Between Foragers and Farmers: Climate Change and Human Strategies in Northwestern Patagonia
Abstract
:1. Introduction
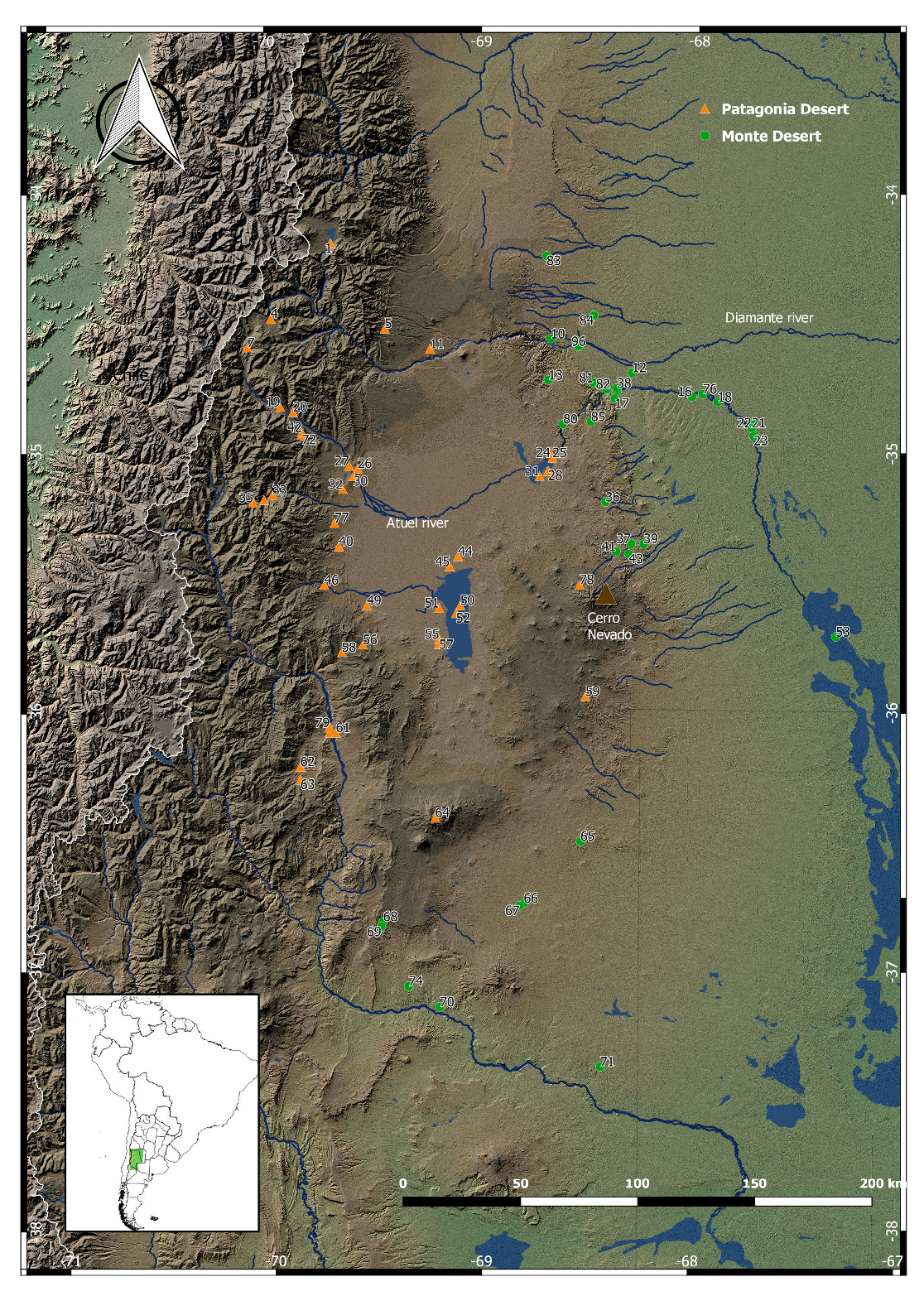
1.1. Climate and Environment in Northwestern Patagonia during the Last Millennium
1.2. Human Population Dynamic and Climate in Northwestern Patagonia: Background and Expectations
2. Materials and Methods
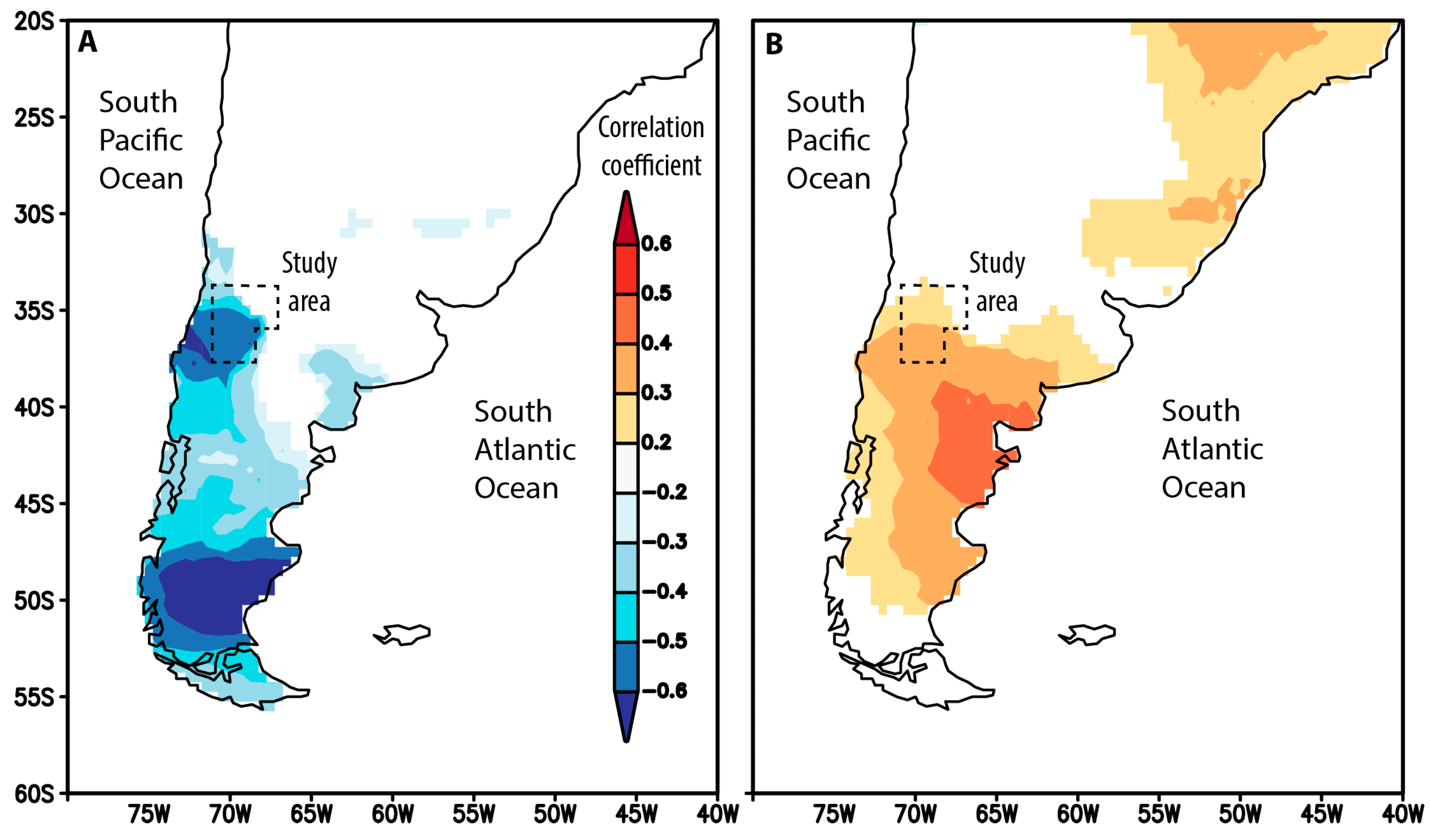
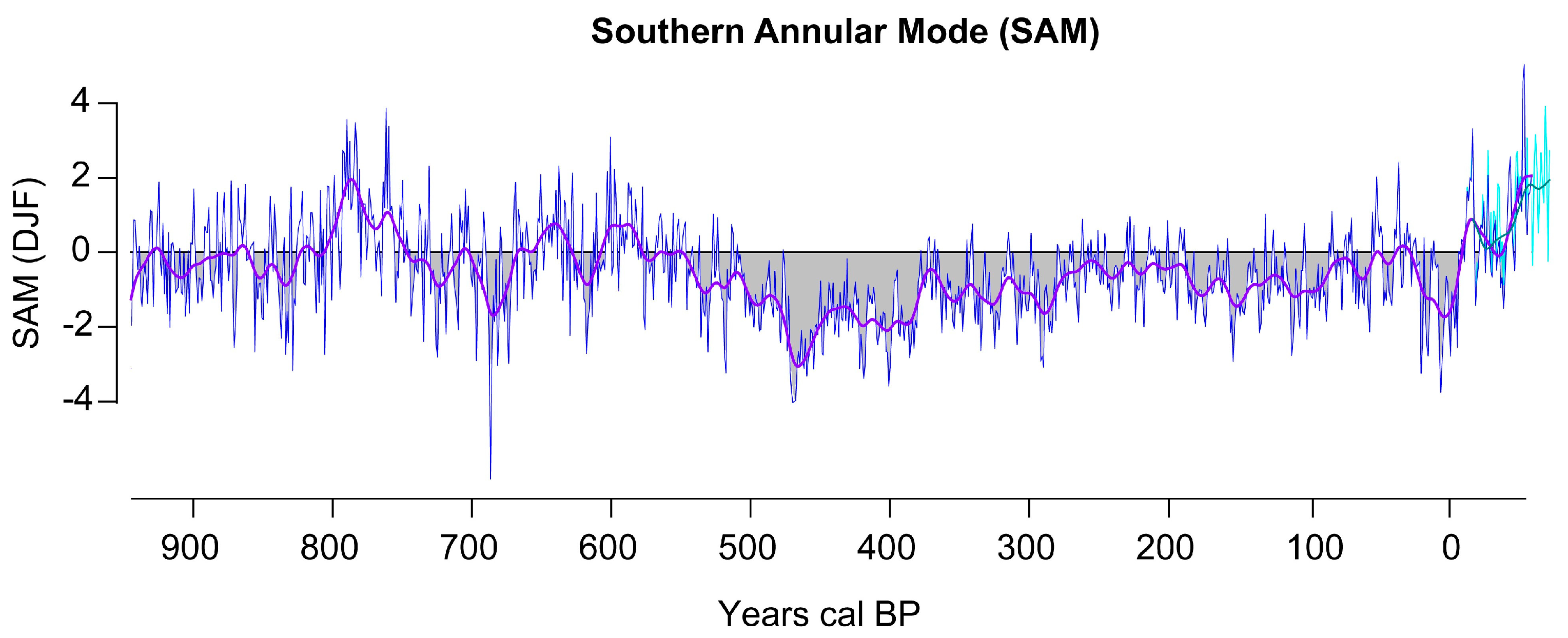
2.1. SAM Reconstruction
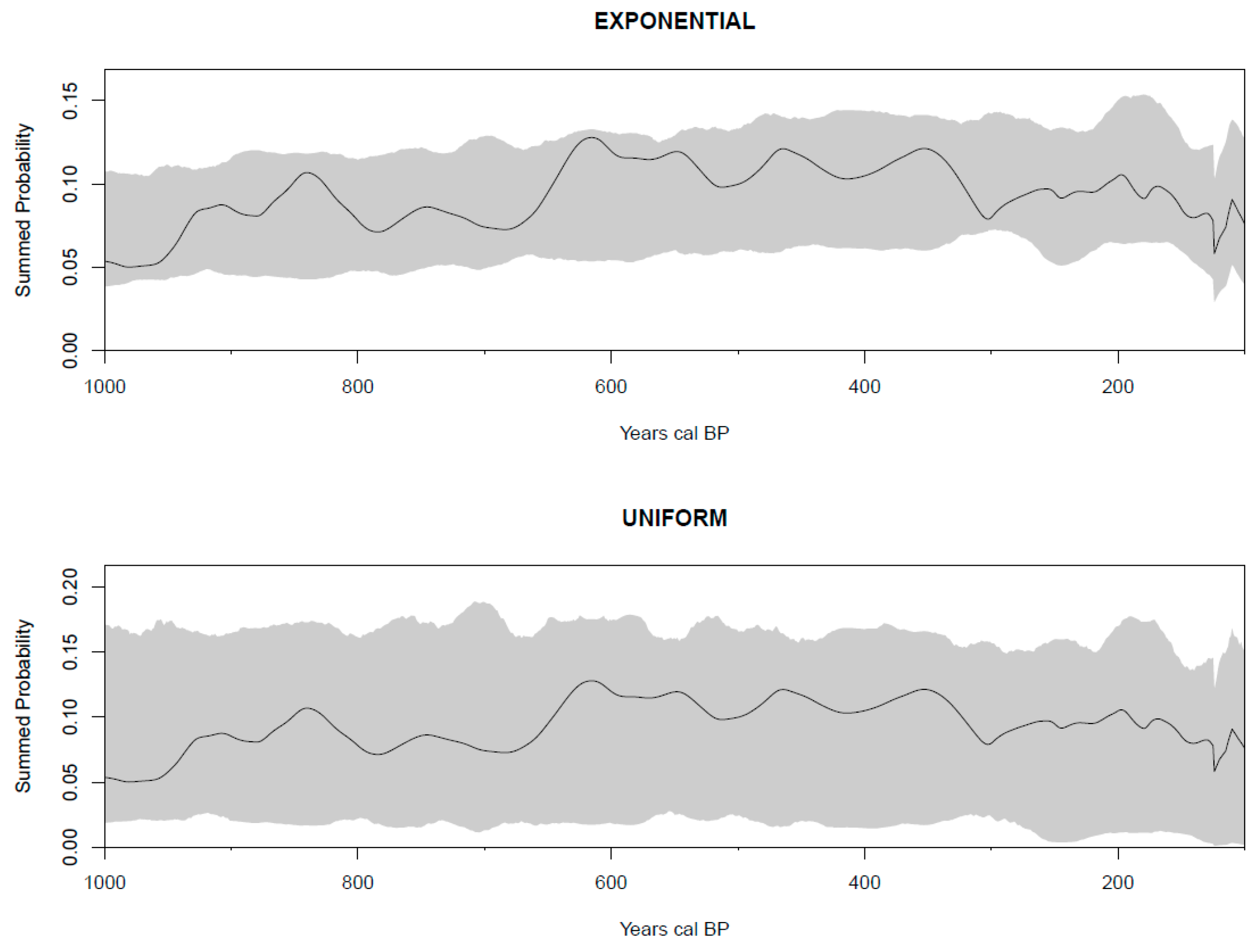
2.2. Radiocarbon Dates as Data: Summed Probability Distribution (SPD)
2.3. Bone Collagen Stable Isotope Carbon and Nitrogen
2.4. Artiodactyl Index
3. Results
3.1. SAM Reconstruction for the Last 1000 Years
3.2. Human Populations in Northwestern Patagonia: Trends in Radiocarbon SPD
3.3. Stable Isotopes (C and N) in Human Bone Collagen
3.4. Artiodactyl Index
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fagan, B. The Little Ice Age: How Climate Made History 1300-1850; Basic Books: Hachette, UK, 2000. [Google Scholar]
- Bettinger, R.L. Orderly Anarchy: Sociopolitical Evolution in Aboriginal California; University of California Press: Berkeley, CA, USA, 2015; Volume 8. [Google Scholar]
- Brothwell, D.R.; Cohen, M.N. The Food Crisis in Prehistory. Overpopulation and the Origins of Agriculture. Popul. Stud. 1978, 32, 593. [Google Scholar] [CrossRef]
- Shennan, S. The First Farmers of Europe; Cambridge University Press (CUP): Cambridge, UK, 2018. [Google Scholar]
- Kroeber, A.L. Cultural and Natural Areas of Native North America; University of California Press: Berkeley, CA, USA, 1939; Volume 38. [Google Scholar]
- Sauer, C. American Agriculture Origins: A Consideration of Nature and Culture. In Essays in Anthropology Presented to A. L. Kroeber in Celebration of His Sixtieth Birthday; University of California Press: Berkeley, CA, USA, 1936. [Google Scholar]
- Svizzero, S.; Tisdell, C.A. The Persistence of Hunting and Gathering Economies. Soc. Evol. Hist. 2015, 14, 3–26. [Google Scholar]
- Freeman, J. Alternative adaptive regimes for integrating foraging and farming activities. J. Archaeol. Sci. 2012, 39, 3008–3017. [Google Scholar] [CrossRef]
