Abstract
Background: Prostate cancer is a significant global health concern, with rising incidence and disease burden in the Middle East (ME). This review aims to explore the current state of prostate cancer epidemiology in the ME, particularly in low- to middle-income settings, investigating trends in incidence and mortality, assessing challenges related to de novo metastatic prostate cancer, and evaluating the need for region-specific screening guidelines. Methods: We conducted a comprehensive narrative review of epidemiological data on prostate cancer in the ME, examining trends in incidence and mortality, de novo metastatic cases, and current screening practices. Additionally, we assessed the applicability of international guidelines for prostate cancer screening to the ME context. Results: The ME exhibits a rising trend in prostate cancer incidence, with a mortality-to-incidence ratio of 0.3–0.4, compared to 0.1 in the United States, reflecting significant differences in healthcare access and quality that contribute to poorer outcomes. The incidence rates are particularly high in Lebanon, reaching 37.2 per 100,000 in 2012. De novo metastatic prostate cancer is also more prevalent in the ME, often exceeding 20–30%, with a value of 23% reported in Lebanon and reaching 54% in a study including six Middle Eastern countries, compared to 4–14% in the United States. Our review identified a critical need for enhanced screening and early detection efforts tailored to the ME’s unique epidemiological and socio-cultural factors. Conclusions: The substantial burden of de novo metastatic prostate cancer in the ME underscores the need for region-specific screening guidelines. Tailored approaches, including increased awareness, early detection, and resource-stratified strategies, are essential to address the unique epidemiological and socio-cultural factors of the ME and improve patient outcomes.
1. Introduction
Prostate cancer represents a significant global health burden, characterized by its high incidence and associated morbidity and mortality [1]. It ranks as the second most frequently diagnosed cancer in men worldwide and stands fifth in cancer-related deaths. Despite the favorable overall survival rates, outcomes vary significantly based on the stage at diagnosis, emphasizing the critical role of early detection.
In the Middle East (ME), the burden of prostate cancer is alarming, especially in low- and lower-middle-income countries. The region exhibits a rising trend in incidence, coupled with a concerning mortality-to-incidence ratio (MIR) [2]. De novo metastatic prostate cancer, defined by the presence of distant metastases at initial diagnosis, poses a substantial challenge in the ME due to its aggressive nature and poor prognosis [3,4]. Yet, comprehensive data on prostate cancer epidemiology and de novo metastatic prostate cancer in the region remain scarce.
This review aims to provide insights into the current state of prostate cancer in the ME, considering variations across countries’ income levels, with a specific focus on de novo metastatic disease. We will examine the epidemiological data, explore trends in prostate cancer incidence and mortality, and address the unique challenges faced by this region. Additionally, we will discuss existing international screening guidelines and highlight the need for region-specific guidelines.
2. Prostate Cancer Epidemiology
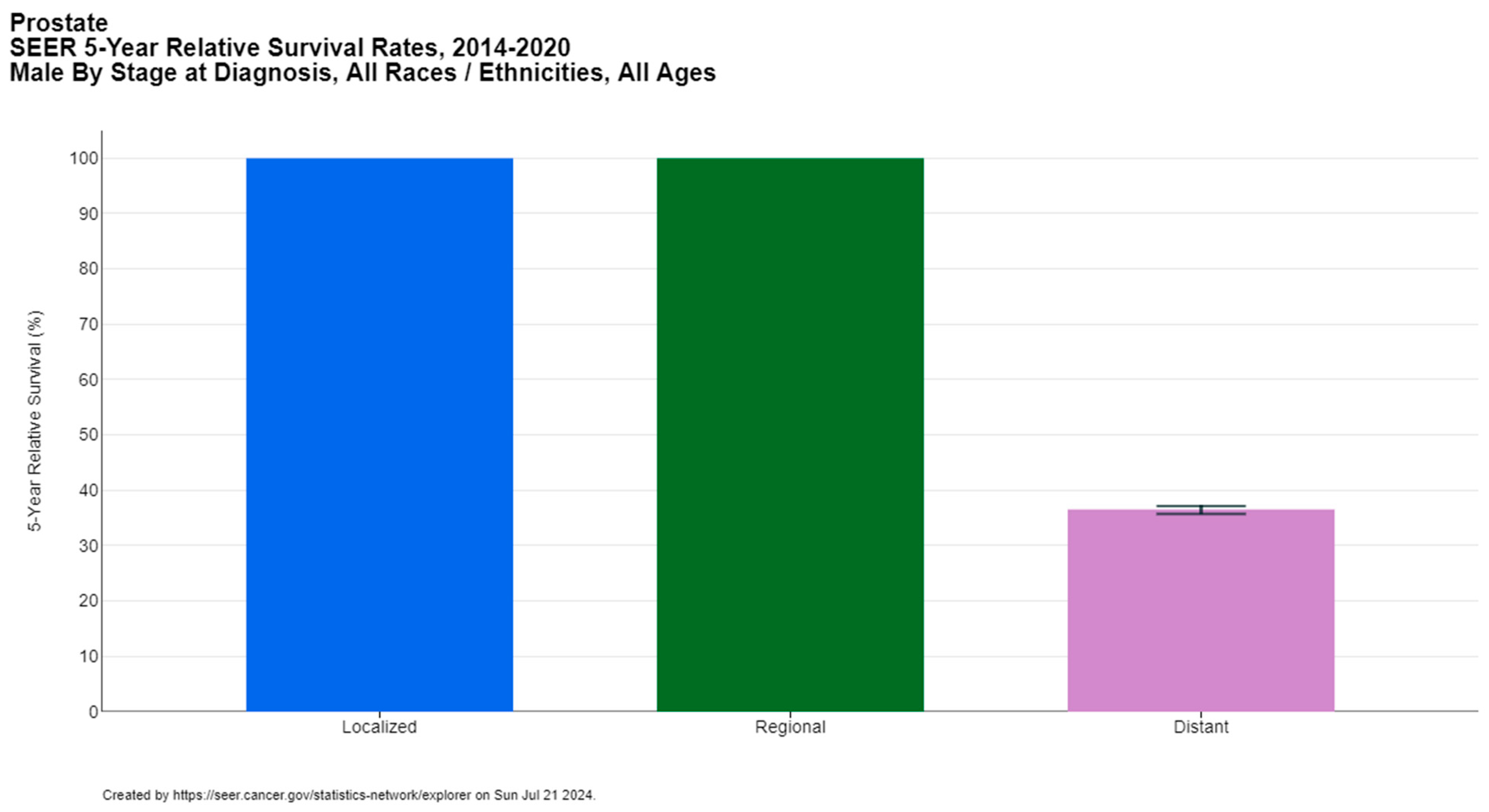
