Abstract
Background/Objectives: Focal therapy (FT) for prostate cancer (PCa) is an alternative to radical treatments that aims to balance cancer control and quality of life preservation in well-selected patients. Understanding its general principles and outcomes is key for its widespread adoption and proper implementation. Methods: The International Consultation on Urological Diseases nominated a committee to review the literature on FT for PCa. A comprehensive PubMed search was conducted to identify articles focused on the different aspects of FT, including patient selection, imaging techniques, treatment modalities, cancer control and safety outcomes, integration with other approaches and future perspectives. Results: FT for PCa was introduced in the 1990s with cryotherapy and high-intensity focused ultrasound (HIFU) as pioneering modalities. Though initially guided by transrectal ultrasound (TRUS) and large biopsy templates, FT implementation expanded significantly with the advent of multiparametric magnetic resonance imaging (MRI) and the validation of the index lesion concept. Appropriate patient selection is key for FT and relies on prostate-specific antigen (PSA) metrics, MRI findings and targeted biopsy information. Multiple energy sources are now available, each with specific technical characteristics. Cancer control rates vary by energy modality, tumor characteristics, and institutional experience, demonstrating comparable outcomes to radical treatments in well-selected patients. The safety profile is excellent, with high rates of urinary continence and sexual function preservation. Post-treatment surveillance integrates PSA measurements, imaging, and histological assessment. Future directions for further FT adoption include the availability of long-term data, protocol standardization and technological improvements to enhance patient selection and treatment planning and delivery. Conclusions: FT is a valuable therapeutic option for selected patients with localized PCa, demonstrating promising oncological outcomes and better functional preservation compared to radical treatments. Understanding its principles and technical aspects is essential for offering comprehensive PCa care.
1. Introduction
Focal therapy (FT) for prostate cancer (PCa) represents a targeted approach for treating localized disease that aims to strike a balance between cancer control and quality of life preservation. Although radical treatments—radical prostatectomy (RP) and radiation therapy (RT)—have been the historical standard of care, they carry significant risks of urinary incontinence, erectile dysfunction, and bowel function alterations [1]. FT addresses this challenge by attempting to be as oncologically efficient as whole-gland treatments while preserving surrounding healthy tissue and minimizing morbidity [2,3].
The evolution of FT began in the mid-1990s with cryoablation emerging as a salvage option for radiotherapy failures, followed by high-intensity focused ultrasound (HIFU) at the century’s end [4]. Technological improvements have since enabled precise treatment planning and monitoring for both whole-gland and focal ablation approaches. The field has also witnessed the development of various energy modalities including cryotherapy, HIFU, focal laser ablation (FLA), photodynamic therapy (PDT), and irreversible electroporation (IRE).
The emergence of multiparametric (mp) magnetic resonance imaging (MRI) has been critical to FT’s implementation. This imaging modality enhances the ability to detect and precisely localize clinically significant (cs) PCa lesions. Evidence increasingly shows that the largest tumor focus within the prostate, known as the “index lesion”, drives cancer natural history and impacts prognosis [5]. The accuracy of mpMRI in identifying these index lesions enables their selective targeting while preserving surrounding healthy tissue, providing the fundamental rationale for FT [6,7]. This article investigates current evidence for focal ablation of prostate cancer, focusing on patient selection, technical aspects, outcomes, and future perspectives.
2. Materials and Methods
An international committee was nominated by the International Consultation on Urological Diseases (ICUD) to review the current literature covering the role of FT in localized PCa management. A non-systematic literature search was performed through the PubMed database which focused on the following topics: patient selection, imaging techniques, treatment modalities, cancer control and safety outcomes, integration with other approaches and future perspectives. The results of this analysis were first presented during a joint international consultation of the ICUD and the Société Internationale d’Urologie (SIU) held in New Deli (India) in October 2024. The PubMed search was updated to add the most recent publications.
3. Results
3.1. Patient Selection Criteria
Proper patient selection is key for successful FT. Several international consensuses recommend FT for patients with MRI-visible intermediate-risk organ-confined PCa [8,9]. FT is also endorsed by international guidelines, which emphasize its implementation within clinical trials or prospective registries due to the current lack of high-quality comparative data versus radical treatments [10,11].
While specific prostate-specific antigen (PSA) cutoff values are not strictly defined as eligibility criteria, levels exceeding 20 ng/mL raise concern for non-localized disease [12]. PSA density, which accounts for prostate volume, serves as a valuable tool for patient risk stratification. Despite being promising, the role of biomarkers beyond PSA is not currently recommended in routine clinical practice [13].
Multiparametric MRI plays a crucial dual role in patient selection for FT. First, it guides the initial diagnostic biopsy, which should incorporate both targeting of MRI-visible lesions and systematic sampling. Second, it helps evaluate factors that could impact ablation efficacy, including the presence of calcifications and the proximity of the index lesion to critical structures such as the urethra, urethral sphincter, or rectum.
Preoperative assessment includes the evaluation of baseline functional status using validated questionnaires that measure urinary symptoms, sexual function, and bowel status, establishing a baseline for monitoring post-treatment outcomes [9].
Finally, patient counseling needs to emphasize the importance of rigorous post-ablation follow-up, which is generally more intense during the first year and then gradually becomes less frequent.
3.2. Prostate Cancer Lesion Identification and Localization
Successful FT requires accurate localization of the index lesion within the prostate. Historically, transrectal ultrasound (TRUS) was the standard imaging modality for PCa detection. PCa typically appears hypoechoic on B-mode imaging due to the replacement of normal loose glandular tissue with densely packed tumor cells [14]. However, the positive predictive value of a hypoechoic area ranges only from 18% to 42% [14,15]. Moreover, over 30% of cancers are isoechoic and thus not visible on conventional TRUS [16]. Due to these limitations, while TRUS remains optimal for guiding prostate biopsies due to its cost-effectiveness, it fails as a standalone diagnostic approach.