- Gil, A.F.; Menendez, L.P.; Atencio, J.P.; Peralta, E.A.; Neme, G.A.; Ugan, A. Estrategias Humanas, Estabilidad Y Cambio en la Frontera Agricola Sur Americana. Lat. Am. Antiq. 2017, 29, 6–26. [Google Scholar] [CrossRef] [Green Version]
- Simms, S.R. Ancient Peoples of the Great Basin and Colorado Plateau; Left Coast Press: Walnut Creek, CA, USA, 2016. [Google Scholar]
- Lagiglia, H. Actas del V Congreso Nacional de Arqueología Argentina; Universidad Nacional de San Juan: San Juan, Argentina, 1982; Volume 1, pp. 231–252. [Google Scholar]
- Gil, A.F. Cultígenos Prehispánicos En El Sur de Mendoza. Relac. Soc. Argent. Antropol. 1998, 22, 295–318. [Google Scholar]
- Johnson, A.; Gil, A.; Neme, G.; Freeman, J. Hierarchical method using ethnographic data sets to guide archaeological research: Testing models of plant intensification and maize use in Central Western Argentina. J. Anthr. Archaeol. 2015, 38, 52–58. [Google Scholar] [CrossRef]
- Lagiglia, H. Dinámica Cultural En El Centro Oeste y Sus Relaciones Con Áreas Aledañas Argentinas y Chilenas. In Actas del VII Congreso de Arqueología Chilena. Tomo II; Sociedad Chilena de Arqueologia: Santiago de Chile, Chile, 1977; pp. 531–560. [Google Scholar]
- Gil, A.F.; Giardina, M.A.; Neme, G.A.; Ugan, A. Demografía Humana e Incorporación de Cultígenos En El Centro Occidente Argentino: Explorando Tendencias En Las Fechas Radiocarbónicas; Universidad Complutense de Madrid: Madrid, Spain, 2015; Volume 44. [Google Scholar] [CrossRef]
- Lagiglia, H.A. Primeros Contactos Hispanico-Indígenas de Mendoza:(La Arqueología Histórica y Su Periodificación); Museo de Historia Natural de San Rafael: San Rafael, Argentina, 1983. [Google Scholar]
- Prieto, M. Formación y Consolidación de Una Sociedad de Frontera En Un Área Marginal Del Reino de Chile: La Provincia de Cuyo En El Siglo XVII. An. Arqueol. Etnol. 2000, 52, 17–366. [Google Scholar]
- Neme, G.A. Un Enfoque Regional En Cazadores-Recolectores Del Oeste Argentino: El Potencial de La Ecología Humana. In Perspectivas acutales en Arqueología Argentina; Dunken: Buenos Aires, Argentina, 2009; pp. 306–326. [Google Scholar]
