Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments
Abstract
:1. Introduction
2. Materials and Methods
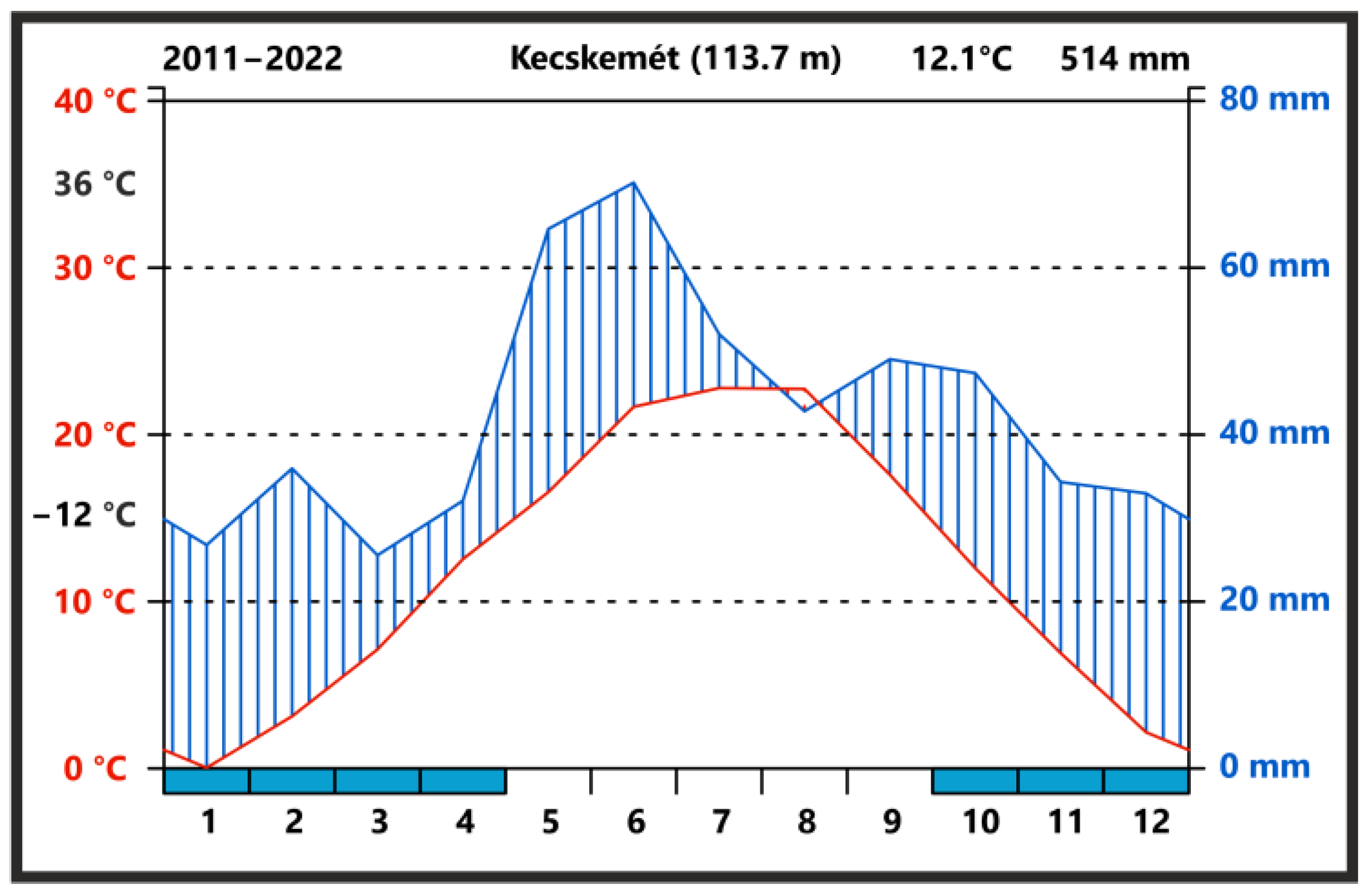
2.1. Study Area
2.2. Sampling and Stratigraphy
2.3. Radiocarbon Dating
2.4. Magnetic Susceptibility, Grain Size, and Loss on Ignition
2.5. Geochemical Analysis
- Water extraction to obtain water-soluble ions from weathered minerals, carbonates, salts, and precipitates, and ions bound slightly on mineral surfaces;
- Extraction with an NH4-acetate/acetic acid buffer to obtain the ions of carbonates, precipitates, gels and allophans;
- DCB extraction to obtain the ions of Al, Fe, Mn, and Ti-containing oxy-hydroxy-gels;
- Extraction with acid Na2EDTA to obtain the ions of the remaining carbonates, gels, allophanes, and ions bound strongly on mineral surfaces;
- Wet total digestion of the cleaned crystalline fraction with 65% HNO3 and 35% HF to obtain ions of well-crystallized carbonate-, gel-, and allophan-free minerals.