Prostate cancer is a significant global health concern, affecting 1.5 million men worldwide, with approximately 397,000 reported deaths according to GLOBOCAN 2022 [1]. It ranks as the second most common cancer among men after lung cancer (14.2% of new cancer cases in men) and the fifth leading cause of cancer-related mortality in males (7.3% of male cancer deaths). The median age of prostate cancer diagnosis is 67 years, with the majority of cases diagnosed between the ages of 65 and 74, according to the Surveillance, Epidemiology, and End Results (SEER) database [5]. The five-year relative survival rate of prostate cancer is 97.5%, but it is strongly dependent on the stage at diagnosis, ranging from nearly 100% for locoregional disease to 36.6% for metastatic cases (Figure 1) [5,6].
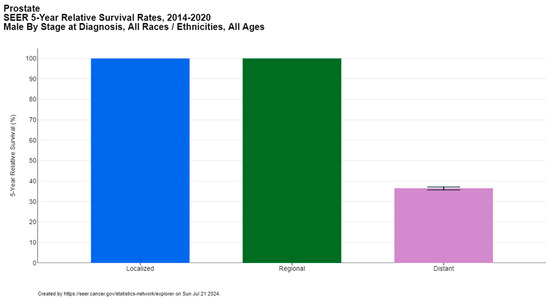
Figure 1.
Five-year relative survival rates of prostate cancer per stage at diagnosis. Created with SEER database.
Prostate cancer incidence is notably high in developed countries, particularly in Europe and North America, where it ranks first in cancer incidence among males. Specific countries within the ME, such as Lebanon, Kuwait, Oman, Qatar, and the United Arab Emirates, also report prostate cancer as the most common cancer in men (Table 1) [1]. Notably, Lebanon has one of the highest incidence rates of prostate cancer in the region, as highlighted by Lakkis et al. [7], with the highest incidence rate observed in 2012 (37.2 per 100,000), surpassing the global average (31.1 per 100,000) [2,7]. Of note, Kuwait, Oman, Qatar, and the United Arab Emirates are classified as high-income countries according to the World Bank classification, while Lebanon transitioned from an upper-middle-income to a lower-middle-income country in 2021 [8].

Table 1.
Top four cancer sites by incidence in males in Middle Eastern countries in 2022, compared to the United States of America and the world.
The variations in prostate cancer incidence and mortality can be correlated with the Human Development Index (HDI). Higher HDI countries, such as North American and European countries, tend to have higher incidence rates of prostate cancer, likely due to better screening practices, differences in lifestyle and environmental risk factors, and longer life expectancy, as this disease tends to affect older age groups. Conversely, mortality rates are highest in countries with the lowest HDI, especially in Africa, reflecting disparities in healthcare access and quality [9,10,11]. The MIR further illustrates these disparities. Hilal et al. [2] reported an MIR of 0.5 in the ME and North Africa (MENA) region compared to 0.09 in the United States. Recent data indicate that the MIR in the MENA region has improved to 0.3–0.4, yet it remains significantly higher than the 0.1 observed in the USA (Table 2) [1]. Disparities also exist among Middle Eastern countries. High-income nations (such as Cyprus, Qatar, Bahrain, and the United Arab Emirates) experienced an MIR of 0.2–0.3 in 2022, except for Oman and Saudi Arabia which had an MIR of 0.4. Upper-middle-income countries (like Iraq and Turkey) and recently transitioned lower-middle-income countries (such as Jordan and Lebanon) had an MIR of 0.3–0.4. In contrast, low- and lower-middle-income countries (including Egypt, Iran, Syria, and Yemen) exhibited an MIR of 0.5 or higher (reaching 0.7 in Yemen).

Table 2.
Age-standardized incidence and mortality rates in Middle Eastern countries in 2022, with income level in 2022/2023, compared to the United States of America and the world.