- Bernal, V.; Gonzalez, P.N.; Gordón, F.; Perez, S.I. Exploring Dietary Patterns in the Southernmost Limit of Prehispanic Agriculture in America by Using Bayesian Stable Isotope Mixing Models. Curr. Anthr. 2016, 57, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Corbat, M.; Zangrando, A.F.J.; Gil, A.F.; Chiavazza, H.D. Explotacion de Peces e intensificación en ambientes áridos: Comparando el registro en humedales del centro-occidente de argentina. Lat. Am. Antiq. 2017, 28, 196–212. [Google Scholar] [CrossRef]
- Neme, G. Cazadores-recolectores de altura en los Andes meridionales: El alto valle del río Atuel, Argentina; BAR Publishing: Oxford, UK, 2007; Volume 1591. [Google Scholar]
- Abraham, E.; Del Valle, H.; Roig, F.; Torres, L.M.; Ares, J.; Coronato, F.; Godagnone, R. Overview of the geography of the Monte Desert biome (Argentina). J. Arid Environ. 2009, 73, 144–153. [Google Scholar] [CrossRef]
- Cabrera, A.L. Regiones Fitogeográficas Argentinas; Acme: Buenos Aires, Argentina, 1976. [Google Scholar]
- Roig, F.; Roig-Juñent, S.; Corbalán, V. Biogeography of the Monte Desert. J. Arid Environ. 2009, 73, 164–172. [Google Scholar] [CrossRef]
- Otaola, C.; Ugan, A.; Gil, A. Environmental diversity and stable isotope variation in faunas: Implications for human diet reconstruction in Argentine mid-latitude deserts. J. Archaeol. Sci. Rep. 2018, 20, 57–71. [Google Scholar] [CrossRef]
- Labraga, J.C.; Villalba, R. Climate in the Monte Desert: Past trends, present conditions, and future projections. J. Arid Environ. 2009, 73, 154–163. [Google Scholar] [CrossRef]
- Marshall, G.J. Trends in the Southern Annular Mode from Observations and Reanalyses. J. Clim. 2003, 16, 4134–4143. [Google Scholar] [CrossRef]
- Gillet, C.; Quétin, P. Effect of temperature changes on the reproductive cycle of roach in Lake Geneva from 1983 to 2001. J. Fish Boil. 2006, 69, 518–534. [Google Scholar] [CrossRef]
- Earthdata. Available online: https://earthdata.nasa.gov (accessed on 15 April 2020).