2.6. Statistical Analysis
3. Results
3.1. Radiocarbon, Magnetic Susceptibility, Grain Size, and Loss-on-Ignition Analysis
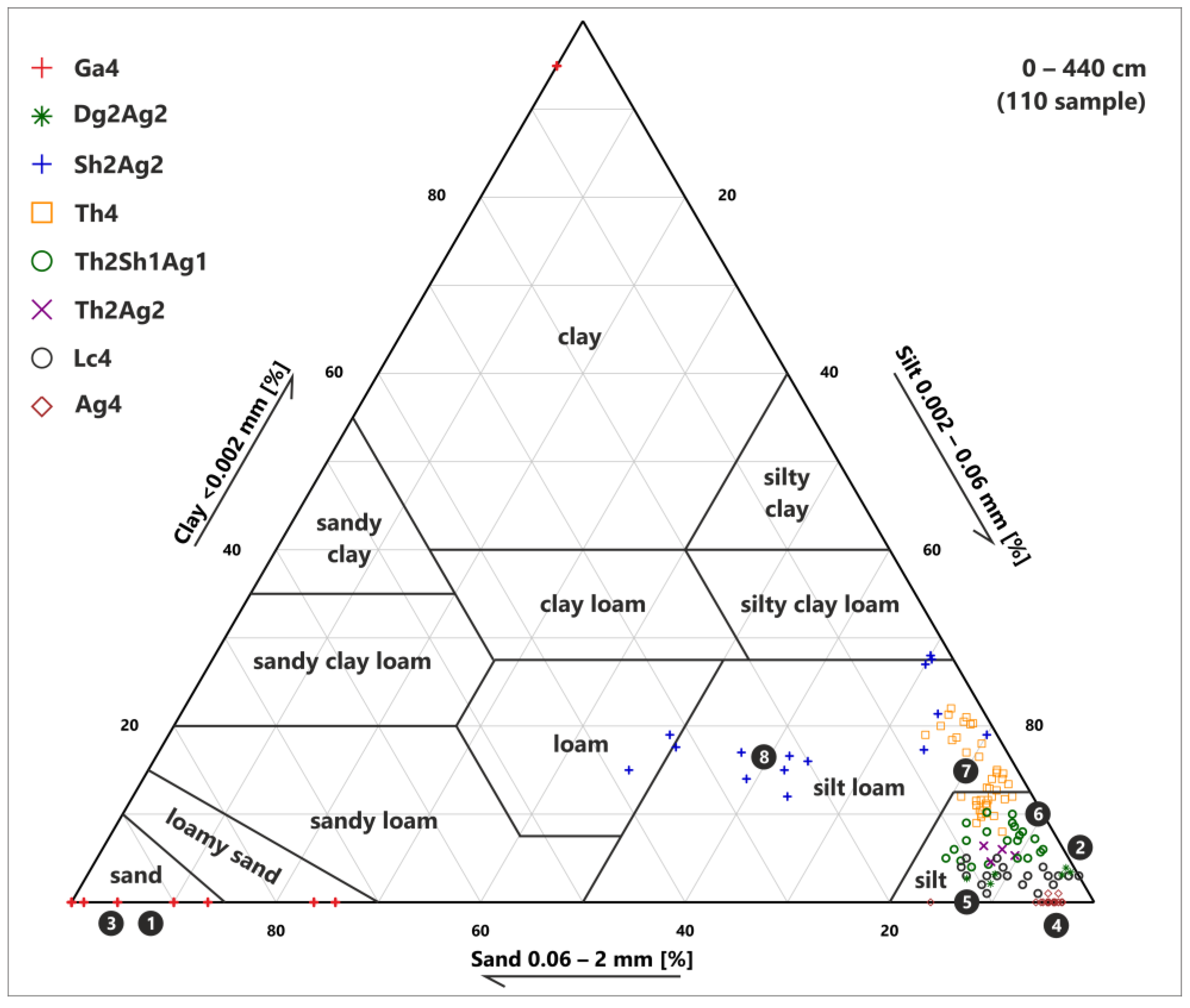
3.2. Sediment Classification
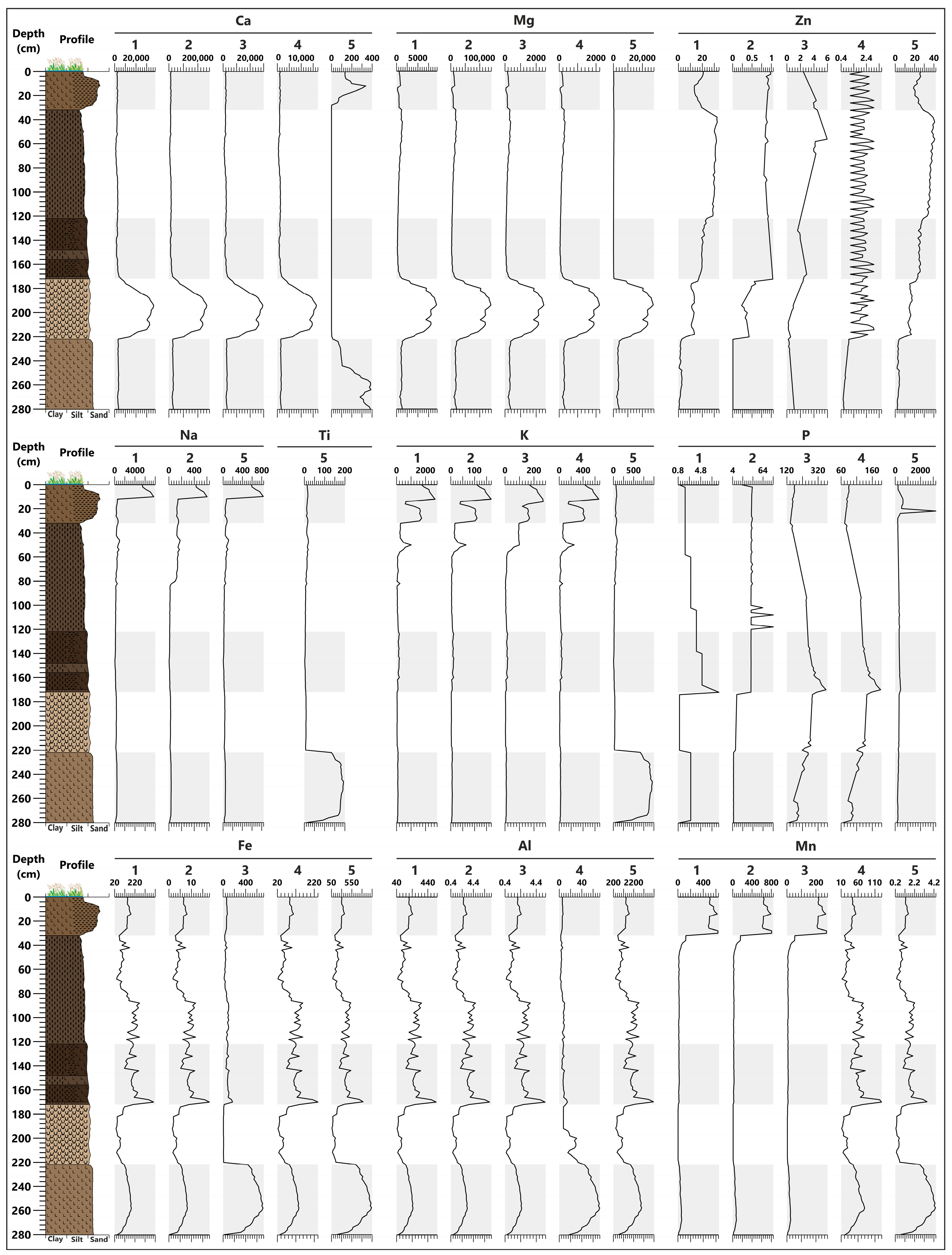
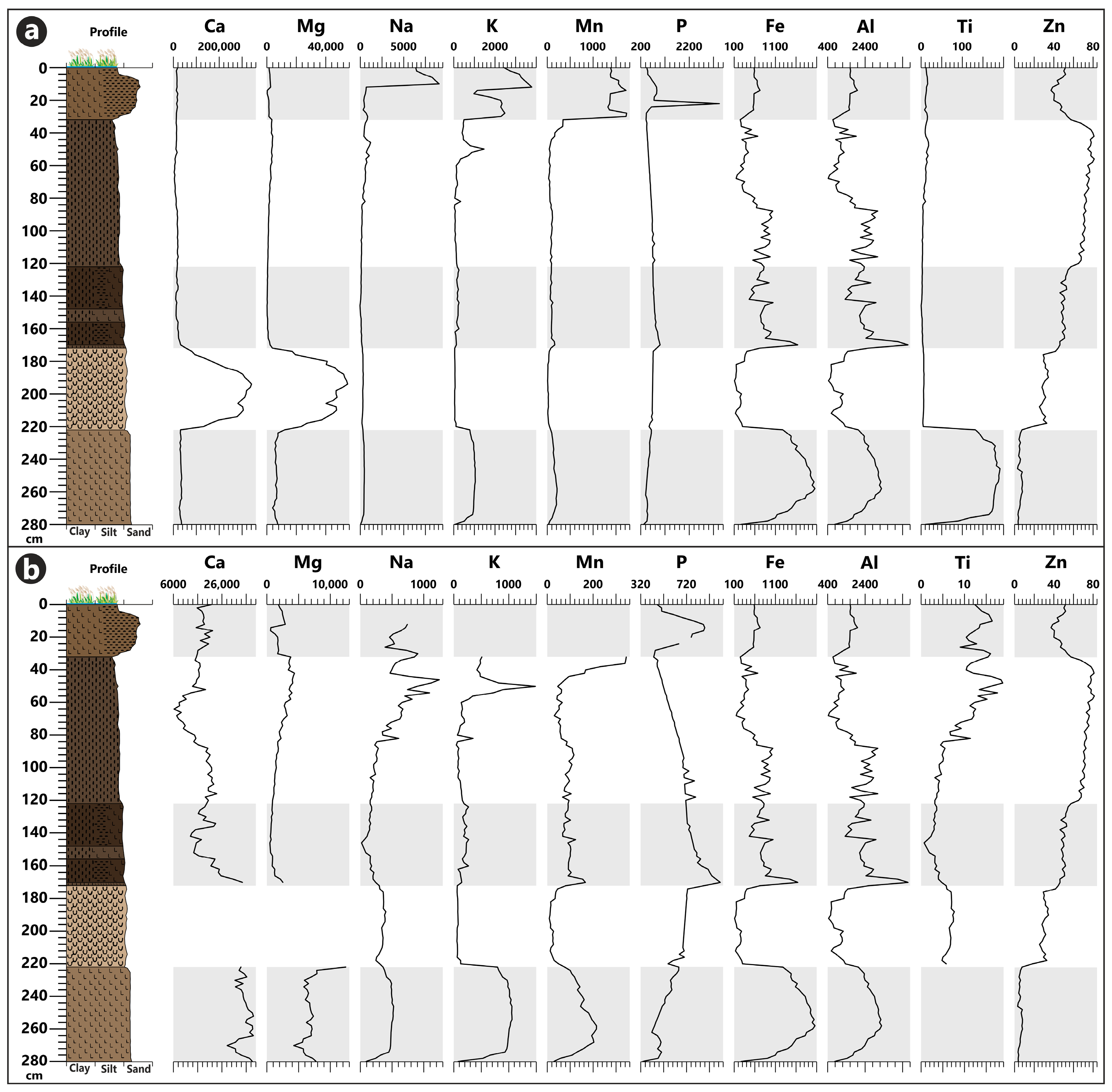
3.3. Geochemical Analysis
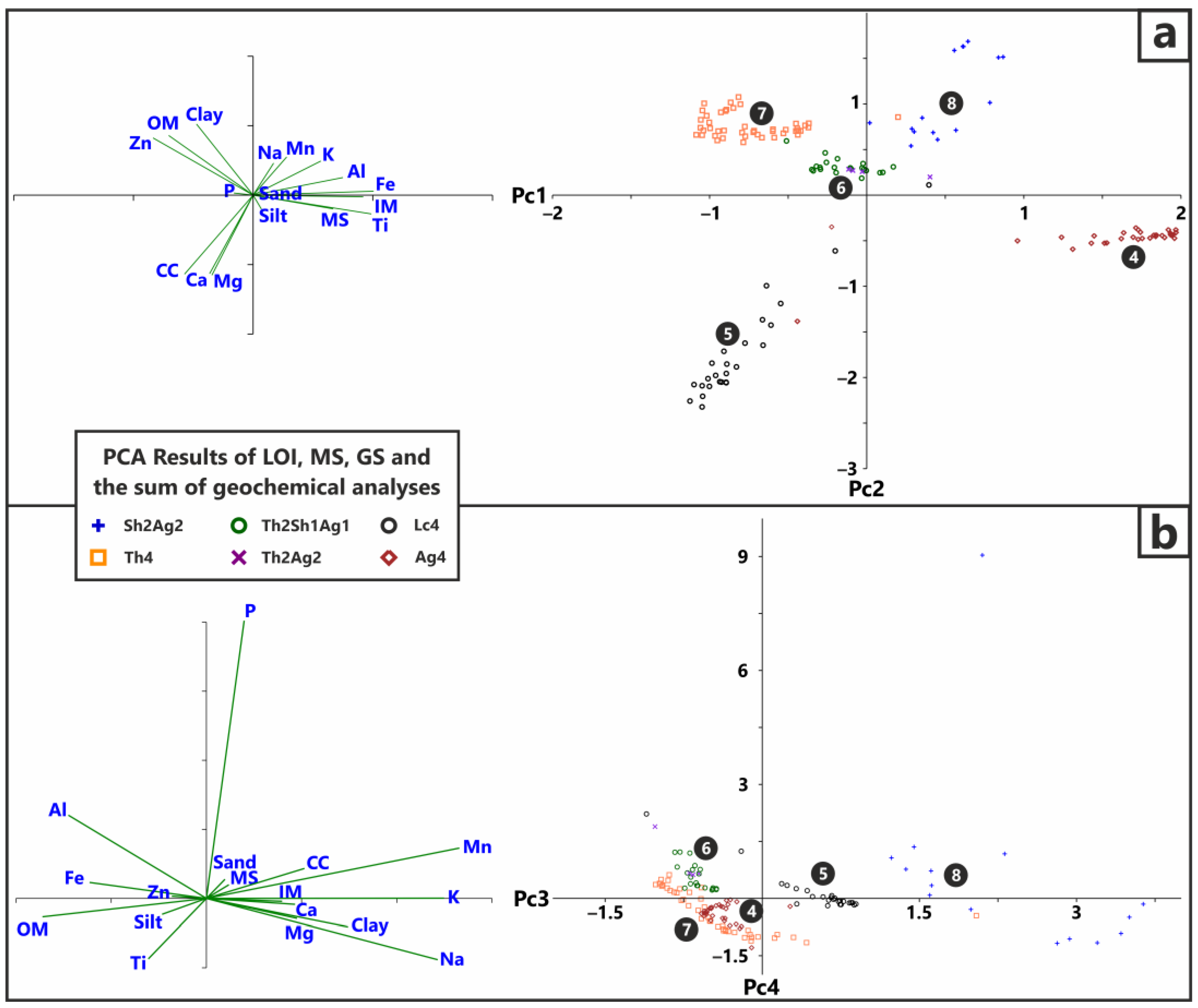
3.4. Statistical Analysis
4. Discussion
4.1. Paleoenvironment Changes during the Pleistocene
4.2. Paleoenvironment Changes during the Holocene
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sümegi, P.; Molnár, M.; Jakab, G.; Persaits, G.; Majkut, P.; Páll, D.; Gulyás, S.; Jull, A.J.T.; Törőcsik, T. Radiocarbon-Dated Paleoenvironmental Changes on a Lake