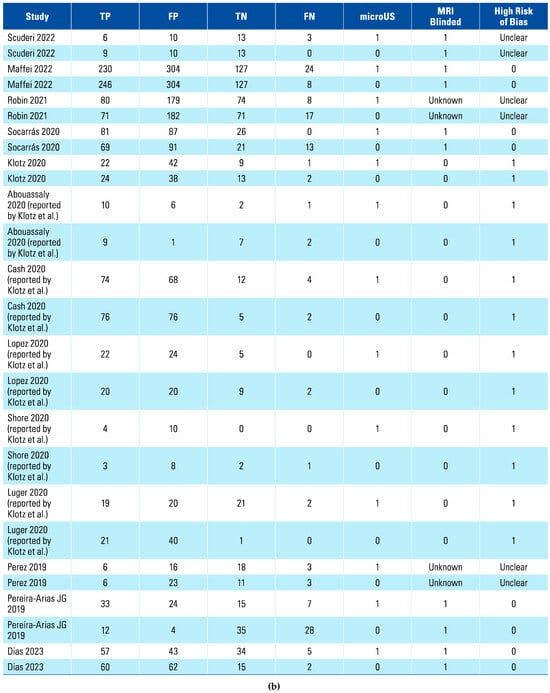
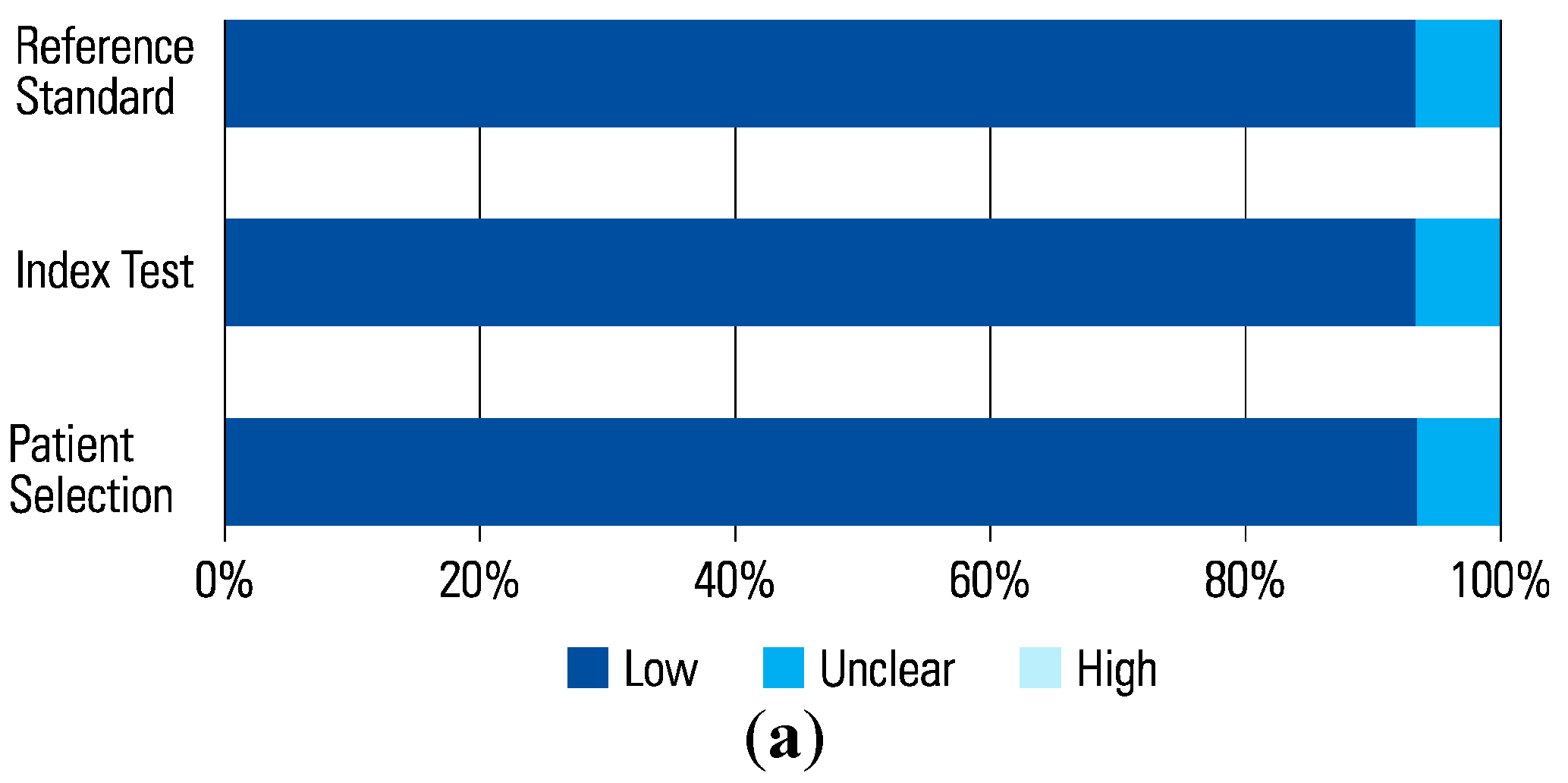
Figure 2.
(a) Applicability Concerns. (b) Data Updated with Subgroup + Bias 2023.
To date there have been a limited number of prospective studies comparing the diagnostic performance of micro-ultrasound and mpMRI-guided biopsies. The 3 studies excluded from the meta-analysis provided data for the detection rate of micro-ultrasound and mpMRI-guided biopsies only[23,24,25]. Hofbauer et al. reported that micro-ultrasound was non-inferior to mpMRI in the diagnosis of clinically significant prostate cancer, detecting 97% of the cases detected by mpMRI-guided biopsy[25].