- Perez, S.I.; Gonzalez, P.N.; Bernal, V. Past population dynamics in Northwest Patagonia: An estimation using molecular and radiocarbon data. J. Archaeol. Sci. 2016, 65, 154–160. [Google Scholar] [CrossRef]
- Giardina, M.; Corbat, M.; Otaola, C.; Salgán, L.; Ugan, A.; Neme, G.; Gil, A. Recursos y Dietas Humanas en Laguna Llancanelo (Mendoza, Nordpatagonia): Una Discusión Isotópica del Registro Arqueológico. Magallania (Punta Arenas) 2014, 42, 111–131. [Google Scholar] [CrossRef] [Green Version]
- Tripaldi, A.; Zárate, M.; Neme, G.; Gil, A.; Giardina, M.; Salgán, M.L. Archaeological site formation processes in northwestern Patagonia, Mendoza Province, Argentina. Geoarchaeology 2017, 32, 605–621. [Google Scholar] [CrossRef]
- Barberena, R.; Borrazzo, K.; Rughini, A.A.; Romero, G.; Pompei, M.P.; Llano, C.; De Porras, M.E.; Durán, V.; Stern, C.R.; Re, A.; et al. Perspectivas arqueológicas para Patagonia Septentrional: Sitio Cueva Huenul 1 (Provincia del Neuquén, Argentina). Magallania (Punta Arenas) 2015, 43, 137–163. [Google Scholar] [CrossRef] [Green Version]
- Neme, G.; Gil, A.; Otaola, C.; Giardina, M. Resource Exploitation and Human Mobility: Trends in the Archaeofaunal and Isotopic Record from Central Western Argentina. Int. J. Osteoarchaeol. 2013, 25, 866–876. [Google Scholar] [CrossRef]
- Gil, A.F. Arqueología de La Payunia (Mendoza, Argentina): El poblamiento humano en los márgenes de la agricultura. Payunia (Mendoza, Argentina) 2006, 1477. [Google Scholar] [CrossRef]
- Neme, G.; Gil, A.; Durán, V. Late Holocene in Southern Mendoza (Northwestern Patagonia) Radiocarbon Pattern and Human Occupation. Before Farming 2005, 2005, 1–18. [Google Scholar] [CrossRef]
- Martínez, G. Arqueología y Pobladores Antiguos de La Cuenca Del Río Colorado; Serie Aportes al Desarrollo Nacional de la Fundacion ArgenINTA: Buenos Aires, Argentina, 2015. [Google Scholar]
- Berón, M. Integración de Evidencias Para Evaluar Dinámica y Circulación de Poblaciones En Las Fronteras Del Río Colorado; CEQUA: Punta Arena, Chile, 2007. [Google Scholar]
- Franchetti, F. Hunter-Gatherer Adaptation in the Deserts of Northern Patagonia; University of Pittsburgh: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Wolverton, S.; Otaola, C.; Neme, G.; Giardina, M.; Gil, A. Patch Choice, Landscape Ecology, and Foraging Efficiency: The Zooarchaeology of Late Holocene Foragers in Western Argentina. J. Ethnobiol. 2015, 35, 499–518. [Google Scholar] [CrossRef]
- Rindel, D. Explorando La Variabilidad En El Registro Zooarqueológico de La Provincia Del Neuquén: Tendencias Cronológicas y Patrones de Uso Antrópico; Gordon, F., Barberena, R., Bernal, V., Eds.; Aspha: Buenos Aires, Argentina, 2017. [Google Scholar]
- Otaola, C.; Giardina, M.; Corbat, M.; Fernández, F. Zooarqueología en el sur de Mendoza: Integrando Perspectivas en un Marco Biogeográfico; Neme, G., Gil, A., Eds.; Sociedad Argentina de Antropología: Buenos Aires, Argentina, 2012. [Google Scholar]
- Corbat, M.; Gil, A.; Zangrando, A.F. Ranking de Recursos y El Rol de Los Peces En Las Dietas Humanas Del Centro Occidente Argentino. In MC Salemme et al. (Comps.), Libro de Resúmenes del IV Congreso Nacional de Zooarqueología Argentina; Congreso Nacional de Zooarqueologia: Buenos Aires, Argentina, 2016; p. 92. Available online: https://www.researchgate.net/publication/309459807_IV_Congreso_Nacional_de_Zooarqueologia_Argentina_Libro_de_Resumenes (accessed on 15 January 2020).