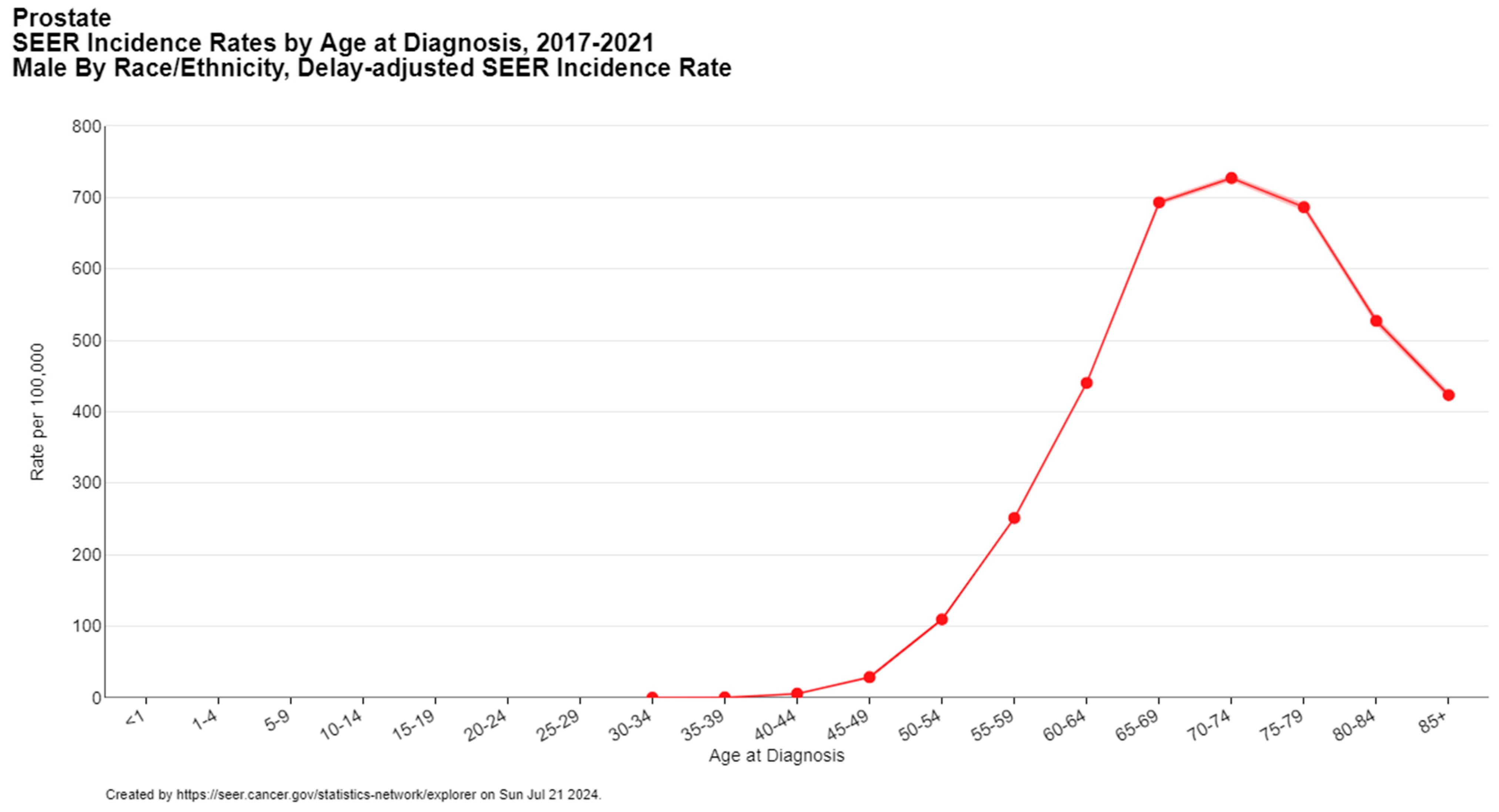
Several risk factors contribute to the incidence of prostate cancer, including age, family history, and genetic predisposition. The risk of developing prostate cancer increases with age, with a notable rise in men over the age of 50 (Figure 2) [5]. A family history of prostate cancer and certain genetic mutations, namely homologous DNA repair genes such as BRCA1/2 and ATM (hereditary breast and ovarian cancer syndrome) and DNA mismatch repair genes (Lynch syndrome), also elevate the risk [12].
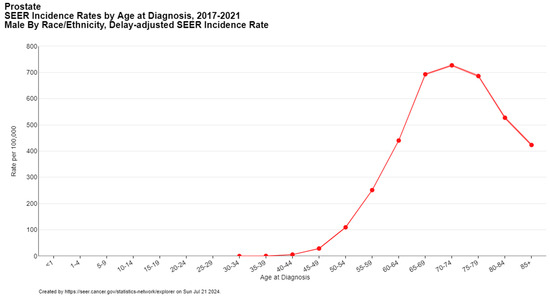
Figure 2.
Prostate cancer incidence by age at diagnosis. Created with SEER database.
In the ME, certain protective factors may contribute to the epidemiology of prostate cancer. The region’s young age structure, characterized by a lower median age compared to other parts of the world, likely plays a role in the lower overall incidence. Additionally, lower levels of androgens and prostate-specific antigen (PSA) in the population, as reported in several Gulf countries, may offer some protective effects. The Mediterranean diet, rich in fruits, vegetables, and healthy fats, is postulated to have a protective role against prostate cancer [2,7,13,14]. Studies have shown that the incidence of prostate cancer in Arab men who have immigrated to Western countries remained lower than that of the non-Arab population in the host countries. This observation can be partly attributed to these population-related factors [2,15]. Moreover, local differences in diet and lifestyle habits can contribute to higher incidence of other cancers compared to prostate cancer. In 2020, Kulhánová et al. [16] analyzed the proportion of cancer attributable risk factors in men in the Eastern Mediterranean Region. Smoking was identified as the main risk factor in Bahrain, Iraq, Jordan, Palestine, Syria, and Turkey, which explains the predominance of lung cancer in these countries (Table 1). In Iranian men, diet was identified as the major cancer risk factor, contributing to a higher prevalence of gastric cancer. In Egypt, liver cancer ranks first due to the high prevalence of HCV infection, whereas colorectal cancer leads in Saudi Arabia and Yemen, likely due to infection and chronic inflammation.