and Peat Sediment Sequence from the Central Great Hungarian Plain (Central Europe) During the Last 25,000 Years. Radiocarbon 2011, 53, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Sümegi, P.; Molnár, D.; Náfrádi, K.; Makó, L.; Cseh, P.; Törőcsik, T.; Molnár, M.; Zhou, L. Vegetation and land snail-based reconstruction of the palaeocological changes in the forest steppe eco-region of the Carpathian Basin during last glacial warming. Glob. Ecol. Conserv. 2022, 33, e01976. [Google Scholar] [CrossRef]
- Iványosi-Szabó, A. Az Izsáki Kolon-tó üledék- és környezetföldtani vizsgálata. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 1984. (In Hungarian). [Google Scholar]
- European Environment Agency. Average Annual Precipitation in the EEA Area. Available online: https://www.eea.europa.eu/data-and-maps/figures/average-annual-precipitation (accessed on 7 June 2023).
- Birks, H.J.B.; Birks, H.H. Quaternary Palaeoecology; Edward Arnold: London, UK, 1980; pp. 1–280. [Google Scholar]
- Gaillard, M.-J.; Birks, H.H. Paleolimnological applications. In Encyclopedia of Quatermary Science; Elias, S.A., Ed.; Elsevier: New York, NY, USA, 2007; Volume 3, pp. 2337–2355. [Google Scholar]
- Berglund, B.E. Handbook of Holocene Palaeoecology and Palaeohydrology; John Wiley & Sons Ltd.: Chichester, UK, 1986. [Google Scholar]
- Kolon-tó [Lake Kolon]. Available online: www.kolon-to.com (accessed on 15 May 2023).