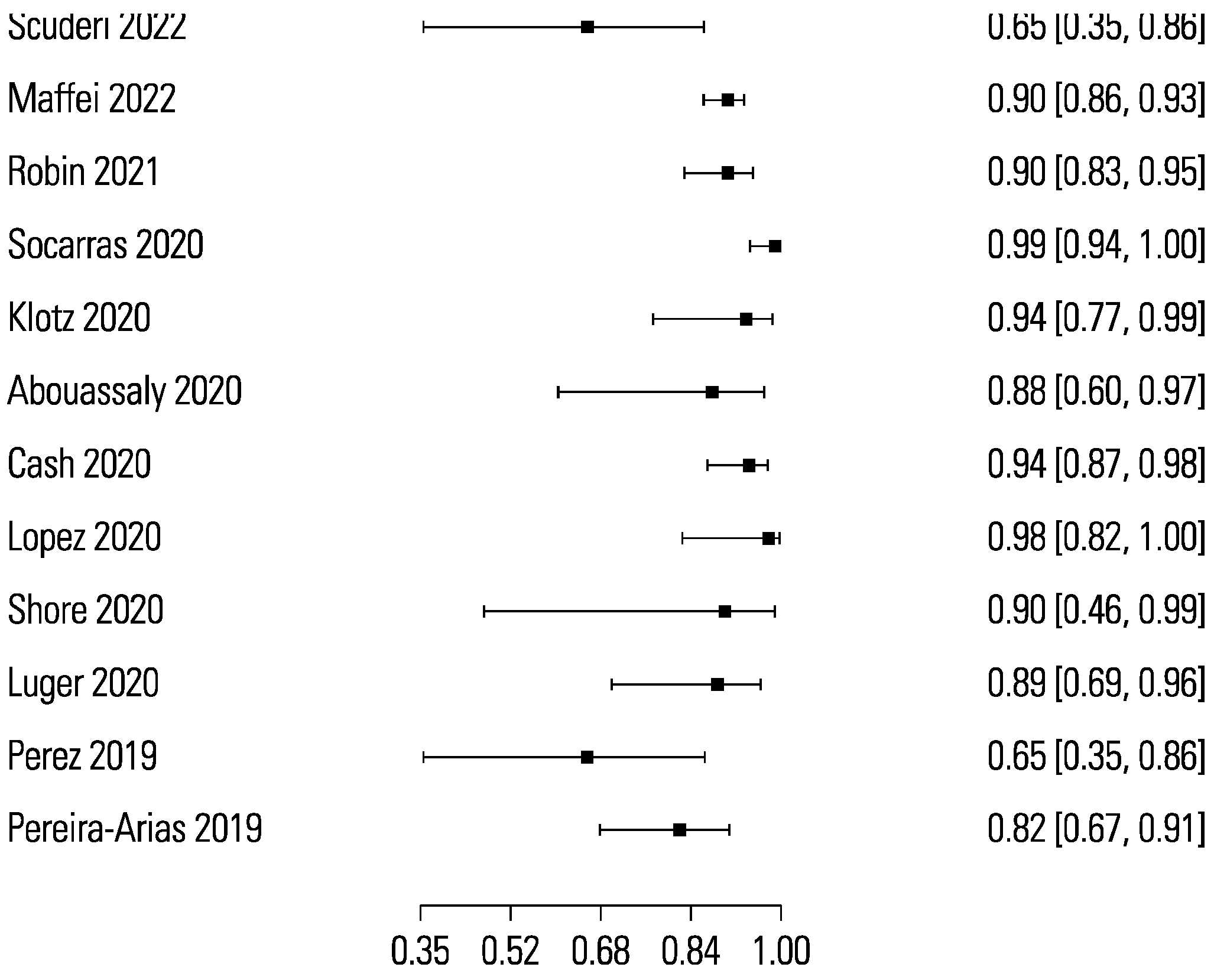
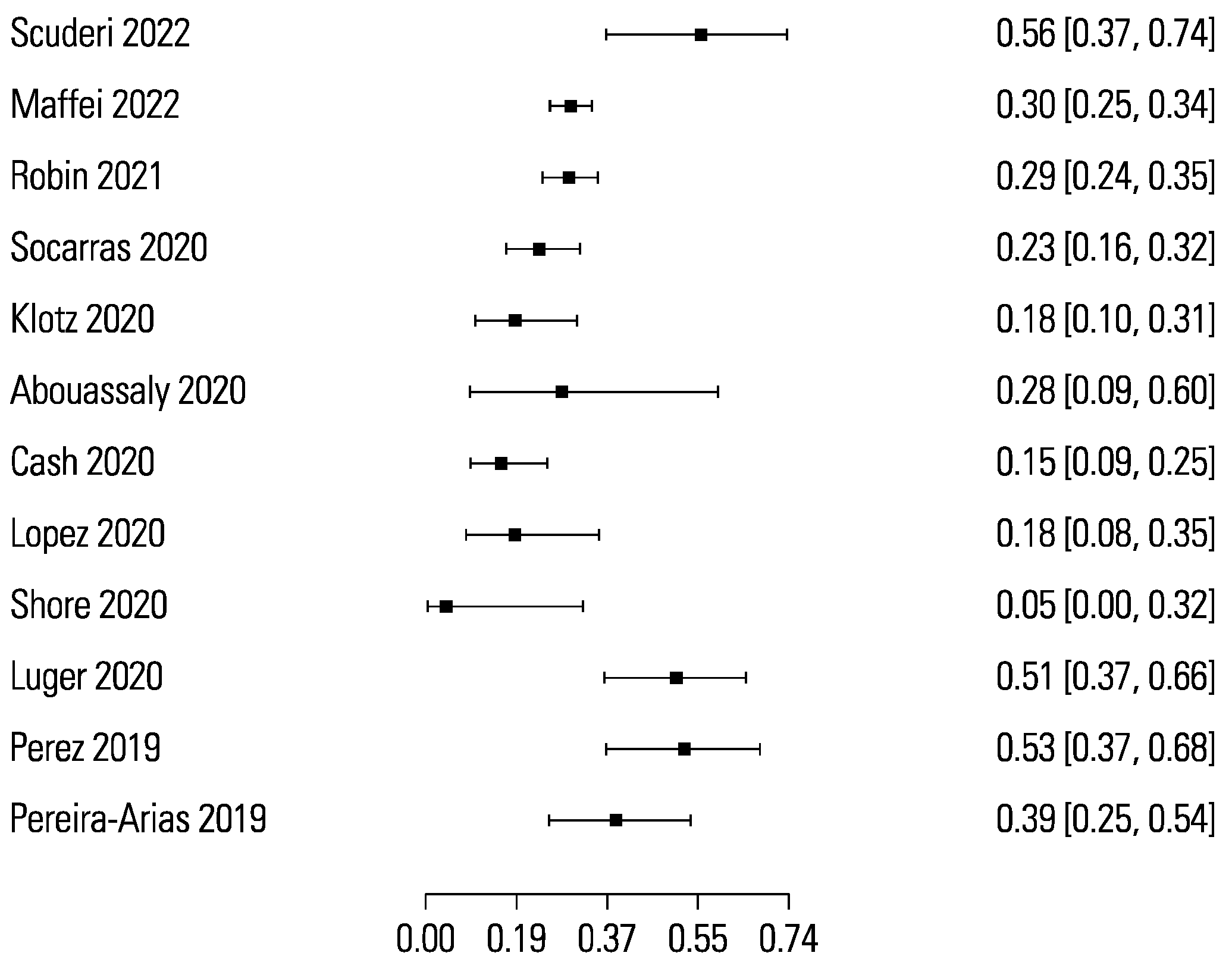
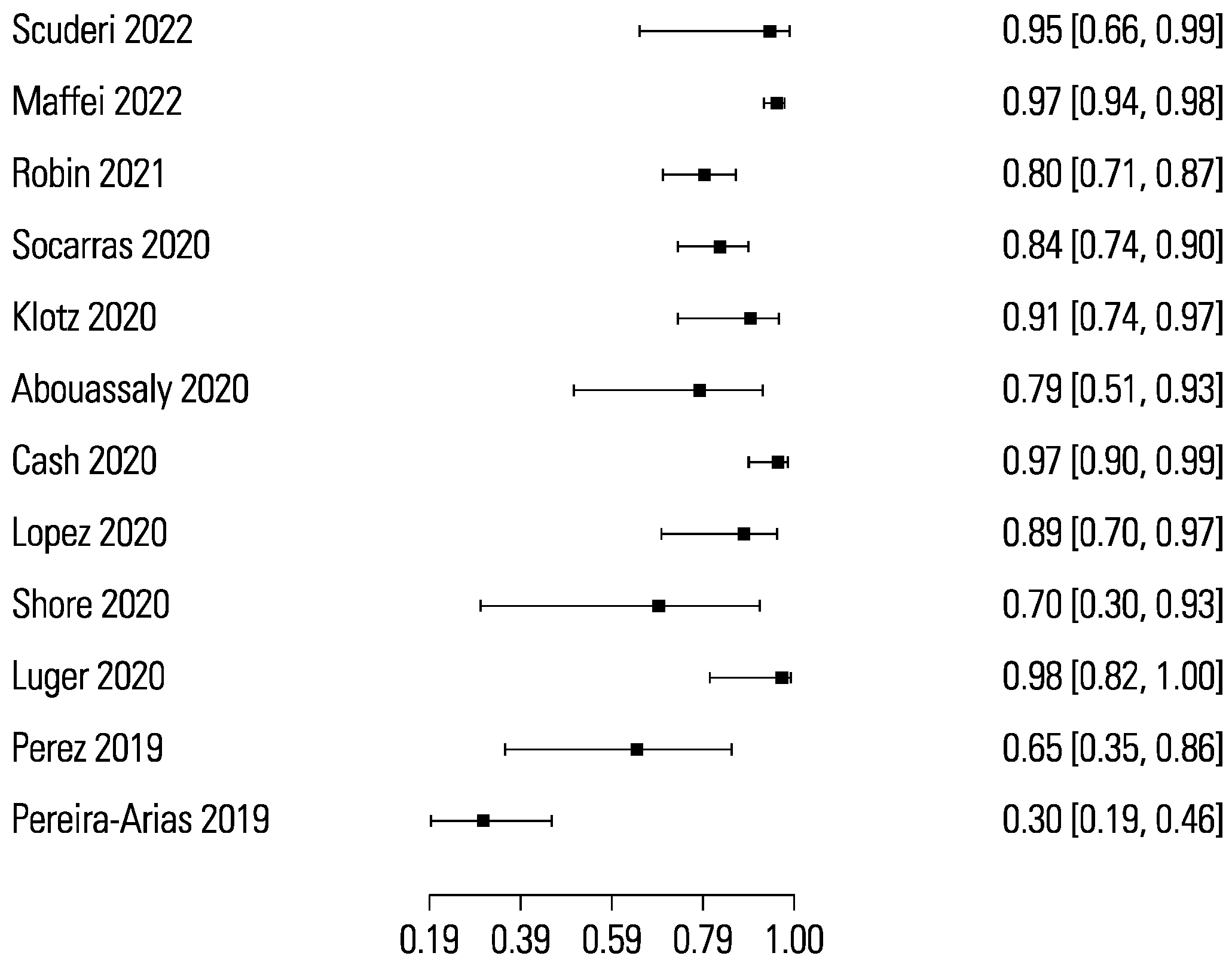
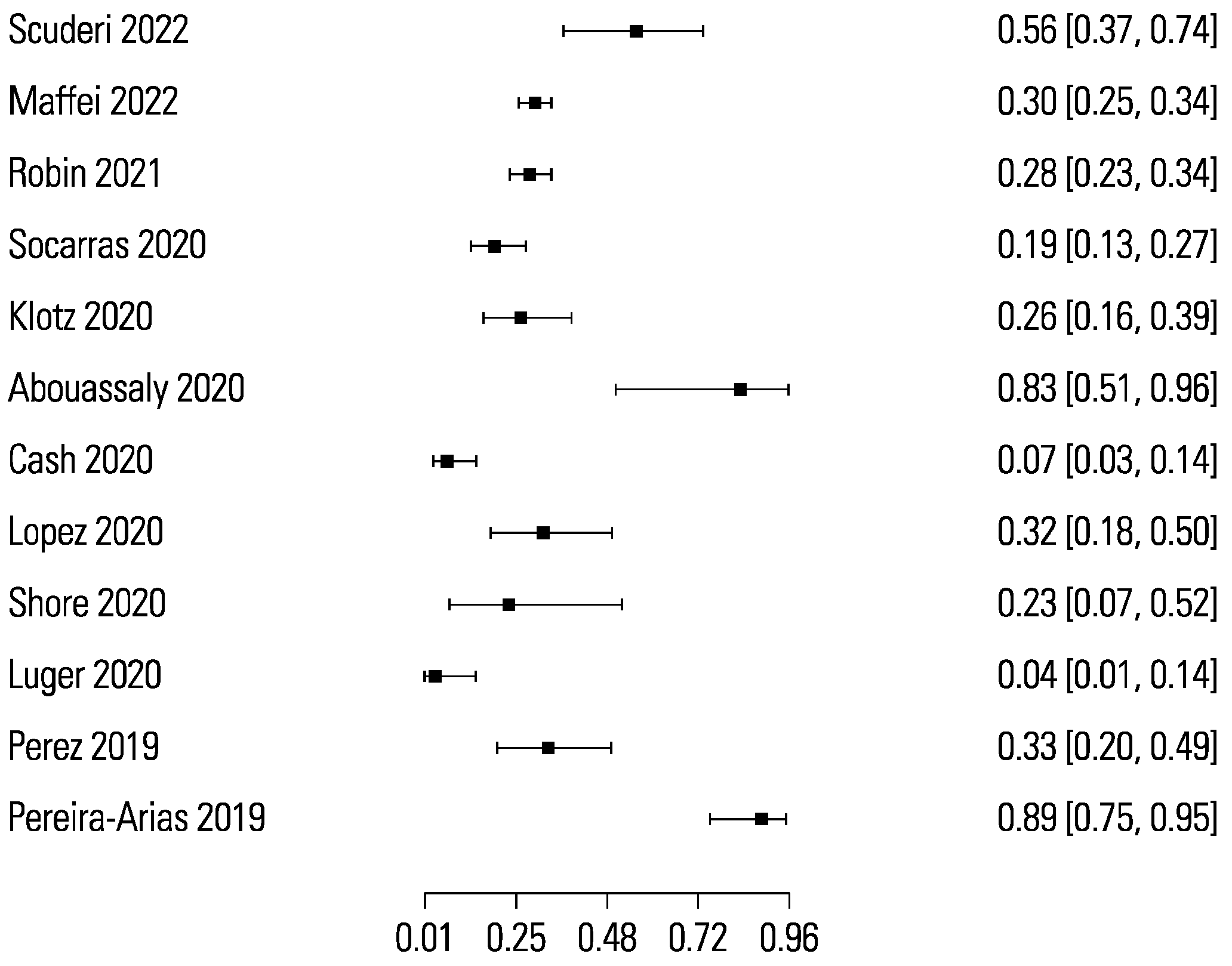
The pooled sensitivity and specificity for micro-ultrasound-guided biopsies detecting clinically significant prostate cancer were 89% (95% CI 83 to 93) and 31% (95% CI 23 to 40), respectively (Figure 3 and Figure 4). The area under the sROC curve for micro-ultrasound was 0.665. In comparison, the pooled sensitivity and specificity for mpMRI-targeted biopsies detecting clinically significant prostate cancer were 86% (95% CI 73 to 93) and 32% (95% CI 18 to 50), respectively (Figure 5 and Figure 6). The area under the sROC curve for mpMRI was 0.667.