- Neme, G.A.; Gil, A.F. Faunal exploitation and agricultural transitions in the South American agricultural limit. Int. J. Osteoarchaeol. 2008, 18, 293–306. [Google Scholar] [CrossRef]
- Otaola, C.; Wolverton, S.; Giardina, M.; Neme, G. Geographic scale and zooarchaeological analysis of Late Holocene foraging adaptations in western Argentina. J. Archaeol. Sci. 2015, 55, 16–25. [Google Scholar] [CrossRef]
- Giardina, M.A. El Aprovechamiento de La Avifauna Entre Las Sociedades Cazadoras-Recolectoras Del Sur de Mendoza: Un Enfoque Arqueozoológico; Universidad Nacional de La Plata: La Plata Argentina, Argentina, 2010. [Google Scholar]
- Llano, C. On optimal use of a patchy environment: Archaeobotany in the Argentinean Andes (Argentina). J. Archaeol. Sci. 2015, 54, 182–192. [Google Scholar] [CrossRef]
- Smith, B.D. Low-Level Food Production. J. Archaeol. Res. 2001, 9, 1–43. [Google Scholar] [CrossRef]
- Neme, G. El Indígeno and High-Altitude Human Occupation in the Southern Andes, Mendoza (Argentina). Lat. Am. Antiq. 2016, 27, 96–114. [Google Scholar] [CrossRef]
- Winterhalder, B.; Kennett, D.J. Behavioral Ecology and the Transition to Agriculture; University of California Press: Berkeley, CA, USA, 2006; Volume 1. [Google Scholar]
- Lema, V.S.; Della Negra, C.; Bernal, V. Explotación de recursos vegetales silvestres y domesticados en Neuquén: Implicancias del hallazgo de restos de maíz y algarrobo en artefactos de molienda del holoceno tardío. Magallania (Punta Arenas) 2012, 40, 229–247. [Google Scholar] [CrossRef] [Green Version]
- Llano, C.; Barberena, R. Explotación de Especies Vegetales En La Patagonia Septentrional: El Registro Arqueobotánico de Cueva Huenul 1 (Provincia de Neuquén, Argentina). Darwiniana 2013, 1, 5–19. [Google Scholar]
- Freeman, J.; Peeples, M.; Anderies, J.M. Toward a theory of non-linear transitions from foraging to farming. J. Anthr. Archaeol. 2015, 40, 109–122. [Google Scholar] [CrossRef]
- Zahid, H.J.; Robinson, E.; Kelly, R.L. Agriculture, population growth, and statistical analysis of the radiocarbon record. Proc. Natl. Acad. Sci. USA 2015, 113, 931–935. [Google Scholar] [CrossRef] [Green Version]
- Simms, S.R. Behavioral Ecology and Hunter-Gatherer Foraging: An example from the Great Basin; BAR Publishing: Oxford, UK, 1987; Volume 381. [Google Scholar]
- Bettinger, R.L. Hunter-Gatherer Foraging; Eliot Werner Publications: New York, NY, USA, 2009. [Google Scholar]
- Winterhalder, B.; Kennett, D.J. Seven Behavioral Ecology Reasons for the Persistence of Foragers with Cultivars. In Cowboy Ecologist: Essays in Honor of Robert L. Bettinger; Delacorte, M., Terry, J.L., Eds.; CARD (Center for Archaeological Research at Davis): Davis, CA, USA, 2020; pp. 93–110. [Google Scholar]
- Lupo, K. Evolutionary Foraging Models in Zooarchaeological Analysis: Recent Applications and Future Challenges. J. Archaeol. Res. 2007, 15, 143–189. [Google Scholar] [CrossRef]
- Dätwyler, C.; Neukom, R.; Abram, N.J.; Gallant, A.J.E.; Grosjean, M.; Jacques-Coper, M.; Karoly, D.J.; Villalba, R. Teleconnection stationarity, variability and trends of the Southern Annular Mode (SAM) during the last millennium. Clim. Dyn. 2017, 51, 2321–2339. [Google Scholar] [CrossRef]
- Bianchi, E.; Villalba, R.; Solarte, A. NDVI Spatio-temporal Patterns and Climatic Controls over Northern Patagonia. Ecosystem 2019, 23, 84–97. [Google Scholar] [CrossRef]
- Crema, E.R.; Bevan, A.; Shennan, S. Spatio-temporal approaches to archaeological radiocarbon dates. J. Archaeol. Sci. 2017, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Robinson, E.; Nicholson, C.; Kelly, R.L. The Importance of Spatial Data to Open-Access National Archaeological Databases and the Development of Paleodemography Research. Adv. Archaeol. Pr. 2019, 7, 395–408. [Google Scholar] [CrossRef] [Green Version]
- Rick, J.W. Dates as Data: An Examination of the Peruvian Preceramic Radiocarbon Record. Am. Antiq. 1987, 52, 55. [Google Scholar] [CrossRef]
- Bevan, A.; Colledge, S.; Fuller, D.Q.; Fyfe, R.; Shennan, S.; Stevens, C.J. Holocene fluctuations in human population demonstrate repeated links to food production and climate. Proc. Natl. Acad. Sci. USA 2017, 114, E10524–E10531. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.; A Byers, D.; Robinson, E.; Kelly, R.L. Culture Process and the Interpretation of Radiocarbon Data. Radiocarbon 2017, 60, 453–467. [Google Scholar] [CrossRef] [Green Version]
- Shennan, S.; Downey, S.S.; Timpson, A.; Edinborough, K.; Colledge, S.; Kerig, T.; Manning, K.; Thomas, M.G. Regional population collapse followed initial agriculture booms in mid-Holocene Europe. Nat. Commun. 2013, 4, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surovell, T.A.; Brantingham, P.J. A note on the use of temporal frequency distributions in studies of prehistoric demography. J. Archaeol. Sci. 2007, 34, 1868–1877. [Google Scholar] [CrossRef]
- Timpson, A.; Colledge, S.; Crema, E.R.; Edinborough, K.; Kerig, T.; Manning, K.; Thomas, M.G.; Shennan, S. Reconstructing regional population fluctuations in the European Neolithic using radiocarbon dates: A new case-study using an improved method. J. Archaeol. Sci. 2014, 52, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Gordón, F.; Béguelin, M.; Rindel, D.; Della Negra, C.; Hajduk, A.; Vazquez, R.; Cobos, V.; Perez, S.I.; Bernal, V. Estructura Espacial y Dinámica Temporal de La Ocupación Humana de Neuquén (Patagonia Argentina) Durante El Pleistoceno Final-Holoceno. Intersecc. Antropol. 2019, 20, 93–105. [Google Scholar]
- Kelly, R.L.; Surovell, T.A.; Shuman, B.N.; Smith, G.M. A continuous climatic impact on Holocene human population in the Rocky Mountains. Proc. Natl. Acad. Sci. USA 2012, 110, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peros, M.C.; Munoz, S.E.; Gajewski, K.; Viau, A. Prehistoric demography of North America inferred from radiocarbon data. J. Archaeol. Sci. 2010, 37, 656–664. [Google Scholar] [CrossRef]
- Riede, F. Climate and Demography in Early Prehistory: Using Calibrated 14 C Dates as Population Proxies. Hum. Boil. 2009, 81, 309–337. [Google Scholar] [CrossRef] [Green Version]
- Surovell, T.A.; Finley, J.B.; Smith, G.M.; Brantingham, P.J.; Kelly, R.L. Correcting temporal frequency distributions for taphonomic bias. J. Archaeol. Sci. 2009, 36, 1715–1724. [Google Scholar] [CrossRef]
- Bevan, A.; Crema, E.R. Rcarbon v1. 2.0: Methods for Calibrating and Analy. Available online: https://CRAN.R-project.org/package=rcarbon (accessed on 31 March 2020).