Therefore, today, mpMRI represents the gold standard for PCa lesion identification. A complete mpMRI protocol combines anatomic (T1- and T2-weighted) and functional sequences (diffusion-weighted imaging and dynamic contrast-enhanced imaging). These sequences work synergistically to detect suspicious areas within the prostate [17]. Several studies confirmed the high sensitivity of mpMRI in localizing the index lesion, with accuracy improving as Gleason grade and lesion size increase [18,19].
Alternative imaging modalities continue to emerge. Multiparametric ultrasound, combining various ultrasound (US) techniques including B-mode, color-doppler, contrast-enhanced ultrasound and elastography, has shown higher sensitivity compared to individual ultrasound modalities [20]. Prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA-PET/CT) has also shown promise, revealing a sensitivity comparable to mpMRI in a head-to-head comparison with the maximum standardized uptake value (SUVmax) value potentially correlating with cancer aggressiveness [21,22]. PSMA PET/mpMRI can further improve diagnostic accuracy, though cost and availability considerations currently limit widespread adoption.
The optimal biopsy approach to assess focal therapy eligibility combines both MRI-targeted and systematic biopsy. While MRI-targeted biopsy enhances the detection of clinically significant prostate cancer by 20–40%, systematic sampling of the entire gland provides precise information about tumor distribution and helps identify the small cancer foci not seen by the MRI outside the planned treatment area [19,23].
3.3. Focal Therapy Techniques
Various energy modalities have been developed for FT for PCa, each with distinct approaches and mechanisms of action (Table 1).

Table 1.
Overview of the main focal therapy techniques, energy sources, mechanisms of action and approaches. HIFU = high-intensity focused ultrasound, TR = transrectal, TP = transperineal and TU = transurethral, TULSA = transurethral ultrasound ablation.
Cryotherapy operates through rapid freezing cycles (below −40 °C) followed by slow thawing, causing cell death through intracellular ice formation, membrane disruption, and microvascular damage. HIFU employs ultrasound waves (0.8–4 MHz) at high intensity (>5 W/cm2) to generate thermal energy (up to 80 °C) and cavitation effects, leading to coagulative necrosis. FLA utilizes laser-induced thermal destruction, with temperatures above 60 °C causing quasi-instantaneous protein denaturation and coagulation. Vascular PDT employs photosensitizing agents that, when activated by specific wavelengths of light, generate intravascular free radicals causing complete vascular arrest. IRE offers non-thermal ablation through high-voltage electrical pulses that create permanent nanopores in cell membranes. Focal brachytherapy delivers targeted radiation through permanently implanted radioactive seeds to a specific area of the prostate.
Several novel approaches are under investigation. Nanoparticle ablation employs gold-silica nanoparticles that generate thermal energy when excited by near-infrared light. Transurethral ultrasound ablation (TULSA) offers real-time MRI-guided therapy with continuous temperature monitoring. Microwave ablation uses radio waves to generate frictional heating, while water vapor ablation represents an adaptation of benign prostatic hyperplasia treatment technology.
Treatment delivery varies by modality and approach. The transperineal approach is typical for cryotherapy, IRE and focal brachytherapy and common for FLA, vascular PDT, nanoparticles and microwave ablation. These procedures generally employ grid-guided probe insertion under TRUS guidance, often utilizing MRI-US fusion for precise targeting. Cryotherapy requires thermocouple probe placement for temperature monitoring, while FLA may incorporate real-time temperature feedback. IRE uniquely requires deep muscle relaxation to prevent unwanted muscle contractions. The transrectal approach is primarily utilized by HIFU, for which rectal cooling systems are essential to prevent thermal injury. Finally, TULSA and water vapor ablation typically adopt a transurethral approach.
Ablation success hinges on several technical considerations. Correct probe placement is essential, with MRI-US fusion technology aiding precise targeting. For multi-probe techniques, such as cryotherapy or IRE, careful probe configuration prevents the undertreatment of the tumor zone. Urethral warming catheters and rectal cooling devices are crucial for protecting critical structures. Special attention must be paid to lesions near the apex, urethra, or rectum. Calcifications can shield HIFU energy, potentially requiring prior transurethral resection. Large prostates may benefit from volume reduction strategies before treatment. The anesthesia choice should balance procedural requirements with patient factors, as movement can compromise targeting accuracy.
3.4. Treatment Planning and Execution
Lesion targeting and margin planning are key aspects of treatment success. On MRI images, the cancer lesion can be identified through cognitive, MRI-US software-based fusion, or in-bore approaches. While in-bore FT is extremely precise but expensive, cognitive approaches may result in excessively wide treatment areas to compensate for the lower accuracy. MRI-US software-based fusion represents an ideal compromise to enhance cancer control outcomes without compromising functional results. Treatment margins of 5–10 mm beyond the index lesion are recommended to ensure adequate treatment coverage and optimize oncological outcomes [8]. The adoption of software enabling the visualization of prior biopsy trajectories and 3D surgical navigation of the prostate can be extremely useful in avoiding undertreatment.
Real-time monitoring during treatment delivery is performed under TRUS guidance. For most FTs, the prostatic parenchyma reacts to treatment becoming hyperintense (popcorn effect), allowing monitoring of treatment coverage. In cryoablation, monitoring focuses on ice ball extension. Integration of sagittal, coronal, and axial TRUS planes is essential for achieving oncologically safe treatment. Less commonly, treatment can be performed under intraoperative MRI guidance, which increases precision but also costs. Some energy modalities, such as FLA, HIFU, and TULSA, incorporate MRI thermometry for real-time temperature feedback [24].
Anesthesia considerations vary by technique. While HIFU typically requires general anesthesia, IRE demands both general anesthesia and deep muscle relaxation to prevent muscle contractions from electrical pulses. FLA and cryoablation offer greater flexibility, potentially allowing office-based treatment under local anesthesia with mild sedation. The choice between local, spinal, or general anesthesia must balance procedural requirements with patient factors.