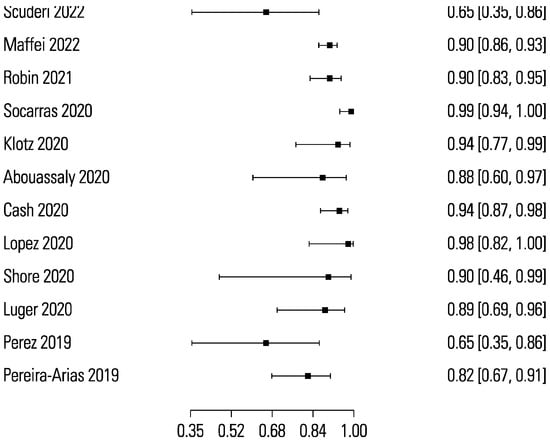
Figure 3.
Forest plot of micro-ultrasound sensitivity.
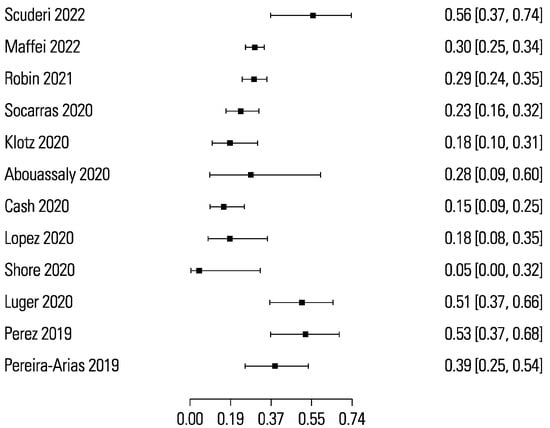
Figure 4.
Forest plot of micro-ultrasound specificity.
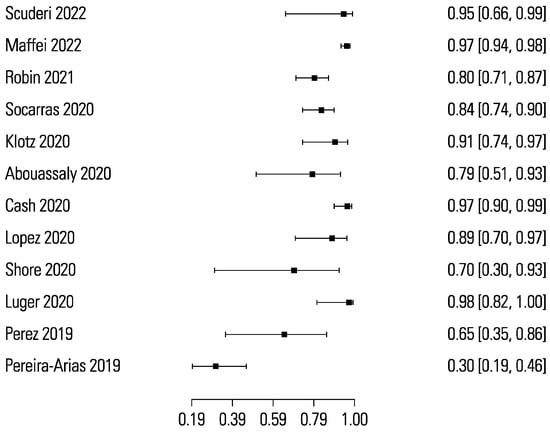
Figure 5.
Forest plot of mpMRI sensitivity.
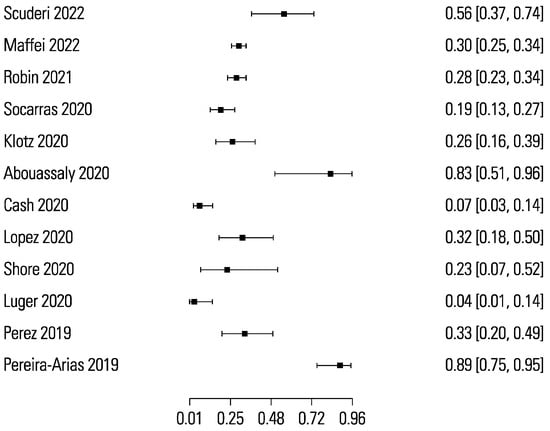
Figure 6.
Forest plot of mpMRI specificity.