3. Prostate Cancer Trends
Prostate cancer trends worldwide exhibit an interesting dichotomy. While the incidence of prostate cancer has been on the rise, mortality rates have generally been decreasing [6,17,18]. This trend is more pronounced in regions with robust screening programs and advanced healthcare systems like the United States.
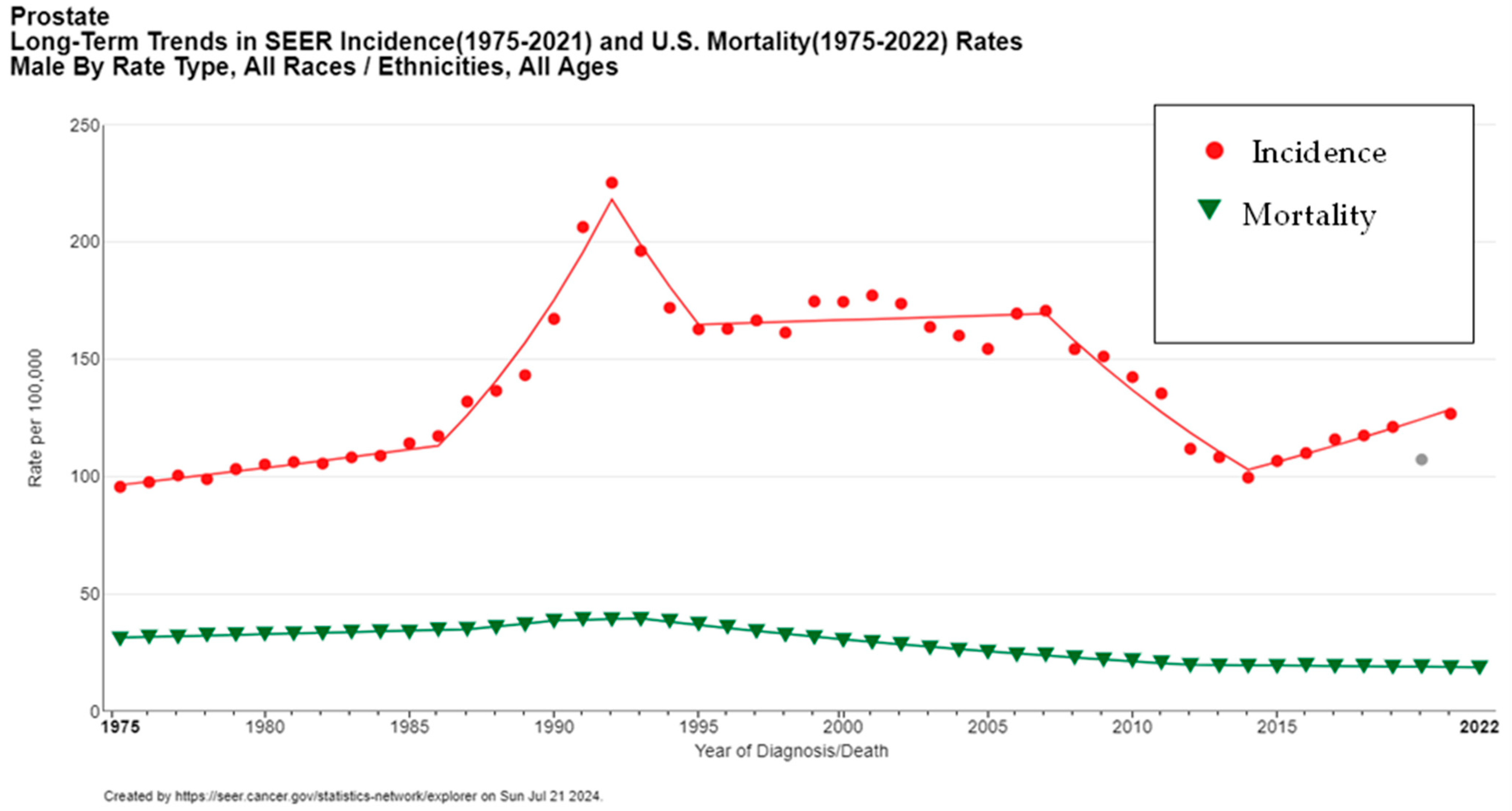
Globally, the increasing incidence of prostate cancer can largely be attributed to enhanced screening and diagnostic techniques. For instance, the widespread adoption of prostate-specific antigen (PSA) testing in the early 1990s in the United States led to a significant rise in detected cases. This surge in incidence was followed by a stabilization and eventual decline from 2007 to 2014 (Figure 3), correlating with evolving screening guidelines and recommendations aimed at reducing overdiagnosis and overtreatment [5].
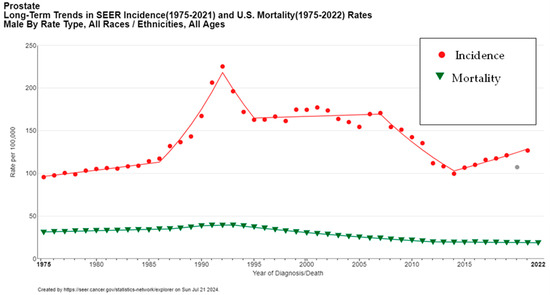
Figure 3.
Long-term trends in prostate cancer incidence and mortality (1975–2022). Created with SEER database. The gray data point represents the 2020 incidence rate, which was not included in the trend line due to the impact of COVID-19 on SEER Cancer Incidence data.