3.5. Outcomes and Follow-Up Strategies
3.5.1. Focal Therapy Success
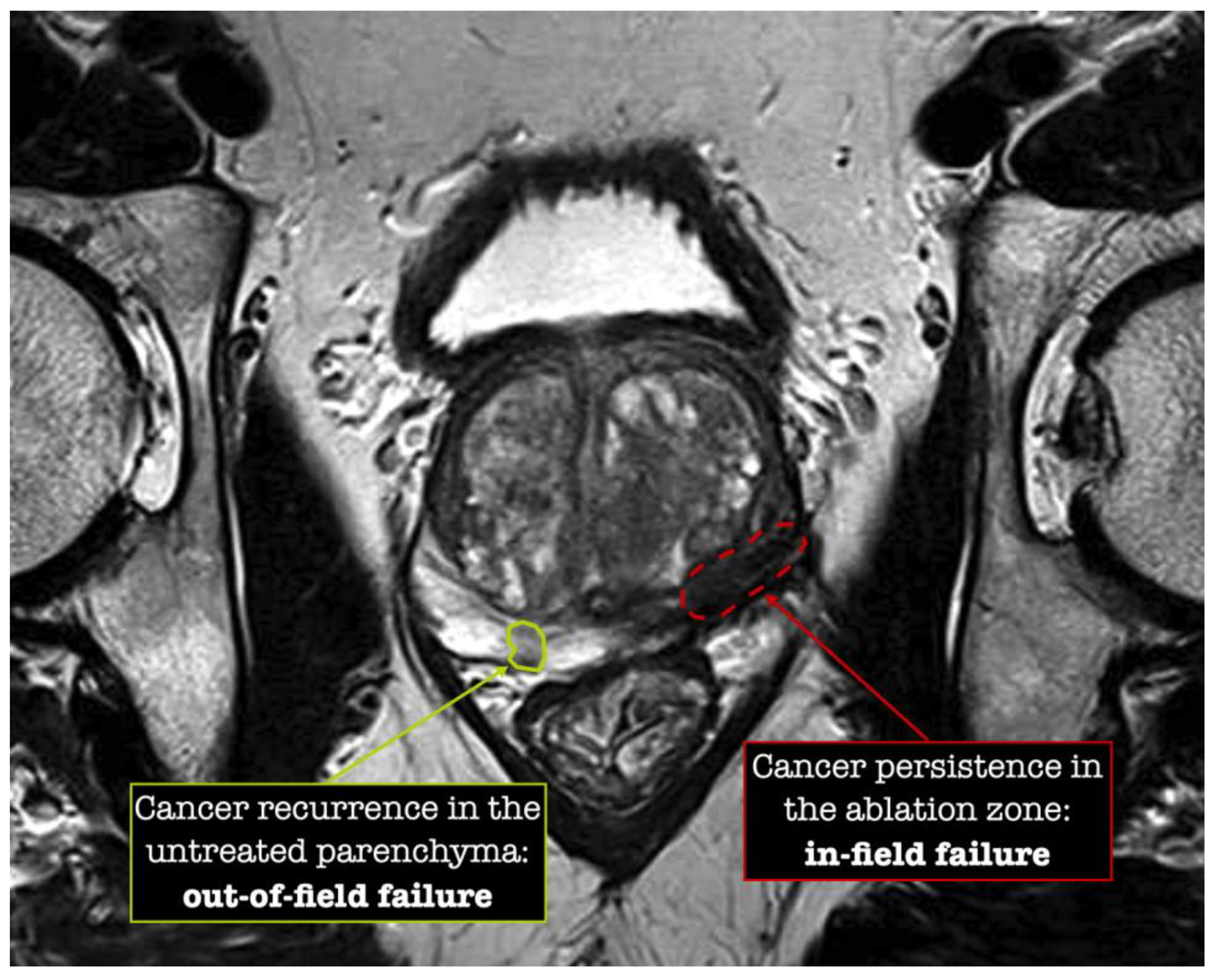
The definition of FT success varies across studies and institutions. From a strict histological perspective, success is primarily defined as the absence of csPCa at post-ablation control biopsy. In-field failures arise from potential inadequacies in energy delivery or targeting precision, while out-of-field failures include progression in pre-existing undiagnosed tumors or the onset of new malignancies, potentially reflecting suboptimal initial patient selection Figure 1 [25,26].
Figure 1.
Patterns of focal therapy failure.
From a broader clinically relevant perspective, success is based on the overall FT strategy, which aims to control csPCa while avoiding or delaying radical and/or systemic treatments. Under this paradigm, neither subsequent focal re-treatments nor surveillance of non-csPCa is considered a failure. This viewpoint raises interesting questions about the interpretation of long-term outcomes—particularly whether progression to radical treatment after 5–10 years should be viewed as a true failure of FT or rather a successful delay of more invasive intervention [27]. Despite these promising concepts, however, FT still requires robust long-term data and comparative studies with standard treatments before it can be widely adopted as a standard of care.
3.5.2. Oncological Outcomes
In-field-failure rates after primary FT range from 8.5% to 17% across different energy modalities (8.5% for IRE, 10% for PDT, 14.7% for HIFU, 15% for cryoablation, and 17% for FLA), although follow-up duration was relatively short in most series [28].
A large UK registry study of 649 patients receiving focal HIFU or cryotherapy reported failure-free survival (no progression to radical treatment, need for systemic therapy, metastatic disease, or cancer-specific death) of 82% at 5 years [29]. The comparative analysis using a pair-matched radical treatments cohort showed lower 5-year failure-free survival for FT (82% vs. 96%), though this difference might be overestimated because 93% of radical treatment patients received RT with its associated use of androgen deprivation therapy.
A recent systematic review of more than 8000 men—enrolled using different inclusion criteria, treated with different FT energies and evaluated along different follow-up times—reported a salvage treatment rate ranging from 0–66.7%, highlighting the high heterogeneity of FT outcomes (Table 2) [30].