- Manning, K.; Timpson, A. The demographic response to Holocene climate change in the Sahara. Quat. Sci. Rev. 2014, 101, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Crema, E.R.; Habu, J.; Kobayashi, K.; Madella, M. Summed Probability Distribution of 14C Dates Suggests Regional Divergences in the Population Dynamics of the Jomon Period in Eastern Japan. PLoS ONE 2016, 11, e0154809. [Google Scholar] [CrossRef] [Green Version]
- Durán, V. Nuevas Consideraciones Sobre La Problemática Arqueológica Del Río Grande (Malargüe, Mendoza); Gil, A.F., Neme, G.A., Eds.; Sociedad Argentina de Antropología: Buenos Aires, Argentina, 2002. [Google Scholar]
- Roberts, N.; Woodbridge, J.; Bevan, A.; Palmisano, A.; Shennan, S.; Asouti, E. Human responses and non-responses to climatic variations during the last Glacial-Interglacial transition in the eastern Mediterranean. Quat. Sci. Rev. 2018, 184, 47–67. [Google Scholar] [CrossRef]
- Katzenberg, M.A. Stable Isotope Analysis: A Tool for Studying Past Diet, Demography, and Life History. In Biological Anthropology of the Human Skeleton; Wiley: Hoboken, NJ, USA, 2008; pp. 411–441. [Google Scholar]
- Gil, A.F.; Ugan, A.; Neme, G.A. More Carnivorous than Vegetarian: Isotopic Perspectives on Human Diets in Late Holocene Northwestern Patagonia. J. Archaeol. Sci. Rep 2019. in review. [Google Scholar]
- Gil, A.; Neme, G.; Tykot, R.H. Stable isotopes and human diet in central western Argentina. J. Archaeol. Sci. 2011, 38, 1395–1404. [Google Scholar] [CrossRef]
- Coltrain, J.B.; Janetski, J.C.; Carlyle, S.W. The Stable and Radio-Isotope Chemistry of Eastern Basketmaker and Pueblo Groups in the Four Corners Region of the American Southwest. In Histories of Maize; Elsevier BV: Amsterdam, The Netherlands, 2006; pp. 275–287. [Google Scholar]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Hedges, R.E.M. On bone collagen?apatite-carbonate isotopic relationships. Int. J. Osteoarchaeol. 2003, 13, 66–79. [Google Scholar] [CrossRef]
- Coltrain, J.B.; Leavitt, S.W. Climate and Diet in Fremont Prehistory: Economic Variability and Abandonment of Maize Agriculture in the Great Salt Lake Basin. Am. Antiq. 2002, 67, 453–485. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Norr, L. Experimental Evidence for the Relationship of the Carbon Isotope Ratios of Whole Diet and Dietary Protein to Those of Bone Collagen and Carbonate; Springer Science and Business Media LLC: Berlin, Germany, 1993; pp. 1–37. [Google Scholar]
- Tieszen, L.L.; Fagre, T. Effect of Diet Quality and Composition on the Isotopic Composition of Respiratory CO2, Bone Collagen, Bioapatite, and Soft Tissues; Springer Science and Business Media LLC: Berlin, Germany, 1993; pp. 121–155. [Google Scholar]
- Fernandes, R.; Nadeau, M.-J.; Grootes, P.M. Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol. Anthr. Sci. 2012, 4, 291–301. [Google Scholar] [CrossRef]
- Ambrose, S.H. Controlled Diet and Climate Experiments on Nitrogen Isotope Ratios of Rats. In Advances in Archaeological and Museum Science; Springer Science and Business Media LLC: Berlin, Germany, 2006; Volume 5, pp. 243–259. [Google Scholar]
- Hedges, R.E.; Reynard, L.M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 2007, 34, 1240–1251. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D.G. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- Tykot, R.H.; Falabella, F.; Planella, M.T.; Aspillaga, E.; Sanhueza, L.; Becker, C. Stable isotopes and archaeology in central Chile: Methodological insights and interpretative problems for dietary reconstruction. Int. J. Osteoarchaeol. 2009, 19, 156–170. [Google Scholar] [CrossRef]
- Froehle, A.; Kellner, C.; Schoeninger, M. FOCUS: Effect of diet and protein source on carbon stable isotope ratios in collagen: Follow up to. J. Archaeol. Sci. 2010, 37, 2662–2670. [Google Scholar] [CrossRef]
- Keeling, C.D. The Suess effect: 13Carbon-14Carbon interrelations. Environ. Int. 1979, 2, 229–300. [Google Scholar] [CrossRef]