- Kiskunsági Nemzeti Park [Kiskunság National Park]. Available online: https://www.knp.hu/hu/kolon-to (accessed on 15 May 2023).
- Walter, H.; Lieth, H. Klimadiagramm Weltatlas; G. Fischer: Jena, Germany, 1960. [Google Scholar]
- The Climatol R Package. Available online: http://www.climatol.eu (accessed on 15 May 2023).
- RStudio Desktop—Posit. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 15 May 2023).
- Országos Meteorológiai Szolgálat. Available online: https://www.met.hu (accessed on 15 May 2023).
- Országos Meteorológiai Szolgálat—Meteorológiai Adattár. Available online: https://odp.met.hu (accessed on 15 May 2023).
- Aaby, B.; Digerfeldt, G. Sampling techniques for lakes and mires. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley & Sons: Chichester, UK, 1986; pp. 181–194. [Google Scholar]
- Vleeschouwer, F.; Chambers, F.; Swindles, G. Coring and sub-sampling of peatlands for palaeoenvironmental research. Mires Peat 2010, 7, 10. [Google Scholar]
- Troels-Smith, J. Characterization of unconsolidated sediments. Dan. Geol. Unders. 1955, 4, 10. (In Danish) [Google Scholar]
- Munsell Color. Munsell Soil Color Charts; Munsell Color Company. Inc.: Baltimore, MD, USA, 1954. [Google Scholar]
- rbacon: Age-Depth Modelling Using Bayesian Statistics. Available online: https://cran.r-project.org/web/packages/rbacon/index.html (accessed on 15 May 2023).