Table 2.
Salvage treatment outcomes stratified by focal therapy modality [30].
3.5.3. Functional Outcomes and Complications
FT demonstrates favorable functional outcomes compared to radical treatments. All energy modalities exhibit low toxicity in terms of functionality (Table 3) [31,32,33]. Post-ablation urinary incontinence is a rare event. Erectile dysfunction scores show variable decline across modalities, with IRE potentially offering better preservation of neurovascular tissues. In contrast, radical prostatectomy carries considerable risks of postoperative urinary incontinence (10–30%) and erectile dysfunction (48–83%), while radiotherapy patients face risks of urge incontinence (3–6%) and rectal toxicity (5–16%) [34,35,36]. Assessment through validated questionnaires, such as the International Prostate Symptom Score (IPSS), International Index of Erectile Function (IIEF), Sexual Health Inventory for Men (SHIM) or Expanded Prostate cancer Index Composite (EPIC) scores, is advocated [31].

Table 3.
Functional outcomes stratified by focal therapy modality [33].
Complications primarily occur within the first month post-treatment and include hematuria, hematospermia, infections, and catheter-related issues such as pain and discomfort. Patients should be adequately prepared for such complications to facilitate coping mechanisms [12]. Treatment-induced swelling often necessitates catheterization for 1–7 days, with alpha-blockers potentially aiding prevention of urinary retention. Urethral sloughing varies in frequency by energy modality, being related to the involvement of the prostatic urethra in the ablation zone. Recto-urethral fistula represents the most feared complication, today being rare after primary treatment (0–1%) [37]. Characteristic complications of RP (intraoperative surgical complications, need for transfusion, urinary leakage, lymphocele) and RT (hemorrhagic cystitis, bladder or colorectal fistula, or cancer) are not observed in patients treated with FT [37].
3.5.4. Long-Term Follow-Up Strategies
Post-treatment monitoring combines PSA measurement, imaging, and biopsy. PSA interpretation remains complex due to the untreated prostate parenchyma that continues to produce it.
Multiparametric MRI plays a crucial role in follow-up. In this setting, dynamic contrast-enhanced (DCE) images are the leader sequence and typically exhibit characteristic early enhancement patterns. Heterogeneous and hypointense areas on T2-weighted imaging are also observed. Two scoring systems, the Prostate Imaging after Focal Ablation (PI-FAB) and the Transatlantic Recommendations for Prostate Gland Evaluation with Magnetic Resonance Imaging After Focal Therapy (TARGET), have been proposed to standardize post-focal therapy MRI assessment, both prioritizing DCE [38,39]. However, both scores are awaiting validation.
Given the suboptimal performance of both PSA and MRI in detecting residual disease, histological confirmation through biopsy remains the gold standard and is recommended by most consensus panels [27,40]. Control biopsies should include both sampling of the ablated area and systematic cores and can be performed either according to a predetermined protocol (“for protocol”) or when clinically indicated due to suspicious findings (“for cause”) [27].
Alternative imaging modalities such as PSMA PET/CT show promise in detecting recurrence, though evidence in the post-focal therapy setting remains limited [41,42].
3.6. Management of Focal Therapy Failure
FT failure requires histological confirmation through prostate biopsy and restaging to exclude metastatic disease. In non-metastatic recurrences, standard management includes salvage radiation therapy (sRT) or salvage radical prostatectomy (sRP), with sRP surgical complexity correlating with the extent of previous ablation [43]. Interestingly, although limited, available evidence suggests that both salvage treatments can achieve oncological and functional outcomes comparable or only marginally inferior to the same treatment in a primary setting [44].
In selected patients with localized recurrent disease, a re-do FT may be a feasible option. However, this approach requires careful evaluation of the initial treatment failure and assessment of whether additional FT could maintain cancer control without compromising the timing of definitive salvage treatment in aggressive disease [12].
3.7. Integration with Other Treatment Approaches
FT can be strategically integrated into various stages of PCa management.
While active surveillance (AS) remains safe for very low-risk disease, with 10-year cancer-specific survival rates of 98–100%, FT could serve patients with visible tumors during surveillance, who carry a higher risk of histological progression and conversion to whole-gland treatment [45,46,47]. The prospective randomized PCM301 trial demonstrated that partial ablation of low-risk PCa by PDT significantly reduced subsequent detection of higher-grade cancer and conversion to radical treatment compared to AS [48]. When AS is selected for intermediate-risk PCa, FT can be used to destroy the index tumor while maintaining surveillance protocols. This strategy of “super active surveillance” has emerged as an innovative approach aiming to implement the adoption of AS by safely eliminating or postponing the need for radical treatment [49]. Early experiences with various energy sources have shown promising results in reducing disease upgrading and the need for radical treatment, supporting super-active surveillance as a viable strategy to expand the role of tissue-preserving approaches in PCa management [48,49].
In the salvage setting, FT presents a promising alternative for radiorecurrent disease. While 15–20% of patients experience recurrence within 5 years following radiation therapy, traditional salvage options such as radical prostatectomy or re-irradiation or androgen deprivation therapy carry significant morbidity [50]. Salvage FT, guided by advances in imaging for precise recurrence localization, including mpMRI and PSMA PET/CT scan, offers a chance for local control with minimal burden on patient quality of life. Current evidence supports various energy modalities in this setting, with adjusted 5-year recurrence-free survival ranging from 50% after cryotherapy to 60% after high-dose brachytherapy and stereotactic body RT, with no significant differences compared to RP [51].
Emerging evidence suggests potential benefits in combining FT with systemic treatments, particularly immunotherapy. The local tissue destruction and antigen release during FT may enhance immune responses, potentially affecting both treated and untreated disease sites [52,53]. Early studies combining cryoablation with checkpoint inhibitors have shown promising results in experimental models, though clinical validation remains ongoing [54,55]. Finally, the Comparative Healthcare Research Outcomes of Novel Surgery (CHRONOS)-B arm of the ongoing CHRONOS trial, comparing FT alone versus FT with neoadjuvant agents such as finasteride or bicalutamide, will provide some evidence about this combination [56].