In the ME, a similar pattern is emerging as countries adopt more comprehensive screening programs and advanced diagnostic tools. The introduction of PSA testing has improved the detection rates of prostate cancer, leading to an increase in diagnosed cases. The NCCN guidelines advise the concomitant use of diagnostic imaging or biomarkers with the PSA test to increase the specificity of the latter and identify cases at earlier stages while avoiding overtreatment [12].
Conversely, the global decline in prostate cancer mortality can be attributed to several factors. Early detection through the implementation of cancer screening allows for diagnosis at less advanced stages, improving treatment outcomes. Additionally, advancements in treatment, particularly the development of doublet and combination therapies for metastatic hormone-sensitive prostate cancer, have significantly enhanced survival rates [4,19,20]. These therapies offer more effective management of the disease, reducing mortality even among patients with advanced cancer.
However, it is important to note that the magnitude of these trends in incidence and mortality has seen some attenuation in recent years [9]. This could be due to several factors, including changes in screening practices. Moreover, the incidence and mortality data highly depend on the quality and accuracy of cancer registries in each country [3,18].
4. De Novo Metastatic Prostate Cancer
De novo metastatic prostate cancer, as defined by the American Urological Association (AUA) and the Society of Urologic Oncology (SUO) guidelines, refers to prostate cancer that presents with distant metastases at the time of initial diagnosis, rather than metastasizing after the treatment of localized disease [21]. This type of prostate cancer is distinct because it is identified at an advanced stage and is associated with an aggressive disease course and poor overall survival [4].
The most common sites of metastasis for prostate cancer are the bones and lymph nodes [22]. Bone metastases are highly prevalent and can lead to significant morbidity, including pain, fractures, and spinal cord compression. Lymph node involvement is also frequent, and both types of metastases contribute to the overall prognosis and disease course.
The prognosis of de novo metastatic prostate cancer is strongly influenced by the stage at diagnosis. Even patients with stage III prostate cancer can have relatively good outcomes, with studies reporting a 5-year survival rate of around 93%. However, once the disease progresses to stage IV, the survival rate drops significantly to 30–40%, and the disease course becomes more aggressive [5,6].
The percentage of prostate cancer cases that are metastatic at diagnosis varies significantly by region. In the United States, this percentage typically ranges from 4 to 14% [2,5]. In contrast, the ME experiences much higher rates, often exceeding 20–30% [22] (Table 3). A retrospective analysis of 559 patients diagnosed with prostate cancer and treated at a tertiary center in Lebanon, a lower-middle-income country, revealed 23% of patients with stage IV disease [22]. This percentage was significantly elevated among Iraqi patients (52%). An observational multicenter retrospective study on 615 patients from four Middle Eastern countries (Lebanon, Saudi Arabia, Iraq, and Kuwait) demonstrated 230 (37.4%) patients with metastatic castrate-sensitive prostate cancer and 25 (4.1%) patients with metastatic castration--resistant prostate cancer (41.5% of patients with stage IV disease) [23]. Sayan et al. [3] even reported that, among 1136 prostate cancer patients from six countries in the ME (Turkey, Lebanon, Iraq, Syria, Bahrain, and Jordan), 35% had clinical T3 or T4, 54% presented with stage IV disease, and 50% had a Gleason score ≥ 8. Remarkably, 78% of these patients received their diagnosis based on symptom presentation rather than routine PSA screening. This finding partly explains the poor outcomes observed in this region and highlights the urgent need for enhanced prostate cancer screening and early detection efforts.

Table 3.
Summary of studies reporting rates of de novo metastatic prostate cancer in the ME.
To address the high burden of de novo metastatic prostate cancer in the ME, especially given the low to middle socio-economic status of many countries in the region (Egypt, Iran, Jordan, Lebanon, Palestine, Syria, and Yemen), tailored guidelines for treatment have been developed. Mukherji et al. [24] stratified resources into two levels based on financial resources and availability of expertise. This framework aimed to provide a practical approach for healthcare providers with varying resources to deliver optimal care for prostate cancer patients. The guidelines emphasize a resource-stratified approach to accommodate varying levels of healthcare infrastructure and expertise. Key recommendations include the use of MRI and targeted biopsies for diagnosis where available, and the implementation of multidisciplinary discussions for treatment planning. For localized disease, options include radical prostatectomy with pelvic lymph node dissection and external-beam radiation therapy with androgen deprivation therapy (ADT). In advanced cases, systemic therapies such as docetaxel or abiraterone are recommended, particularly for high-volume metastatic disease. The guidelines also stress the importance of early salvage radiation therapy post-prostatectomy and the use of osteoclast-targeted therapies to manage bone health in metastatic castration-resistant prostate cancer (mCRPC). These recommendations aim to optimize patient outcomes by tailoring treatment strategies to the available resources and expertise in the region. The guidelines were designed to help healthcare participants offer the best possible treatment within their means, thereby improving patient outcomes despite resource limitations.
Data on de novo metastatic prostate cancer in the ME are scarce, highlighting a critical need for further investigation. There is a significant lack of studies on barriers to prostate cancer screening in the region. Poor awareness and inadequate physician counseling are notable issues, with many healthcare providers not routinely recommending PSA testing [15,18,25]. A cross-sectional survey among 4431 healthy male participants in 14 Middle Eastern countries demonstrated a critically low level of awareness of prostate cancer in the region, concerning disease outcomes, risk factors, and cancer screening. Notably, 22% of participants thought that prostate cancer affects both men and women, around 30% recognized advancing age and family history as risk factors for prostate cancer, and only 19% were aware of PSA as a screening method for prostate cancer [25]. In Saudi Arabia, only 54.7% of healthcare physicians were practicing counseling and referring prostate cancer patients when needed [18].