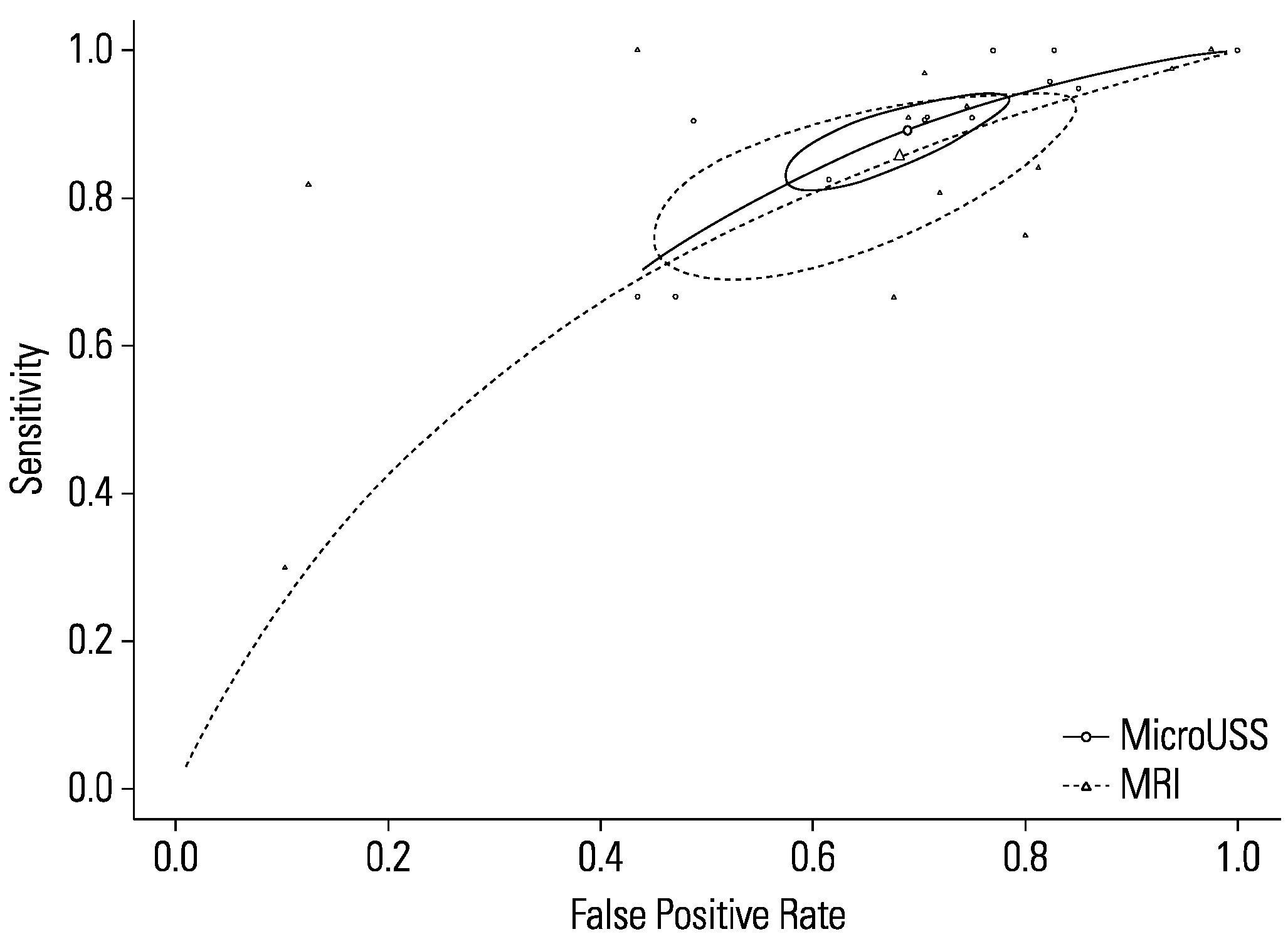
Comparing the sensitivity between micro-ultrasound and mpMRI (P = 0.46) showed no statistically significant difference (Figure 7). Likewise, the specificity between micro-ultrasound and mpMRI (P = 0.88) demonstrated no statistically significant difference. Heterogeneity for both micro-ultrasound (I2 = 0%) and mpMRI (I2 = 16%) were low. MRI subgroup analysis based on blinding of mpMRI results demonstrated no significant difference (P = 0.383).
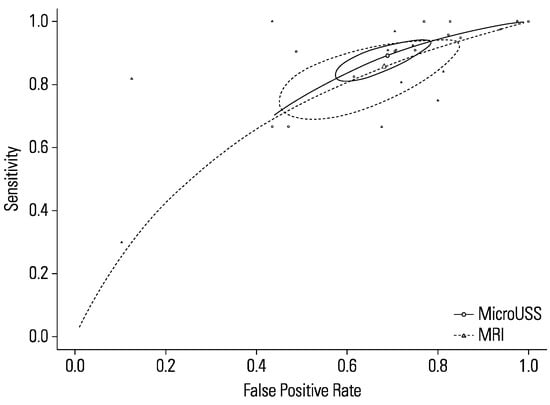
Figure 7.
Comparison of micro-ultrasound and mpMRI.
Discussion
Adding micro-ultrasound to the mpMRI diagnostic pathway
Ghai et al. proposed adding both micro-ultrasound and mpMRI to the diagnostic pathway to optimise the detection of clinically significant prostate cancer[23]. Micro-ultrasound can potentially identify clinically significant prostate cancer not recognised on MRI[23].
Additionally, Avolio et al. suggested that micro-ultrasound could potentially stratify patients with an equivocal mpMRI[26]. Using micro-ultrasound, they assessed patients with a PI-RADS 3 lesion on MRI and found clinically significant prostate cancer in 38.7% of cases with an overall sensitivity and NPV of 100%[26]. Avolio et al. looked at using micro-ultrasound in patients with a negative MRI but persistent suspicion of prostate cancer and found clinically significant prostate cancer in 25% of patients with at least 1 PRI-MUS ≥ 3 lesion[27].
Abouassaly et al. reported that replacing conventional transrectal ultrasound with micro-ultrasound resulted in a relative increase in the detection rate of 26.7% (P < 0.09) and significantly increased the average grade of prostate cancer detected (P < 0.01)[28]. The widespread use of micro-ultrasound instead of conventional ultrasound could optimise outcomes with prostate biopsy and potentially alter the risk–benefit profile of prostate screening, although at a potentially higher cost. Future studies will be needed to conduct cost-benefit analyses to determine if the use of micro-ultrasound in this setting on a large scale is viable.
The ongoing OPTIMUM trial is a 3-arm randomized controlled trial exploring how microUS guided biopsy compares with MRI/US fusion and MRI/MicroUS “contour-less” fusion. It will examine the diagnostic performance of micro-ultrasound by itself and in combination with mpMRI in the detection of clinically significant prostate cancer as well assessing its economic impact[29].
Previous meta-analyses
Two previous meta-analyses showed micro-ultrasound-guided biopsies to be comparable to mpMRI-guided prostate biopsies in the detection of prostate cancer[10,12]. However, both included studies with patients on active surveillance protocol, possibly overestimating the sensitivity of micro-ultrasound. Sountoulides et al. found similar detection rates of both clinically significant and insignificant prostate cancer as well as the overall detection rate for prostate cancer between micro-ultrasound-guided and mpMRI-targeted prostate biopsies[12]. However, they did include studies that required patients to have an equivocal or positive MRI (PI-RADS ≥ 3) in their meta-analysis, which were excluded from this meta-analysis[30,31,32]. You et al. also concluded from their meta-analysis that micro-ultrasound and mpMRI-guided biopsies had similar detection rates of prostate cancer[10]. However, they also included studies that required an equivocal or positive MRI[30,32].
Current meta-analysis
This meta-analysis demonstrates that there is no difference in detection of clinically significant prostate cancer between micro-ultrasound-guided biopsies and mpMRI-targeted biopsies.