3.8. Future Perspectives and Challenges
The field of FT for PCa keeps evolving rapidly, with several key areas of development shaping its future. Artificial intelligence (AI) and machine learning are expected to play a crucial role in standardizing imaging interpretation, potentially reducing the current inter-reader variability in MRI assessment. Current AI-powered models enhancing lesion detection and risk assessment by analyzing both imaging features and patient-specific characteristics, including demographics and biopsy results, are already under investigation [57].
Great variability exists for FT protocols among different institutions, with the standardization of FT protocols remaining a significant challenge for the future. While short- and intermediate-term data demonstrate favorable effectiveness, newer treatment modalities still require long-term validation. As urologic surgeons gain more experience with FT and robust long-term data become available, greater consensus regarding appropriate candidate selection and monitoring can be expected.
Patient education and awareness is another field requiring development. For decades, RP and RT have dominated PCa treatment discussions. Increasing patient awareness about FT begins at the provider level, requiring comprehensive instruction in prostate MRI interpretation and FT techniques during urologic training. As more institutions employ urologists trained in multimodal PCa treatment approaches, patients will routinely be offered FT options when appropriate.
Finally, several challenges need to be addressed before FT can be widely adopted in clinical practice. Key priorities include establishing standardized follow-up protocols, defining uniform criteria for treatment success, and providing robust long-term oncological data. In this regard, the results of the ongoing CHRONOS trial, an randomized controlled trial (RCT) comparing radical treatment versus FT, are awaited to provide high-level evidence [56]. Additionally, improved tools for patient selection and outcome prediction are needed to optimize treatment strategies. Finally, despite promising cost-effectiveness analyses, broader implementation will require adequate insurance coverage and integration into healthcare systems [58,59,60].
4. Conclusions
FT has emerged as a viable treatment option for carefully selected patients with localized PCa, particularly those with MRI-visible intermediate-risk disease. The evolution of imaging technology, especially MRI, alongside various energy modalities, has enabled precise lesion targeting and ablation while preserving surrounding healthy tissue. Current evidence demonstrates promising oncological control with a significantly lower impact on functional outcomes compared to radical treatments.
The success of FT relies on proper patient selection, which requires expertise in both MRI interpretation and MRI-US fusion biopsy techniques to accurately identify suitable candidates. The choice of ablative energy must be tailored to both patient and tumor characteristics, while accurate post-treatment monitoring is essential to evaluate oncological outcomes through MRI and biopsy, functional results using validated questionnaires, and early recognition of potential complications.
Several challenges need to be addressed for broader implementation, including the standardization of protocols, the establishment of clear success criteria, and the accumulation of long-term oncological data. Additionally, increased provider education and healthcare system integration will be crucial. As imaging technology continues to advance and artificial intelligence applications enhance diagnostic accuracy, FT is poised to play an increasingly important role in the personalized management of PCa.
Author Contributions
Conceptualization: A.R.R., E.B., G.M., H.U.A., M.E., A.V. and T.J.P.; methodology: all authors; validation: all authors; investigation: all authors; resources: all authors; data curation: all authors; writing—original draft preparation: A.M., J.O., T.G.R.B., E.B. and C.D.; writing—review and editing: all authors; visualization: A.M.; supervision: A.R.R., G.M., A.V. and T.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to Scott Eggener, Mack Roach, 3rd, and Laurence Klotz for the opportunity to contribute to the remarkable project of the 3rd WUOF/SIU International Consultation on Urological Diseases (ICUD) on Localized PCa.
Conflicts of Interest
Giancarlo Marra was paid for sponsored lectures and travel expenses by Angiodynamics Inc. Hashim U. Ahmed receives infrastructure support from the NIHR Imperial Biomedical Research Centre and Imperial College Experimental Cancer Medicine Centre; receives core funding from the UK NIHR Imperial Biomedical Research Centre (BRC), the Wellcome Trust, the UK NIHR, the UK Medical Research Council, Cancer Research UK, Prostate Cancer UK, The Urology Foundation, the British Medical Association Foundation, Imperial Health Charity, Sonablate, Trod Medical, and Sophiris Biocorp; has received travel allowance from Sonablate; was a paid consultant for Sophiris Biocorp; sponsored conference attendance by Angiodynamics; medical advisory board for Janssen previously in last 3 years; is a proctor for Rezum treatment and cryotherapy for Boston Scientific and a paid proctor for HIFU by Sonablate. Mark Emberton receives research support from the United Kingdom National Institute of Heath Research (NIHR) via the UCLH/UCL Biomedical Research Centre. He acts as a consultant/lecturer/proctor for Sonacare Inc; Angiodynamics Inc; Early Health and Albermale Medical. Arnauld Villers is involved in studies supported by Astellas, Janssen. Thomas J. Polascik is involved in studies supported by Janssen Bladder Cancer, Myovant Sciences, Prostatype Genomics, Merck, Astellas mCSPC and Astellas mHSPC. Ardeshir R. Rastinehad is a speaker and consultant for Philips Healthcare (Best, The Netherlands) and Nanospectra Biosciences (Houston, TX, USA).