Additionally, patient and societal perceptions play a crucial role in the late detection of prostate cancer. Cultural beliefs about cancer can deter individuals from seeking timely medical advice and screening. The lack of a robust primary care system in many low- to middle-income Middle Eastern countries further exacerbates this issue, as it leads to delayed screening and late detection of prostate cancer [3]. Implementing national prostate cancer screening programs in the ME could increase the detection of localized and early-stage disease. However, the availability of urologists and radiation oncologists to treat localized disease varies significantly across the ME. High-income countries such as the United Arab Emirates, Qatar, and Kuwait generally have better access to specialized healthcare professionals. In contrast, low-income countries like Yemen and Syria face a shortage of trained urologists and radiation oncologists, which can hinder effective prostate cancer treatment.
Moreover, PSA levels vary between regions and should be interpreted based on ethnicity, age, and genetic and environmental profiles, which further illustrates the need for tailored screening protocols that account for these risk factors [9,26]. Current guidelines encourage the combination of PSA with MRI and biomarker tests (such as the 4Kscore test) to increase sensitivity and specificity, improving the detection of high-grade prostate cancer while reducing overdiagnosis and unnecessary prostate biopsies [27]. However, the availability of these tests remains an issue in some low-income settings.
Addressing these barriers through targeted awareness campaigns, improving physician education, strengthening primary care systems, and establishing regional collaboration and incentive programs that support the training and retention of healthcare professionals in low-income countries can significantly improve early detection and outcomes for prostate cancer patients in the ME [25].
Active surveillance is now increasingly recognized as the preferred management strategy for low-risk prostate cancer. It involves closely monitoring the patient’s condition without immediate treatment unless the cancer shows signs of progression, to avoid overtreatment. Although regular monitoring is required, the intensity of active surveillance can be modulated based on an individual’s risk parameters, which can be suitable for countries with limited resources [28].
5. Screening Guidelines for Prostate Cancer
Current guidelines for prostate cancer screening vary by region (Table 4). In the United States, the American Urological Association (AUA) recommends baseline PSA testing in men aged 45–50 and routine screening with shared decision-making for men aged 55–69. Clinicians should offer prostate cancer screening for men 40 to 45 years with increased risk (Black ancestry, germline mutations, strong family history of prostate cancer). Routine screening for men aged 70 and older is generally not recommended unless they are in excellent health with a life expectancy of more than 10–15 years [29]. Similarly, the U.S. Preventive Services Task Force (USPSTF) supports individualized, informed decision-making for men aged 55–69 and does not recommend routine screening for men older than age 70 years [30]. The National Comprehensive Cancer Network (NCCN) suggests baseline PSA testing at age 45, with follow-up every 1–2 years if PSA is ≥1.0 ng/mL and every 2–4 years if PSA is <1.0 ng/mL, recommending earlier screening for high-risk groups such as African American men and those with a family history [31]. In Europe, the European Association of Urology (EAU) advises PSA testing every 2–4 years for men aged 50–70 with a life expectancy of 10 to 15 years, with earlier screening starting at 40–45 years for high-risk individuals (positive family history, of African descent, or carrying the BRCA2 mutation) [32]. The UK’s National Health Service (NHS) focuses on risk-informed decision-making for men over 50 who request screening [33].

Table 4.
Summary of international guidelines for prostate cancer screening.
Several studies have evaluated the effect of prostate cancer screening on mortality (Table 5). The largest is the European Randomized Study of Screening for Prostate Cancer (ERSPC), which included 182,000 men aged 50 to 74 years. It showed an absolute risk reduction in prostate cancer mortality of 1.28 per 1000 men randomized to PSA screening, over a median follow-up of 13 years. After 16 years of follow-up, the study found that 570 men need to be invited to screening and 18 need to be diagnosed in order to prevent one death from prostate cancer [34,35]. Similarly, the Goteborg trial from Sweden reported an absolute cumulative risk reduction in death from prostate cancer at 14 years of 0.40% (95% CI 0.17–0.64) with biennial PSA-based screening. Therefore, at 14 years of follow-up, 293 men have to be invited for screening and 12 must be diagnosed to prevent one mortality case from prostate cancer [36]. In contrast, the U.S.-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial did not find a significant difference in mortality with PSA screening. However, some factors may have contributed to the lack of mortality benefit from screening, such as the high rates of contamination where participants received PSA tests outside the study protocol, the PSA threshold of 4 ng/mL which may have had a lower sensitivity compared to 3 ng/mL used in the ERSPC trial, and the advances in prostate cancer treatment which may have decreased mortality in both groups [37]. Collectively, these studies emphasize the potential benefits of PSA screening in reducing mortality, especially in European populations, but also highlight the need to balance benefits with risks such as overdiagnosis.

Table 5.
Summary of studies evaluating the effect of prostate cancer screening on mortality.