- Athfield, N.B.; Green, R.C.; Craig, J.; McFadgen, B.; Bickler, S. Influence of marine sources on 14 C ages: Isotopic data from Watom Island, Papua New Guinea inhumations and pig teeth in light of new dietary standards. J. R. Soc. N. Z. 2008, 38, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Telford, R.J.; Heegaard, E.; Birks, H.J.B. The intercept is a poor estimate of a calibrated radiocarbon age. Holocene 2004, 14, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Gil, A.; Ugan, A.; Otaola, C.; Neme, G.; Giardina, M.; Menendez, L.P. Variation in camelid δ13C and δ15N values in relation to geography and climate: Holocene patterns and archaeological implications in central western Argentina. J. Archaeol. Sci. 2016, 66, 7–20. [Google Scholar] [CrossRef]
- Ascough, P.; Mainland, I.; Newton, A. From Isoscapes to Farmscapes: Introduction to the Special Issue. Environ. Archaeol. 2018, 23, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bowen, G.J. Isoscapes: Spatial Pattern in Isotopic Biogeochemistry. Annu. Rev. Earth Planet. Sci. 2010, 38, 161–187. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Fan, J.; Zhong, H.; Li, Y.; Zhu, H.; Qiao, Y.; Zhang, H. A multi-factor weighted regression approach for estimating the spatial distribution of soil organic carbon in grasslands. Catena 2019, 174, 248–258. [Google Scholar] [CrossRef]
- Barrientos, G.; Catella, L.; Morales, N.S. A journey into the landscape of past feeding habits: Mapping geographic variations in the isotope (δ15N) -inferred trophic position of prehistoric human populations. Quat. Int. 2020. [Google Scholar] [CrossRef]
- Janetski, J.C. Fremont Hunting and Resource Intensification in the Eastern Great Basin. J. Archaeol. Sci. 1997, 24, 1075–1088. [Google Scholar] [CrossRef] [Green Version]
- Bayham, F.E. Factors Influencing the Archaic Pattern of Animal Exploitation. KIVA 1979, 44, 219–235. [Google Scholar] [CrossRef]
- Broughton, J.M. Late Holocene Resource Intensification in the Sacramento Valley, California: The Vertebrate Evidence. J. Archaeol. Sci. 1994, 21, 501–514. [Google Scholar] [CrossRef]
- Broughton, J.M. Declines in Mammalian Foraging Efficiency during the Late Holocene, San Francisco Bay, California. J. Anthr. Archaeol. 1994, 13, 371–401. [Google Scholar] [CrossRef]
- Lyman, R.L. Quantitative Paleozoology by R. Lee Lyman; Cambridge University Press (CUP): Cambridge, UK, 2008. [Google Scholar]
- Neme, G.A.; Gil, A.F.; Abbona, C.C.; Otaola, C.; Nagaoka, L.; Giardina, M.; Wolverton, S.; Johnson, J. Exploring the Environmental Rebound in Northwest Patagonia: Zooarchaeological, Stable Isotope, Radiocarbon Frequency and DNA Trends; Book Unpublished; 2020. [Google Scholar]
- Dieguez, S.; Gil, A.; Neme, G.; Zárate, M.; De Francesco, C.; Strasser, E. Cronoestratigrafía Del Sitio Rincón Del Atuel-1 (San Rafael, Mendoza): Formación Del Sitio y Ocupación Humana. Intersecc. Antropol. 2004, 5, 71–80. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Baxter, M.J. Statistics in Archaeology; Arnold: London, UK, 2003. [Google Scholar]
- Espizua, L.E.; Pitte, P. The Little Ice Age glacier advance in the Central Andes (35° S), Argentina. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 281, 345–350. [Google Scholar] [CrossRef]
- Villalba, R. Climatic Fluctuations in Northern Patagonia during the Last 1000 Years as Inferred from Tree-Ring Records. Quat. Res. 1990, 34, 346–360. [Google Scholar] [CrossRef]
- Masiokas, M.H.; Rivera, A.; Espizua, L.E.; Villalba, R.; Delgado, S.; Aravena, J.C. Glacier fluctuations in extratropical South America during the past 1000 years. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 281, 242–268. [Google Scholar] [CrossRef]
- IPCC. The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Volume 996, p. 2007. [Google Scholar]
- Benson, L. Factors Controlling Pre-Columbian and Early Historic Maize Productivity in the American Southwest, Part 1: The Southern Colorado Plateau and Rio Grande Regions. J. Archaeol. Method Theory 2010, 18, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Claessens, L.; Antle, J.M.; Stoorvogel, J.J.; Valdivia, R.; Thornton, P.; Herrero, M. A method for evaluating climate change adaptation strategies for small-scale farmers using survey, experimental and modeled data. Agric. Syst. 2012, 111, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Demeritt, D. Agriculture, Climate, and Cultural Adaptation in the Prehistoric Northeast. Archaeol. East. N. Am. 1991, 19, 183–202. [Google Scholar]