References
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- Bass, E.J.; Ahmed, H.U. Focal therapy in prostate cancer: A review of seven common controversies. Cancer Treat. Rev. 2016, 51, 27–34. [Google Scholar] [CrossRef]
- Valerio, M.; Cerantola, Y.; Eggener, S.E.; Lepor, H.; Polascik, T.J.; Villers, A.; Emberton, M. New and Established Technology in Focal Ablation of the Prostate: A Systematic Review. Eur. Urol. 2017, 71, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Avallone, A.; Jones, J.S. Current state of urological cryosurgery: Prostate and kidney. BJU Int. 2010, 105, 590–600. [Google Scholar] [CrossRef]
- Liu, W.; Laitinen, S.; Khan, S.; Vihinen, M.; Kowalski, J.; Yu, G.; Chen, L.; Ewing, C.M.; Eisenberger, M.A.; Carducci, M.A.; et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat. Med. 2009, 15, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U. The index lesion and the origin of prostate cancer. N. Engl. J. Med. 2009, 361, 1704–1706. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.; Moschini, M.; Montorsi, F.; Cathelineau, X.; Sanchez-Salas, R. Focal therapy for prostate cancer—Index lesion treatment vs. hemiablation. A matter of definition. Int. Braz. J. Urol. 2019, 45, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, I.A.; Alonzi, R.; Barratt, D.; Barret, E.; Berge, V.; Bott, S.; Bottomley, D.; Eggener, S.; Ehdaie, B.; Emberton, M.; et al. Focal Therapy: Patients, Interventions, and Outcomes—A Report from a Consensus Meeting. Eur. Urol. 2015, 67, 771–777. [Google Scholar] [CrossRef]
- Bos, W.v.D.; Muller, B.G.; Ahmed, H.; Bangma, C.H.; Barret, E.; Crouzet, S.; Eggener, S.E.; Gill, I.S.; Joniau, S.; Kovacs, G.; et al. Focal Therapy in Prostate Cancer: International Multidisciplinary Consensus on Trial Design. Eur. Urol. 2014, 65, 1078–1083. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Tilki, D.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part. II—2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024, 86, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Marquis, A.; Suberville, M.; Woo, H.; Govorov, A.; Hernandez-Porras, A.; Bhatti, K.; Turkbey, B.; Katz, A.E.; Polascik, T.J. Surveillance after Focal Therapy—A Comprehensive Review. Prostate Cancer Prostatic Dis. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Laguna, M.P.; Walz, J.; Pavlovich, C.P.; Bianco, F.; Gregg, J.; Lebastchi, A.H.; Lepor, H.; Macek, P.; Rais-Bahrami, S.; et al. Molecular biomarkers in the context of focal therapy for prostate cancer: Recommendations of a Delphi Consensus from the Focal Therapy Society. Minerva Urol. Nephrol. 2022, 74, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Toi, A. Role of Transrectal Ultrasonography in Prostate Cancer. Radiol. Clin. North. Am. 2012, 50, 1061–1073. [Google Scholar] [CrossRef]
- Loch, T.; Eppelmann, U.; Lehmann, J.; Wullich, B.; Loch, A.; Stöckle, M. Transrectal ultrasound guided biopsy of the prostate: Random sextant versus biopsies of sono-morphologically suspicious lesions. World J. Urol. 2004, 22, 357–360. [Google Scholar] [CrossRef]
- Carter, H.B.; Hamper, U.M.; Sheth, S.; Sanders, R.C.; Epstein, J.I.; Walsh, P.C. Evaluation of Transrectal Ultrasound in the Early Detection of Prostate Cancer. J. Urol. 1989, 142, 1008–1010. [Google Scholar] [CrossRef]
- Bonekamp, D.; Jacobs, M.A.; El-Khouli, R.; Stoianovici, D.; Macura, K.J. Advancements in MR Imaging of the Prostate: From Diagnosis to Interventions. RadioGraphics 2011, 31, 677–703. [Google Scholar] [CrossRef]
- Wysock, J.S.; Lepor, H. Multi-parametric MRI imaging of the prostate-implications for focal therapy. Transl. Androl. Urol. 2017, 6, 453–463. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Mannaerts, C.K.; Wildeboer, R.R.; Remmers, S.; van Kollenburg, R.A.A.; Kajtazovic, A.; Hagemann, J.; Postema, A.W.; van Sloun, R.J.G.; Roobol, M.J.; Tilki, D.; et al. Multiparametric Ultrasound for Prostate Cancer Detection and Localization: Correlation of B-mode, Shear Wave Elastography and Contrast Enhanced Ultrasound with Radical Prostatectomy Specimens. J. Urol. 2019, 202, 1166–1173. [Google Scholar] [CrossRef]
- Sonni, I.; Felker, E.R.; Lenis, A.T.; Sisk, A.E.; Bahri, S.; Allen-Auerbach, M.S.; Armstrong, W.R.; Suvannarerg, V.; Tubtawee, T.; Grogan, T.; et al. Head-to-Head Comparison of 68 Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J. Nucl. Med. 2022, 63, 847–854. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, L.; Tamburo, M.; Marletta, G.; Savoca, F.; Pennisi, M.; Fraggetta, F. 68 Ga-PSMA PET/CT and Prostate Cancer Diagnosis: Which SUVmax Value? Vivo 2023, 37, 1318–1322. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Rieke, V.; Kinsey, A.M.; Ross, A.B.; Nau, W.H.; Diederich, C.J.; Sommer, G.; Pauly, K.B. Referenceless MR Thermometry for Monitoring Thermal Ablation in the Prostate. IEEE Trans. Med. Imaging 2007, 26, 813–821. [Google Scholar] [CrossRef]