- Blaauw, M.; Christen, J.A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Oldfield, F.; Thompson, R.; Barber, K.E. Changing atmospheric fallout of magnetic particles recorded in recent ombrotrophic peat sections. Science 1978, 199, 679–680. [Google Scholar] [CrossRef]
- Dearing, J. Environmental Magnetic Susceptibility: Using the Bartington MS2 System; Chi Publishing: Keniloworth, UK, 1994. [Google Scholar]
- Sümegi, P.; Náfrádi, K.; Molnár, D.; Sávai, S. Results of paleoecological studies in the loess region of Szeged-Öthalom (SE Hungary). Quat. Int. 2015, 372, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition; comparison with other methods. J. Sediment. Petrol. 1974, 44, 242–248. [Google Scholar]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolim. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Dániel, P. Results of the geochemical analysis of the samples from Bátoliget II profile. In The Geohistory of Bátorliget Marshland; Sümegi, P., Gulyás, S., Eds.; Archaeolingua Press: Budapest, Hungary, 2004; pp. 95–128. [Google Scholar]
- Dániel, P.; Kovács, B.; Gyori, Z.; Sümegi, P. A Combined Sequential Extraction Method for Analysis of Ions Bounded to Mineral Component. In Proceedings of the 4th Soil and Sediment Contaminant Analysis Workshop. Lausanne, Switzerland, 23–25 September 1996; p. 396. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9–18. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Wold, S. Introduction to Multi- and Megavariate Data Analysis Using Projection Methods (PCA & PLS); Umetrics AB: Umea, Sweden, 1999. [Google Scholar]
- Davis, J.C. Statistics and Data Analysis in Geology; John Wiley & Sons Inc.: New York, NY, USA, 1986; p. 656. [Google Scholar]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th edition—Principles, classification scheme and correlations. Soil Sci. Annu. 2019, 70, 71–97. [Google Scholar] [CrossRef] [Green Version]
- Łachacz, A.; Nitkiewicz, S. Classification of soils developed from bottom lake deposits in north-eastern Poland. Soil Sci. Annu. 2021, 72, 1–14. [Google Scholar] [CrossRef]
- Uggla, H. Charakterystyka gytii i gleb gytiowych Pojezierza Mazurskiego w świetle dotychczasowych badań Katedry Gleboznawstwa WSR w Olsztynie [In Polish with English Summary: Characteristics of gyttja and gyttja soils of Mazurian Lakeland in light of hitherto investigations of the Chair of Pedology, College of Agriculture in Olsztyn]. Zesz. Probl. Postępów Nauk Rol. 1971, 107, 14–25. [Google Scholar]
- Molnár, M.; Futo, I.; Svingor, É.; Sümegi, P. Review of Holocene lacustrine carbonate formation in the light of new radiocarbon data from a site of Csólyospálos, Central Hungary. ATOMKI Annu. Rep. 2006, 21, 52. [Google Scholar]
- Jenei, M.; Gulyás, S.; Sümegi, P.; Molnár, M. Holocene Lacustrine Carbonate Formation; Old Ideas in the Light of New Radiocarbon Data from a Single Site in Central Hungary. Radiocarbon 2007, 49, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Sümegi, P.; Molnár, D.; Sávai, S.; Náfrádi, K.; Novák, Z.; Szelepcsényi, Z.; Törőcsik, T. First radiocarbon dated paleoecological data from the freshwater carbonates of the Danube-Tisza Interfluve. Open Geosci. 2015, 7, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Knipl, I.; Sümegi, P. A Duna-Tisza közi Hátság és a Kalocsai Sárköz Hajós és Császártöltés községek közötti határterületének geoarcheológiai elemzése. In Geoszférák 2014; Unger, J., Pál-Molnár, E., Eds.; Földtani és Őslénytani Tanszék: Szeged, Hungary, 2015; pp. 85–107. [Google Scholar]