Robin et al. found similar accuracy between micro-ultrasound and mpMRI[33]. They noted a small number of cases diagnosed based uniquely on micro-ultrasound (4.1%) and mpMRI (1.7%). The paper by Klotz et al. looking at multiple centres comparing micro-ultrasound and mpMRI provided complete data for 6 of the studies used in the meta-analysis[34]. Some of these centres had later publications; however, they lacked the relevant data for the meta-analysis. Overall, Klotz et al. determined that micro-ultrasound had comparable or higher sensitivity for clinically significant prostate cancer with a specificity similar to mpMRI[34]. Those results were in the context of this being the first exposure to micro-ultrasound for many of the centres involved. They hypothesised that specificity for micro-ultrasound would improve with more experience and validation[34].
Advantages of micro-ultrasound
Previous studies have demonstrated a short learning curve for operating the micro-ultrasound and interpreting the images[17,35]. The receiver operator characteristic curve stabilises for micro-ultrasound after approximately 15 cases[36]. Abouassaly et al. suggest that as operators get more familiar with the use of micro-ultrasound, it may further improve its diagnostic performance[28].
Micro-ultrasound could allow the detection and biopsy of suspicious prostatic lesions using the same modality in the same procedure[36]. This is in contrast to the mpMRI diagnostic pathway, which requires at least 2 procedures: the initial mpMRI and subsequent prostate biopsy. Also, the mpMRI diagnostic pathway requires referral to radiology and radiologists specialised in the interpretation of prostate MRI[29]. As a result, the addition of mpMRI to the diagnostic pathway for prostate cancer has added complexity and the potential for delayed diagnosis. In the UK, the median length of time to diagnosis of prostate cancer is approximately 55.5 days, while the median time to diagnosis for all cancers is 40 days[37]. mpMRI-guided prostate biopsies are usually carried out with the assistance of a conventional transrectal ultrasound probe, introducing a potential source of error when switching between the 2 modalities[12].
Currently, mpMRI is at least 10 times more expensive than conventional ultrasound[38]. The ability to fund MRI before prostate biopsy varies across health care systems, with access to mpMRI in developing countries being particularly limited[38]. In contrast micro-ultrasound is a low-cost, single-session modality for prostate screening and guided biopsy[34]. Like conventional ultrasound, micro-ultrasound has the advantage of being portable, making it considerably more convenient than MRI. Other benefits of micro-ultrasound are that it does not require contrast and that most urologists already possess expertise and familiarity with ultrasound as a modality.
Disadvantages of micro-ultrasound
Micro-ultrasound evaluation of the anterior gland can be limited. This is in part due to the greater depth from the rectum, as well as potential calcification in the corpora amylacea causing shadowing[23]. Another disadvantage of micro-ultrasound is that the PRI-MUS protocol does not currently include evaluation of the anterior or transition zone[23]. In one study, 43% of the cases of clinically significant prostate cancer missed by micro-ultrasound but detected by mpMRI were anterior lesions, while another 29% were in the transitional zone[25]. In another study, 60% of the cases of clinically significant prostate cancer missed by micro-ultrasound were in the anterior transition zone[23]. The depth of tissue penetration with micro-ultrasound is limited to approximately 5 cm presently, limiting its ability to detect suspicious lesions in the anterior and transition zone, especially in larger prostates[36]. Nevertheless, future software updates can be expected to permit enhanced tissue penetration and diagnostic performance for the anterior prostate[36]. Future studies will be needed to methodically characterise suspicious features on micro-ultrasound of the anterior and transition zone to allow it to be validated and eventually incorporated into a future expanded PRI-MUS protocol.
Micro-ultrasound can assess the morphology of the prostate gland at a higher resolution than the T2-weighted imaging sequence with mpMRI[23]. However, mpMRI has a functional assessment component that micro-ultrasound lacks. Dynamic contrast-enhanced sequences and diffusion-weighted imaging allow further evaluation of suspicious areas[23].
Prostatic calcifications or benign prostatic hyperplasia occurring in the transition zone can result in acoustic shadowing posteriorly, obscuring the clinical picture and potentially affecting the accuracy of micro-ultrasound[25].
Limitations
This study has a number of limitations. mpMRI-guided prostate biopsy is not an ideal reference standard. Approximately 15% of cases of clinically significant prostate cancer can go undetected on mpMRI in biopsy-naïve men[39]. There is considerable and widespread inter-reader variability in the interpretation of mpMRI of the prostate[29]. It is unclear to what degree inter-reader variability exists in the interpretation of micro-ultrasound and the use of the PRI-MUS protocol.
Whole-mount pathology following radical prostatectomy has previously been used as a reference standard when testing the diagnostic performance of mpMRI[40]. Indeed, surgical pathology is considered the gold standard[34]. However, there is a paucity of studies comparing micro-ultrasound to whole-mount pathology. Two such studies found its diagnostic performance to be similar to mpMRI[36,41]. Lorusso et al., examining micro-ultrasound and mpMRI performance in patients with biopsy-proven prostate cancer scheduled for radical prostatectomy to whole-mount pathology, found it to be comparable to mpMRI[36]. Likewise, Brisbane et al., investigating micro-ultrasound on MRI-guided biopsy-proven prostate cancer patients, concluded its diagnostic ability was comparable to mpMRI when using whole-mount pathology as a reference standard[41]. Further studies are required to compare micro-ultrasound-targeted biopsy with mpMRI-guided biopsy using whole-mount pathology as a reference standard.
In addition, some studies were conference abstracts that were not peer reviewed. The study protocol differed across the studies. It is not clear how studies interpreted borderline lesions. For instance, in the multicentre paper by Klotz et al., some centres biopsied them comprehensively while other centres did not include them[34]. Another limitation of this meta-analysis is that it does not provide a breakdown of the diagnostic performance of micro-ultrasound versus mpMRI with regard to zones of the prostate. In a number of the studies, the urologists performing the micro-ultrasound-guided biopsies were not blinded to the mpMRI results, which may have given rise to operator bias.
It was hoped to perform further subgroup analysis to investigate heterogeneity in the studies further. However, there were insufficient data to assess patients with ISUP Gleason grade groups, PI-RADS/PRI-MUS grades, and mpMRI specifications. There were insufficient data available to allow for a meta-analysis of the diagnostic performance of micro-ultrasound in the detection of clinically insignificant prostate cancer.
Owing to limited data available from the selected studies, the detection of clinically insignificant prostate cancer was not explored. Only Perez and Socarras et al. had sufficient data to investigate the diagnostic performance of micro-ultrasound in the detection of clinically insignificant prostate cancer[24,42]. Additionally, Maffei et al. and Scuderi et al. had limited data on the detection of clinically insignificant prostate cancer, with Scuderi et al. noting that micro-ultrasound increased its detection by 6%, while mpMRI increased the detection of clinically significant prostate cancer by 9%[43,44].