- Kotamarti, S.; Séguier, D.; Arcot, R.; Polascik, T.J. Assessment after focal therapy: What is the latest? Curr. Opin. Urol. 2022, 32, 260–266. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Hindley, R.G.; Dickinson, L.; Freeman, A.; Kirkham, A.P.; Sahu, M.; Scott, R.; Allen, C.; Van der Meulen, J.; Emberton, M. Focal therapy for localised unifocal and multifocal prostate cancer: A prospective development study. Lancet Oncol. 2012, 13, 622–632. [Google Scholar] [CrossRef]
- Lebastchi, A.H.; George, A.K.; Polascik, T.J.; Coleman, J.; de la Rosette, J.; Turkbey, B.; Wood, B.J.; Gorin, M.A.; Sidana, A.; Ghai, S.; et al. Standardized Nomenclature and Surveillance Methodologies After Focal Therapy and Partial Gland Ablation for Localized Prostate Cancer: An International Multidisciplinary Consensus. Eur. Urol. 2020, 78, 371–378. [Google Scholar] [CrossRef]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef]
- Habashy, D.; Reddy, D.; Peters, M.; Shah, T.T.; van Son, M.; van Rossum, P.S.N.; Tanaka, M.B.; Cullen, E.; Engle, R.; McCracken, S.; et al. Evaluation of Outcomes Following Focal Ablative Therapy for Treatment of Localized Clinically Significant Prostate Cancer in Patients >70 Years: A Multi-institute, Multi-energy 15-Year Experience. J. Urol. 2023, 210, 108–116. [Google Scholar] [CrossRef]
- Nicoletti, R.; Alberti, A.; Castellani, D.; Yee, C.H.; Zhang, K.; Poon, D.M.C.; Chiu, P.K.-F.; Campi, R.; Resta, G.R.; Dibilio, E.; et al. Oncological results and cancer control definition in focal therapy for Prostate Cancer: A systematic review. Prostate Cancer Prostatic Dis. 2023, 27, 623–634. [Google Scholar] [CrossRef]
- Fiard, G.; Chowdhury, A.; Potter, A.R.; Pook, C.J.; Kelly, D.; Emberton, M.; Yap, T. Detailing Sexual Outcomes After Focal Therapy for Localised Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus. 2022, 8, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Chang, J.I.; Böhm, M.; Bos, W.v.D.; Blazevski, A.; Gielchinsky, I.; Kalsbeek, A.M.F.; van Leeuwen, P.J.; Nguyen, T.V.; de Reijke, T.M.; et al. Pair-matched patient-reported quality of life and early oncological control following focal irreversible electroporation versus robot-assisted radical prostatectomy. World J. Urol. 2018, 36, 1383–1389. [Google Scholar] [CrossRef]
- Nicoletti, R.; Alberti, A.; Castellani, D.; Yee, C.H.; Zhang, K.; Poon, D.M.C.; Chiu, P.K.-F.; Campi, R.; Resta, G.R.; Dibilio, E.; et al. Functional outcomes and safety of focal therapy for prostate cancer: A systematic review on results and patient-reported outcome measures (PROMs). Prostate Cancer Prostatic Dis. 2023, 27, 614–622. [Google Scholar] [CrossRef]
- Bridge, J.; Labban, M.; Cole, A.P.; Adebusoye, B.; Smith, S.C.; Protopapa, E.; McCartan, N.; Brew-Graves, C.; Trinh, Q.-D.; Hamer, K.; et al. Urinary and Sexual Impact of Robotic Radical Prostatectomy: Reporting of Patient-reported Outcome Measures in the First Year after Radical Prostatectomy in a Contemporary Multicentre Cohort in the United Kingdom. Eur. Urol. Open Sci. 2024, 64, 11–21. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Penson, D.F.; Zhao, Z.; Huang, L.-C.; Conwill, R.; Laviana, A.A.; Joyce, D.D.; Luckenbaugh, A.N.; Goodman, M.; Hamilton, A.S.; et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020, 3 23, 149. [Google Scholar] [CrossRef]
- Lane, J.A.; Donovan, J.L.; Young, G.J.; Davis, M.; Walsh, E.I.; Avery, K.N.; Blazeby, J.M.; Mason, M.D.; Martin, R.M.; Peters, T.J.; et al. Functional and quality of life outcomes of localised prostate cancer treatments (Prostate Testing for Cancer and Treatment [ProtecT] study). BJU Int. 2022, 130, 370–380. [Google Scholar] [CrossRef]
- Matta, R.; Chapple, C.R.; Fisch, M.; Heidenreich, A.; Herschorn, S.; Kodama, R.T.; Koontz, B.F.; Murphy, D.G.; Nguyen, P.L.; Nam, R.K. Pelvic Complications After Prostate Cancer Radiation Therapy and Their Management: An International Collaborative Narrative Review. Eur. Urol. 2019, 75, 464–476. [Google Scholar] [CrossRef]
- Giganti, F.; Dickinson, L.; Orczyk, C.; Haider, A.; Freeman, A.; Emberton, M.; Allen, C.; Moore, C.M. Prostate Imaging after Focal Ablation (PI-FAB): A Proposal for a Scoring System for Multiparametric MRI of the Prostate After Focal Therapy. Eur. Urol. Oncol. 2023, 6, 629–634. [Google Scholar] [CrossRef]
- Light, A.; Mayor, N.; Cullen, E.; Kirkham, A.; Padhani, A.R.; Arya, M.; Bomers, J.G.; Dudderidge, T.; Ehdaie, B.; Freeman, A.; et al. The Transatlantic Recommendations for Prostate Gland Evaluation with Magnetic Resonance Imaging After Focal Therapy (TARGET): A Systematic Review and International Consensus Recommendations. Eur. Urol. 2024, 85, 466–482. [Google Scholar] [CrossRef]
- Muller, B.G.; van den Bos, W.; Brausi, M.; Fütterer, J.J.; Ghai, S.; Pinto, P.A.; Popeneciu, I.V.; De Reijke, T.M.; Robertson, C.; De La Rosette, J.J.M.C.H.; et al. Follow-up modalities in focal therapy for prostate cancer: Results from a Delphi consensus project. World J. Urol. 2015, 33, 1503–1509. [Google Scholar] [CrossRef]
- Donato, P.; Morton, A.; Yaxley, J.; Ranasinghe, S.; Teloken, P.E.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1843–1851. [Google Scholar] [CrossRef]