- Achterberg, I.; Eckstein, J.; Birkholz, B.; Bauerochse, A.; Leuschner, H. Dendrochronologically dated pine stumps document phase-wise bog expansion at a northwest German site between ca. 6700 and ca. 3400 BC. Clim. Past 2018, 14, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Markowski, S. Struktura i właściwości podtorfowych osadów jeziornych rozprzestrzenionych na Pomorzu Zachodnim jako podstawa ich rozpoznawania i klasyfikacji. Kreda Jeziorna Gytie 1980, 2, 45–55. [Google Scholar]
- Uggla, H. Gleby gytiowe Pojezierza Mazurskiego. I. Ogólna charakterystyka gleb gytiowo-bagiennych i gytiowo-murszowych. Zesz. Nauk. WSR W Olsztynie 1969, 25, 563–582, (In Polish with German Summary). [Google Scholar]
- Uggla, H. „Rędziny” Pojezierza Mazurskiego. Rocz. Glebozn. 1976, 27, 113–125, (In Polish with English Summary). [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Piti, F. A tihanyi monostor alapítólevele (1055). In Írott Források az 1050-1116 közötti Magyar Történelemről; Makk, F., Thoroczkay, G., Eds.; Szegedi Középkortörténeti Könyvtár: Szeged, Hungary, 2006; pp. 16–25. (In Hungarian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual authors and contributors and not of MDPI and/or the editors. MDPI and/or the editors disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |






| PC 1 | PC 2 | PC 3 | PC 4 | |
|---|---|---|---|---|
| Eigenvalue | 4.99 | 4.29 | 2.86 | 1.14 |
| % variance | 34.04 | 29.24 | 19.51 | 7.74 |
| Ca | −0.1449 | −0.4176 | 0.1636 | −0.0188 |
| Mg | −0.1373 | −0.4253 | 0.1672 | −0.0637 |
| Na | 0.0682 | 0.1701 | 0.4267 | −0.1953 |
| K | 0.2253 | 0.1812 | 0.4385 | 0.0007 |
| Fe | 0.4010 | 0.0209 | −0.2155 | 0.0511 |
| Mn | 0.1116 | 0.2027 | 0.4667 | 0.1612 |
| Al | 0.2987 | 0.0934 | −0.2546 | 0.2665 |
| P | −0.0641 | 0.0113 | 0.0697 | 0.8896 |
| Ti | 0.3934 | −0.0999 | −0.1072 | −0.1927 |
| Zn | −0.3330 | 0.3039 | −0.0638 | 0.0072 |
| IM | 0.3664 | −0.0089 | 0.1397 | −0.0092 |
| OM | −0.2809 | 0.3169 | −0.3024 | −0.0588 |
| CC | −0.2282 | −0.4199 | 0.1809 | 0.0960 |
| MS | 0.2665 | −0.0727 | 0.0415 | 0.0437 |
| Clay | −0.1885 | 0.3776 | 0.2612 | −0.0906 |
| Silt | 0.0276 | −0.0743 | −0.0818 | −0.0508 |
| Sand | 0.0044 | 0.0086 | 0.0342 | 0.0613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vári, T.Z.; Gulyás, S.; Sümegi, P. Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments. Quaternary 2023, 6, 39. https://doi.org/10.3390/quat6030039
Vári TZ, Gulyás S, Sümegi P. Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments. Quaternary. 2023; 6(3):39. https://doi.org/10.3390/quat6030039
Chicago/Turabian StyleVári, Tamás Zsolt, Sándor Gulyás, and Pál Sümegi. 2023. "Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments" Quaternary 6, no. 3: 39. https://doi.org/10.3390/quat6030039
APA StyleVári, T. Z., Gulyás, S., & Sümegi, P. (2023). Reconstructing the Paleoenvironmental Evolution of Lake Kolon (Hungary) through Integrated Geochemical and Sedimentological Analyses of Quaternary Sediments. Quaternary, 6(3), 39. https://doi.org/10.3390/quat6030039