An important limitation is that in most studies, micro-ultrasound was performed in MRI-preselected men. Socarras et al. included suspicious DRE, elevated PSA, or visible lesion on mpMRI as their criteria[15]. Robin et al. included patients with or without a suspicious MRI while in studies by Maffei et al. and Perez’s, patients with an available MRI were included; however, it is unclear if the operator was blinded to the MRI results before micro-ultrasound-guided biopsy[33,42,44]. Scuderi et al. had patients with clinical suspicion of prostate cancer receive both mpMRI and micro-ultrasound in a randomized sequence with the radiologist being blinded to the micro-ultrasound result[43]. It is unclear whether they were also blinded to the mpMRI results. Klotz et al. and Abouassaly, Cash, Lopez, Shore, and Luger (as reported by Klotz et al.) required patients with an already completed mpMRI and were all unblinded to the mpMRI results[34]. As a result, there is a significant selection bias in the patients enrolled in these studies. The potential role of micro-ultrasound as an upfront triage tool for men with clinical suspicion of prostate cancer remains unknown. Future studies will need to prospectively enrol patients before they receive mpMRI and ensure the operators are blinded to the results of both mpMRI and micro-ultrasound. The OPTIMUM trial seeks to address this by aiming to have patients receive MRI after study enrolment and randomization unless they had an mpMRI completed before enrolment, in which case the operator will be blinded to results[29].
This study did not seek to determine which modality would avoid more biopsies. Ghai et al. noted that micro-ultrasound had a higher number of false positives compared with mpMRI and that mpMRI resulted in more biopsies being avoided (34% vs 10%)[23]. Lughezzani et al reported that micro-ultrasound has a lower specificity and positive predictive value versus mpMRI in patients with clinical suspicion of prostate cancer and a positive mpMRI[45]. Future studies will need to determine which modality can avoid more biopsy cores being taken.
Conclusion
Micro-ultrasound may be a viable alternative to mpMRI in the detection of prostate cancer. Large prospective multicentre double-blinded studies with homogenous populations of men with clinical suspicion of prostate cancer are required before it could potentially be included in clinical guidelines and practice. Furthermore, future studies will need to assess the diagnostic performance of micro-ultrasound in the detection of clinically insignificant prostate cancer, by zones in the prostate as well as assess the suspicious characteristics in the anterior and transition zones of the prostate to enable the expansion of the PRI-MUS protocol to include these zones.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2563-6499/4/6/465/s1.
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; et al. Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, A.; Alipour, V.; Moradi, N.; Arabloo, J. Cost-effectiveness of multiparametric magnetic resonance imaging and targeted biopsy versus systematic transrectal ultrasound-guided biopsy for prostate cancer diagnosis: A systematic review. Value Health Reg. Issues 2022, 30, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kim, C.K. Paradigm shift in prostate cancer diagnosis: Pre-biopsy prostate magnetic resonance imaging and targeted biopsy. Korean J. Radiol. 2022, 23, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alazeez, M.; Ahmed, H.U.; Arya, M.; Charman, S.C.; Anastasiadis, E.; Freeman, A.; et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level--can it rule out clinically significant prostate cancer? Urol. Oncol. 2014, 32, 45–e17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Bass, E.J.; Pantovic, A.; Connor, M.; Gabe, R.; Padhani, A.R.; Rockall, A.; et al. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2021, 24, 596–611. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; et al. PI-RADS Prostate imaging - reporting and data system: 2015, version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Rajwa, P.; Mori, K.; Huebner, N.A.; Martin, D.T.; Sprenkle, P.C.; Weinreb, J.C.; et al. The prognostic association of prostate MRI PI-RADS™ v2 assessment category and risk of biochemical recurrence after definitive local therapy for prostate cancer: A systematic review and meta-analysis. J. Urol. 2021, 206, 507–16. [Google Scholar] [CrossRef]
- You, C.; Li, X.; Du, Y.; Peng, L.; Wang, H.; Zhang, X.; Wang, A. The microultrasound-guided prostate biopsy in detection of prostate cancer: A systematic review and meta-analysis. J. Endourol. 2022, 36, 394–402. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Cornish, T.C.; Mullins, J.K.; Fradin, J.; Mettee, L.Z.; Connor, J.T.; et al. High-resolution transrectal ultrasound: Pilot study of a novel technique for imaging clinically localized prostate cancer. Urol. Oncol. 2014, 32, 34–e27. [Google Scholar] [CrossRef] [PubMed]
- Sountoulides, P.; Pyrgidis, N.; Polyzos, S.A.; Mykoniatis, I.; Asouhidou, E.; Papatsoris, A.; et al. Micro-ultrasound-guided vs multiparametric magnetic resonance imaging-targeted biopsy in the detection of prostate cancer: A systematic review and meta-analysis. J. Urol. 2021, 205, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.B.; O’Brien, C.; Correas, j.-M.; Ghai, S. Multiparametric ultrasound and micro-ultrasound in prostate cancer: A comprehensive review. Br. J. Radiol. 2022, 95. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.C.M. Can high resolution micro-ultrasound replace MRI in the diagnosis of prostate cancer? Eur. Urol. Focus. 2020, 6, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Socarras, M.; Gomez Rivas, J.; Cuadros, V.; Reinoso Elbers, J.; Llanes Gonzalez, L.; Juarez Del Dago, P.; et al. Prostate mapping for cancer diagnosis: The Madrid protocol. Transperineal prostate biopsies combining micro-ultrasound and mpMRI fusion biopsy. J. Urol. 2020, 204, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.; Hofbauer, S.; Shore, N.; Pavlovich, C.P.; Bulang, S.; Schostak, M.; et al. Prostate cancer detection by novice micro-ultrasound users enrolled in a training program. Soc. Int. Urol. J. 2022, 3, 62–68. [Google Scholar] [CrossRef]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; et al. Assessing cancer risk on novel 29 mhz micro-ultrasound images of the prostate: Creation of the micro-ultrasound protocol for prostate risk identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Ghai, S.; Perlis, N.; Atallah, C.; Jokhu, S.; Corr, K.; Lajkosz, K.; et al. Comparison of Micro-US and multiparametric MRI for prostate cancer detection in biopsy-naive men. Radiology 2022, 0, 212163. [Google Scholar] [CrossRef]