Prostate cancer in the ME is a serious concern, with a higher incidence of de novo metastatic prostate cancer being diagnosed and poorer outcomes compared to other regions in the world. Current international screening guidelines may not be the best fit for the needs of this region. A tailored approach to improve early detection and outcomes has to address the unique epidemiological and socio-cultural factors of the ME. This involves gathering region-specific data on prostate cancer through population-based studies, to collect data on prostate cancer incidence, mortality, and risk factors. These studies can also serve in identifying appropriate PSA cutoff in this population, as studies from the region suggest that a PSA up to 10 ng/mL in Arab men could still be due to benign causes [22,38]. Thus, determining the optimal age to initiate screening and identifying an appropriate PSA cutoff for the region are crucial to avoid overdiagnosis and unnecessary interventions. Possible barriers to implementing Western prostate cancer screening guidelines in the ME include a lack of awareness, which is particularly important as most Western guidelines rely on shared decision-making. This is illustrated in a study from Lebanon and another one from Oman, which identified fear and lack of understanding of the screening procedures as main barriers to prostate cancer screening [39,40]. Therefore, healthcare providers should raise awareness by discussing the option of screening with their patients and address misconceptions regarding the process. At the governmental level, reviewing existing screening practices can help understand the current healthcare landscape and identify gaps. Conducting pilot screening programs will provide preliminary data on the benefits or harms of screening and allow for necessary adjustments before widespread implementation. Furthermore, qualitative research into financial and cultural factors will help policymakers design culturally sensitive, evidence-based screening strategies tailored to the region’s unique needs. An important consideration in the ME is the population shift across different areas due to migrants and refugees, which should be included in screening guidelines to address challenges such as limited healthcare access and increased vulnerability of this population, especially during times of armed conflict.
6. Conclusions
Prostate cancer poses a significant health burden globally, particularly in the ME and low- to middle-income settings. Countries with lower HDI scores face higher mortality rates and a greater disease burden. The epidemiological trends and outcomes indicate a pressing need for region-specific screening guidelines, especially since there is a higher rate in the ME of de novo metastatic prostate cancer at diagnosis. Developing region-specific guidelines should start with acquiring comprehensive epidemiological data from Middle Eastern countries, reviewing current screening practices and barriers to screening, raising awareness, and piloting resource-stratified strategies to balance the benefits and harms of screening.
Author Contributions
Conceptualization, N.A., L.C., Z.A.S. and A.S.; methodology, N.A. and L.C.; validation, Z.A.S. and A.S.; data curation, N.A. and L.C.; writing—original draft preparation, N.A and L.C.; writing—review and editing, N.A., L.C., Z.A.S. and A.S.; visualization, N.A.; supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
Data supporting the findings of this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Hilal, L.; Shahait, M.; Mukherji, D.; Charafeddine, M.; Farhat, Z.; Temraz, S.; Khauli, R.; Shamseddine, A. Prostate Cancer in the Arab World: A View From the Inside. Clin. Genitourin. Cancer 2015, 13, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Langoe, A.; Aynaci, O.; Eren, A.A.; Eren, M.F.; Kazaz, I.O.; Ibrahim, Z.; Al-Akelie, O.T.; Al-Mansouri, L.; Abu-Hijlih, R.; et al. Prostate cancer presentation and management in the Middle East. BMC Urol. 2024, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Piombino, C.; Oltrecolli, M.; Tonni, E.; Pirola, M.; Matranga, R.; Baldessari, C.; Pipitone, S.; Dominici, M.; Sabbatini, R.; Vitale, M.G. De Novo Metastatic Prostate Cancer: Are We Moving toward a Personalized Treatment? Cancers 2023, 15, 4945. [Google Scholar] [CrossRef]
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. Surveillance Research Program, National Cancer Institute. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 15 June 2024).
- Smith-Palmer, J.; Takizawa, C.; Valentine, W. Literature review of the burden of prostate cancer in Germany, France, the United Kingdom and Canada. BMC Urol. 2019, 19, 19. [Google Scholar] [CrossRef]
- Lakkis, N.A.; Osman, M.H. Prostate Cancer in Lebanon: Incidence, Temporal Trends, and Comparison to Countries From Different Regions in the World. Cancer Control 2021, 28, 10732748211055267. [Google Scholar] [CrossRef]
- World Bank [Internet]. World Bank Country Classifications by Income Level for 2024–2025. Available online: https://blogs.worldbank.org/en/opendata/world-bank-country-classifications-by-income-level-for-2024-2025 (accessed on 15 August 2024).
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Hassanipour-Azgomi, S.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Towhidi, F.; Jamehshorani, S.; Salehiniya, H. Incidence and mortality of prostate cancer and their relationship with the Human Development Index worldwide. Prostate Int. 2016, 4, 118–124. [Google Scholar] [CrossRef]