- Spohn, S.; Jaegle, C.; Fassbender, T.F.; Sprave, T.; Gkika, E.; Nicolay, N.H.; Bock, M.; Ruf, J.; Benndorf, M.; Gratzke, C.; et al. Intraindividual comparison between 68Ga-PSMA-PET/CT and mpMRI for intraprostatic tumor delineation in patients with primary prostate cancer: A retrospective analysis in 101 patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Lebdai, S.; Villers, A.; Barret, E.; Nedelcu, C.; Bigot, P.; Azzouzi, A.-R. Feasibility, safety, and efficacy of salvage radical prostatectomy after Tookad® Soluble focal treatment for localized prostate cancer. World J. Urol. 2015, 33, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Valerio, M.; Emberton, M.; Heidenreich, A.; Crook, J.M.; Bossi, A.; Pisters, L.L. Salvage Local Treatments After Focal Therapy for Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 526–538. [Google Scholar] [CrossRef]
- Shill, D.K.; Roobol, M.J.; Ehdaie, B.; Vickers, A.J.; Carlsson, S.V. Active surveillance for prostate cancer. Transl. Androl. Urol. 2021, 10, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.; Li, W.; Nieboer, D.; Helleman, J.; Roobol, M.; Gnanapragasam, V.; Frydenberg, M.; Sugimoto, M.; Carroll, P.; Morgan, T.M.; et al. Prostate Cancer Patients Under Active Surveillance with a Suspicious Magnetic Resonance Imaging Finding Are at Increased Risk of Needing Treatment: Results of the Movember Foundation’s Global Action Plan Prostate Cancer Active Surveillance (GAP3) Consortium. Eur. Urol. Open Sci. 2022, 35, 59–67. [Google Scholar] [CrossRef]
- Stavrinides, V.; Giganti, F.; Trock, B.; Punwani, S.; Allen, C.; Kirkham, A.; Freeman, A.; Haider, A.; Ball, R.; McCartan, N.; et al. Five-year Outcomes of Magnetic Resonance Imaging-based Active Surveillance for Prostate Cancer: A Large Cohort Study. Eur. Urol. 2020, 78, 443–451. [Google Scholar] [CrossRef]
- Azzouzi, A.-R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
- Bloom, J.B.; Gold, S.A.; Hale, G.R.; Rayn, K.N.; Sabarwal, V.K.; Bakhutashvili, I.; Valera, V.; Turkbey, B.; Pinto, P.A.; Wood, B.J. “Super-active surveillance”: MRI ultrasound fusion biopsy and ablation for less invasive management of prostate cancer. Gland. Surg. 2018, 7, 166–187. [Google Scholar] [CrossRef]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.-P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients with Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol 2018, 4, e180039. [Google Scholar] [CrossRef]
- Valle, L.F.; Lehrer, E.J.; Markovic, D.; Elashoff, D.; Levin-Epstein, R.; Karnes, R.J.; Reiter, R.E.; Rettig, M.; Calais, J.; Nickols, N.G.; et al. A Systematic Review and Meta-analysis of Local Salvage Therapies After Radiotherapy for Prostate Cancer (MASTER). Eur. Urol. 2021, 80, 280–292. [Google Scholar] [CrossRef]
- Baust, J.G.; Bischof, J.C.; Jiang-Hughes, S.; Polascik, T.J.; Rukstalis, D.B.; Gage, A.A.; Baust, J.M. Re-purposing cryoablation: A combinatorial “therapy” for the destruction of tissue. Prostate Cancer Prostatic Dis. 2015, 18, 87–95. [Google Scholar] [CrossRef]
- Klossner, D.P.; Robilotto, A.; VanBuskirk, R.G.; Gage, A.A.; Mouraviev, V.; Polascik, T.J.; Baust, J.G. Vitamin D(3) cryosensitization increases prostate cancer susceptibility to cryoablation via mitochondrial-mediated apoptosis and necrosis. BJU Int. 2012, 109, 949–958. [Google Scholar] [CrossRef]
- Yakkala, C.; Chiang, C.L.-L.; Kandalaft, L.; Denys, A.; Duran, R. Cryoablation and Immunotherapy: An Enthralling Synergy to Confront the Tumors. Front. Immunol. 2019, 10, 2283. [Google Scholar] [CrossRef]
- Sidana, A. Cancer immunotherapy using tumor cryoablation. Immunotherapy 2014, 6, 85–93. [Google Scholar] [CrossRef]
- Reddy, D.; Shah, T.T.; Dudderidge, T.; McCracken, S.; Arya, M.; Dobbs, C.; Emberton, M.; Fiorentino, F.; Day, E.; Prevost, A.T.; et al. Comparative Healthcare Research Outcomes of Novel Surgery in prostate cancer (IP4-CHRONOS): A prospective, multi-centre therapeutic phase II parallel Randomised Control Trial. Contemp. Clin. Trials 2020, 93, 105999. [Google Scholar] [CrossRef]
- Turkbey, B.; Haider, M.A. Artificial Intelligence for Automated Cancer Detection on Prostate MRI: Opportunities and Ongoing Challenges, From the AJR Special Series on AI Applications. Am. J. Roentgenol. 2022, 219, 188–194. [Google Scholar] [CrossRef]
- Nabi, J.; Friedlander, D.F.; Chen, X.; Cole, A.P.; Hu, J.C.; Kibel, A.S.; Dasgupta, P.; Trinh, Q.-D. Assessment of Out-of-Pocket Costs for Robotic Cancer Surgery in US Adults. JAMA Netw. Open 2020, 3, e1919185. [Google Scholar] [CrossRef]
- Paravati, A.J.; Boero, I.J.; Triplett, D.P.; Hwang, L.; Matsuno, R.K.; Xu, B.; Mell, L.K.; Murphy, J.D. Variation in the Cost of Radiation Therapy Among Medicare Patients With Cancer. J. Oncol. Pract. 2015, 11, 403–409. [Google Scholar] [CrossRef]
- Reddy, D.; van Son, M.; Peters, M.; Tanaka, M.B.; Dudderidge, T.; Cullen, E.; Ho, C.L.T.; Hindley, R.G.; Emara, A.; McCracken, S.; et al. Focal therapy versus radical prostatectomy and external beam radiotherapy as primary treatment options for non-metastatic prostate cancer: Results of a cost-effectiveness analysis. J. Med. Econ. 2023, 26, 1099–1107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).