- Rodriguez Socarras, M.E.; Elbers, J.R.; Esposito, F.; Greco, I.; Del Alamo, J.F.; Rivera, V.C.; et al. Transperineal prostate biopsies using micro-ultrasound, MRI-guided and systematic biopsies (Madrid Protocol), an update with 482 patients. J. Urol. 2022, 207, e691. [Google Scholar]
- Hofbauer, S.; Harland, N.; Plage, H.; Reimann, M.; Hollenbach, M.; Gusenleitner, A.; et al. A non-inferiority comparative analysis of micro-ultrasonography and MRI-targeted biopsy in men at risk of prostate cancer. BJU Int. 2022, 129, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Avolio, P.P.; Buffi, N.M.; Maffei, D.; Regis, F.; Persico, F.; Lazzeri, M.; et al. The use of 29 MHZ transrectal micro ultrasound to stratify the presence of prostate cancer in patients with an equivocal mpMRI: A single institutional analysis. Eu. Uro Op. Sci. 2020, 20, S46–S47. [Google Scholar] [CrossRef]
- Avolio, P.P.; Fasulo, V.; Saitta, C.; Diana, P.; Uleri, A.; Gobbo, A.; et al. Assessing the role of high-resolution micro-ultrasound among patients with a negative multiparametric MRI and a persistent suspicion of prostate cancer. J. Urol. 2022, 207, e997–e8. [Google Scholar] [CrossRef]
- Abouassaly, R.; Klein, E.A.; El-Shefai, A.; Stephenson, A. Impact of using 29 MHz high-resolution micro-ultrasound in real-time targeting of transrectal prostate biopsies: Initial experience. World J. Urol. 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- Klotz, L.; Andriole, G.; Cash, H.; Cooperberg, M.; Crawford, E.D.; Emberton, M.; et al. Optimization of prostate biopsy - micro-ultrasound versus MRI (OPTIMUM): A 3-arm randomized controlled trial evaluating the role of 29MHz micro-ultrasound in guiding prostate biopsy in men with clinical suspicion of prostate cancer. Contemp. Clin. Trials. 2022, 112. [Google Scholar] [CrossRef] [PubMed]
- Claros, O.R.; Tourinho-Barbosa, R.R.; Fregeville, A.; Gallardo, A.C.; Muttin, F.; Carneiro, A.; et al. Comparison of initial experience with transrectal magnetic resonance imaging cognitive guided micro-ultrasound biopsies versus established transperineal robotic ultrasound magnetic resonance imaging fusion biopsies for prostate cancer. J. Urol. 2020, 203, 918–925. [Google Scholar] [CrossRef]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Maffei, D.; Saita, A.; Paciotti, M.; Diana, P.; Buffi, N.M.; et al. Diagnostic accuracy of microultrasound in patients with a suspicion of prostate cancer at magnetic resonance imaging: A single-institutional prospective study. Eur. Urol. Focus. 2020, 7, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Robin, D.; Branchu, B.; Taha, F.; Joncour, C.; Larré, S.; Staerman, F. Comparison of the accuracy of 29 MHz micro-ultrasound versus multiparametric magnetic resonance imaging for the diagnosis of clinically significant prostate cancer. Eur. Urol. 2021, 79, S1305. [Google Scholar] [CrossRef]
- Klotz, L.; Lughezzani, G.; Maffei, D.; Sánchez, A.; Pereira, J.G.; Staerman, F.; et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: A multicenter, prospective analysis. Can. Urol. Assoc. J. 2020, 15, E11–E6. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; et al. Evolution of targeted prostate biopsy by adding micro-ultrasound to the magnetic resonance imaging pathway. Eur. Urol. Focus. 2021, 7, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Kabre, B.; Pignot, G.; Branger, N.; Pacchetti, A.; Thomassin-Piana, J.; et al. Comparison between micro-ultrasound and multiparametric MRI Regarding the Correct identification of prostate cancer lesions. Clin. Genitourin. Cancer. 2022. [Google Scholar] [CrossRef] [PubMed]
- Swann, R.; McPhail, S.; Witt, J.; Shand, B.; Abel, G.A.; Hiom, S.; et al. Diagnosing cancer in primary care: Results from the National Cancer Diagnosis Audit. Br. J. Gen. Pract. 2018, 68, e63–e72. [Google Scholar] [CrossRef]
- Grey, A.D.R.; Connor, M.J.; Tam, J.; Loch, T. Can transrectal prostate ultrasound compete with multiparametric MRI in the detection of clinically significant prostate cancer? Transl. Androl. Urol. 2020, 9, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Otti, V.C.; Miller, C.; Powell, R.J.; Thomas, R.M.; McGrath, J.S. The diagnostic accuracy of multiparametric magnetic resonance imaging before biopsy in the detection of prostate cancer. BJU Int. 2019, 123, 82–90. [Google Scholar] [CrossRef]
- Borofsky, S.; George, A.K.; Gaur, S.; Bernardo, M.; Greer, M.D.; Mertan, F.V.; et al. What are we missing? false-negative cancers at multiparametric MR imaging of the prostate. Radiology 2018, 286, 186–195. [Google Scholar] [CrossRef]
- Brisbane, W.; Pensa, J.; Sisk, A.; Tran, E.; Priester, A.; Felker, E.; et al. Micro-ultrasound to whole mount image correlation for detection and localization of prostate cancer. J. Urol. 2021, 206, e394. [Google Scholar] [CrossRef]
- Perez, T.V.R. Initial results comparing high resolution micro-ultrasound with multiparametric magnetic resonance imaging for prostate cancer detection. Eur. Urol. Suppl. 2019, 18, e3499. [Google Scholar] [CrossRef]
- Scuderi, S.; Stabile, A.; Sorce, G.; De Angelis, M.; Nocera, L.; Pellegrino, F.; et al. Comparative analyses of micro-ultrasound versus MRI-targeted biopsy for the diagnosis of clinically significant prostate cancer. preliminary results from the prospective US-MIRROR Trial. J. Urol. 2022, 207, e336–e7. [Google Scholar] [CrossRef]
- Maffei, D.; Saitta, C.; Paolo Avolio, P.; Paciotti, M.; Fasulo, V.; Frego, N.; et al. Diagnostic performance of mixed targeted prostate biopsy approaches using micro-ultrasound and MRI-fusion biopsies. J. Urol. 2022, 207, e993. [Google Scholar] [CrossRef]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; et al. Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Pereira-Arias, J.G.; Sánchez-Vázquez, A.; Gamarra-Quintanilla, M.; Mora-Christian, J.A.; Urdaneta-Salegui, L.F.; Astobieta-Odriozola, A.; et al. Prostatic high resolution micro-ultrasound (MUS) imaging [article in Spanish]. Arch. Esp. Urol. 2019, 72, 804–815. [Google Scholar]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2023 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.