- Maddonni, G. Ángel Analysis of the climatic constraints to maize production in the current agricultural region of Argentina—A probabilistic approach. Theor. Appl. Clim. 2011, 107, 325–345. [Google Scholar] [CrossRef]
- Gil, A.; Villalba, R.; Ugan, A.; Cortegoso, V.; Neme, G.; Michieli, C.T.; Novellino, P.; Durán, V. Isotopic evidence on human bone for declining maize consumption during the little ice age in central western Argentina. J. Archaeol. Sci. 2014, 49, 213–227. [Google Scholar] [CrossRef]
- Heaton, T.H.E.; Vogel, J.C.; Von La Chevallerie, G.; Collett, G. Climatic influence on the isotopic composition of bone nitrogen. Nature 1986, 322, 822–823. [Google Scholar] [CrossRef]
- Sealy, J.; Van Der Merwe, N.J.; Thorp, J.A.; Lanham, J.L. Nitrogen isotopic ecology in southern Africa: Implications for environmental and dietary tracing. Geochim. Cosmochim. Acta 1987, 51, 2707–2717. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Deniro, M.J. Climate and Habitat Reconstruction Using Stable Carbon and Nitrogen Isotope Ratios of Collagen in Prehistoric Herbivore Teeth from Kenya. Quat. Res. 1989, 31, 407–422. [Google Scholar] [CrossRef]
- Gil, A.; Otaola, C.; Neme, G.; Peralta, E.A.; Abbona, C.; Quiroga, G.; Dauverné, A.; Seitz, V.P. Lama guanicoe bone collagen stable isotope (C and N) indicate climatic and ecological variation during Holocene in Northwest Patagonia. Quat. Int. 2019. [Google Scholar] [CrossRef]
- De Fina, A.L.; Giannetto, F.; Richard, A.E.; Sabella, L. Difusión Geográfica de Cultivos Índices En La Provincia de Mendoza y Sus Causas. INTA Inst. Suelos Agrotec. Mendoza 1964, 83, 2–87. [Google Scholar]
- Barlow, K.R. Predicting Maize Agriculture among the Fremont: An Economic Comparison of Farming and Foraging in the American Southwest. Am. Antiq. 2002, 67, 65–88. [Google Scholar] [CrossRef]
- Michieli, C.T. Antigua Historia de Cuyo (Siglos XVI a XVIII); Ansilta: San Juan, Argentina, 1994. [Google Scholar]
- Denevan, W.M. The Pristine Myth: The Landscape of the Americas in 1492. Ann. Assoc. Am. Geogr. 1992, 82, 369–385. [Google Scholar] [CrossRef]
- Denevan, W.M. After 1492: Nature Rebounds. Geogr. Rev. 2016, 106, 381–398. [Google Scholar] [CrossRef]
- O’Fallon, B.D.; Fehren-Schmitz, L. Native Americans experienced a strong population bottleneck coincident with European contact. Proc. Natl. Acad. Sci. USA 2011, 108, 20444–20448. [Google Scholar] [CrossRef] [PubMed] [Green Version]

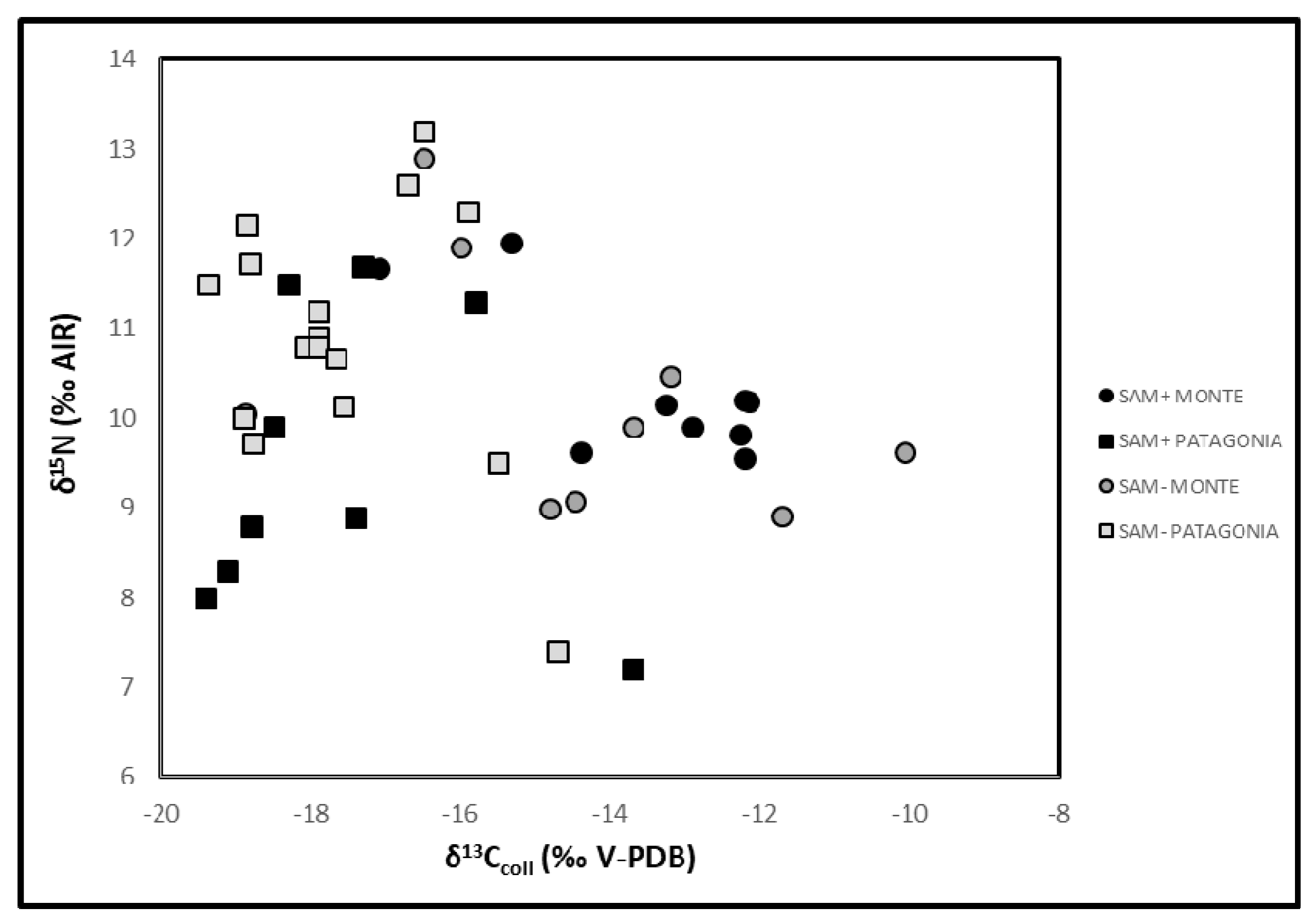
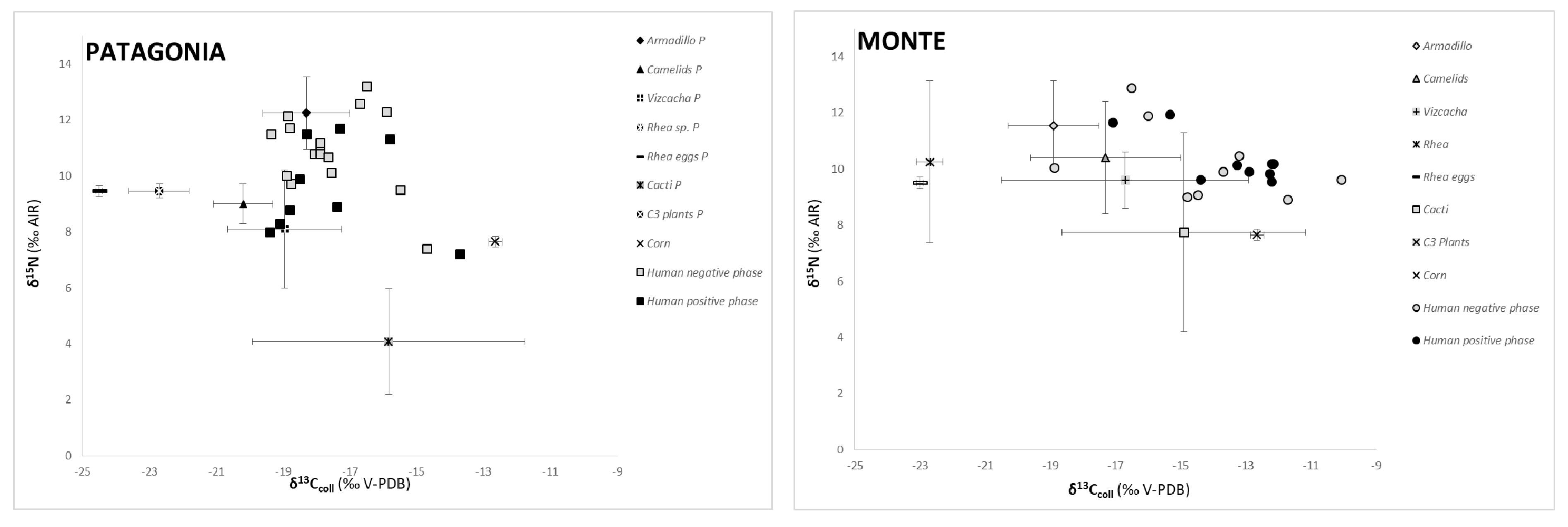


| Descriptive Statistics | SAM Positive Phase | SAM Negative Phase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MONTE | PATAGONIA | MONTE | PATAGONIA | |||||||||
| δ13Ccoll | δ13Ccarb | δ15N | δ13Ccol | δ13Ccarb | δ15N | δ13Ccol | δ13Ccarb | δ15N | δ13Ccol | δ13Ccarb | δ15N | |
| N | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 16 | 16 | 16 |
| Min | −7.1 | −12.3 | 9.6 | −19.4 | −14.5 | 7.2 | −8.9 | −13.5 | 8.9 | 9.4 | −14.9 | 7.4 |
| Max | −12.1 | −6.5 | 12.0 | −13.7 | −7.6 | 11.7 | −10.1 | −5.1 | 12.9 | −14.7 | −8.2 | 13.2 |
| Mean | −13.5 | −8.4 | 10.3 | −17.6 | −12.9 | 9.5 | −14.4 | −8.8 | 10.2 | −17.6 | −12.8 | 10.9 |
| SD | 1.7 | 1.8 | 0.9 | 1.8 | 2.1 | 1.7 | 2.6 | 2.6 | 1.4 | 1.4 | 1.9 | 1.4 |
| Median | −12.9 | −8.0 | 10.2 | −18.3 | −13.6 | 8.9 | −14.5 | −7.9 | 9.9 | −17.9 | −13.1 | 10.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, A.F.; Villalba, R.; Franchetti, F.R.; Otaola, C.; Abbona, C.C.; Peralta, E.A.; Neme, G. Between Foragers and Farmers: Climate Change and Human Strategies in Northwestern Patagonia. Quaternary 2020, 3, 17. https://doi.org/10.3390/quat3020017
Gil AF, Villalba R, Franchetti FR, Otaola C, Abbona CC, Peralta EA, Neme G. Between Foragers and Farmers: Climate Change and Human Strategies in Northwestern Patagonia. Quaternary. 2020; 3(2):17. https://doi.org/10.3390/quat3020017
Chicago/Turabian StyleGil, Adolfo F., Ricardo Villalba, Fernando R. Franchetti, Clara Otaola, Cinthia C. Abbona, Eva A. Peralta, and Gustavo Neme. 2020. "Between Foragers and Farmers: Climate Change and Human Strategies in Northwestern Patagonia" Quaternary 3, no. 2: 17. https://doi.org/10.3390/quat3020017