- Kearney, G.; Chen, M.H.; Mula-Hussain, L.; Skelton, M.; Eren, M.F.; Orio, P.F.; Nguyen, P.L.; D’Amico, A.V.; Sayan, M. Burden of prostate cancer in the Middle East: A comparative analysis based on global cancer observatory data. Cancer Med. 2023, 12, 21419–21425. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Al-Abdin, O.Z.; Al-Beeshi, I.Z. Prostate cancer in the Arab population: An overview. Saudi Med. J. 2018, 39, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Awada, H.; Nassereldine, H.; Zeineddine, M.; Sater, Z.A.; El-Hajj, A.; Mukherji, D. Prostate cancer in the Arab world: Bibliometric review and research priority recommendations. Arab. J. Urol. 2022, 20, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kulhánová, I.; Znaor, A.; Shield, K.D.; Arnold, M.; Vignat, J.; Charafeddine, M.; Fadhil, I.; Fouad, H.; Al-Omari, A.; Al-Zahrani, A.S.; et al. Proportion of cancers attributable to major lifestyle and environmental risk factors in the Eastern Mediterranean region. Int. J. Cancer 2020, 146, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Kangevari, M.; Saeedi Moghaddam, S.; Ghamari, S.H.; Azangou-Khyavy, M.; Malekpour, M.R.; Rezaei, N.; Rezaei, N.; Kolahi, A.A.; Amini, E.; Mokdad, A.H.; et al. The burden of prostate cancer in North Africa and Middle East, 1990–2019: Findings from the global burden of disease study. Front. Oncol. 2022, 12, 961086. [Google Scholar] [CrossRef]
- Arafa, M.A.; Rabah, D.M. With increasing trends of prostate cancer in the Saudi Arabia and Arab World: Should we start screening programs? World J. Clin. Oncol. 2017, 8, 447–449. [Google Scholar] [CrossRef]
- Schoen, M.W.; Montgomery, R.B.; Owens, L.; Khan, S.; Sanfilippo, K.M.; Etzioni, R.B. Survival in Patients with De Novo Metastatic Prostate Cancer. JAMA Netw. Open 2024, 7, e241970. [Google Scholar] [CrossRef]
- Corsini, C.; Garmo, H.; Orrason, A.W.; Gedeborg, R.; Stattin, P.; Westerberg, M. Survival Trend in Individuals with De Novo Metastatic Prostate Cancer After the Introduction of Doublet Therapy. JAMA Netw. Open 2023, 6, e2336604. [Google Scholar] [CrossRef]
- Lowrance, W.; Dreicer, R.; Jarrard, D.F.; Scarpato, K.R.; Kim, S.K.; Kirkby, E.; Buckley, D.I.; Griffin, J.C.; Cookson, M.S. Updates to Advanced Prostate Cancer: AUA/SUO Guideline (2023). J. Urol. 2023, 209, 1082–1090. [Google Scholar] [CrossRef]
- Daher, M.; Telvizian, T.; Dagher, C.; Abdul-Sater, Z.; Massih, S.A.; Chediak, A.E.; Charafeddine, M.; Shahait, M.; Alameddine, R.; Temraz, S.; et al. High rates of advanced prostate cancer in the Middle East: Analysis from a tertiary care center. Urol. Ann. 2021, 13, 418–423. [Google Scholar] [CrossRef]
- El-Karak, F.; Shamseddine, A.; Omar, A.; Haddad, I.; Abdelgawad, M.; Naqqash, M.A.; Kaddour, M.A.; Sharaf, M.; Abdo, E. Prostate cancer across four countries in the Middle East: A multi-centre, observational, retrospective and prognostic study. Ecancermedicalscience 2024, 18, 1695. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, D.; Youssef, B.; Dagher, C.; El-Hajj, A.; Nasr, R.; Geara, F.; Rabah, D.; Al Dousari, S.; Said, R.; Ashou, R.; et al. Management of patients with high-risk and advanced prostate cancer in the Middle East: Resource-stratified consensus recommendations. World J. Urol. 2020, 38, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Eren, A.A.; Tuac, Y.; Langoe, A.; Alali, B.; Aynaci, O.; Mohammadipour, S.; Vahedi, F.; Daneshmand, B.; Abbas, W.; et al. Prostate Cancer Awareness in the Middle East: A Cross-Sectional International Study. JCO Glob. Oncol. 2024, 10, e2400171. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, G.; Wu, F.; Wang, Y.; Liu, Z.; Hu, H.; Xu, K. Global Burden of Prostate Cancer and Association with Socioeconomic Status, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study. J. Epidemiol. Glob. Health 2023, 13, 407–421. [Google Scholar] [CrossRef]
- Auvinen, A.; Tammela, T.L.J.; Mirtti, T.; Lilja, H.; Tolonen, T.; Kenttämies, A.; Rinta-Kiikka, I.; Lehtimäki, T.; Natunen, K.; Nevalainen, J.; et al. Prostate Cancer Screening with PSA, Kallikrein Panel, and MRI: The ProScreen Randomized Trial. JAMA 2024, 331, 1452–1459. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Zheng, Y.; Faino, A.V.; Newcomb, L.F.; Zhu, K.; Cowan, J.E.; Brooks, J.D.; Dash, A.; Gleave, M.E.; Martin, F.; et al. Tailoring Intensity of Active Surveillance for Low-Risk Prostate Cancer Based on Individualized Prediction of Risk Stability. JAMA Oncol. 2020, 6, e203187. [Google Scholar] [CrossRef]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J. Urol. 2023, 210, 46–53. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer Early Detection. Version 2.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf (accessed on 1 August 2024).
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Prostate Cancer Risk Management Programme (PCRMP). NHS; 2016. Available online: https://www.gov.uk/guidance/prostate-cancer-risk-management-programme-overview (accessed on 1 August 2024).
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Carlsson, S.; Aus, G.; Bergdahl, S.; Khatami, A.; Lodding, P.; Pihl, C.G.; Stranne, J.; Holmberg, E.; Lilja, H. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L., 3rd.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Gelmann, E.P.; Kvale, P.A.; Reding, D.J.; et al. Mortality results from a randomized prostate-cancer screening trial. N. Engl. J. Med. 2009, 360, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, E.O.; Sheikh, M.; Mojimoniyi, O.A.; Francis, I.; Anim, J.T.; Nkansa-Dwamena, D.; Al-Awadi, K.A. High serum prostate-specific antigen levels in the absence of prostate cancer in Middle-Eastern men: The clinician’s dilemma. BJU Int. 2003, 91, 618–622. [Google Scholar] [CrossRef]
- Hejase, R.; Saleh, A.M.; Abdel-Aziz, H.R.; Vellaiyan, A.; AlOmari, A.K.; AlOmari, A.A. Factors Influencing Prostate Cancer Screening Intentions in Lebanese Men. Asian Pac. J. Cancer Prev. 2024, 25, 963–970. [Google Scholar] [CrossRef]
- Muliira, J.K.; Al-Saidi, H.S.; Al-Yahyai, A.N. Determinants of Behavioral Intentions to Screen for Prostate Cancer in Omani Men. Asia Pac. J. Oncol. Nurs. 2017, 4, 348–355. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).