Vegetation Dynamics and Megaherbivore Presence of MIS 3 Stadials and Interstadials 10–8 Obtained from a Sediment Core from Auel Infilled Maar, Eifel, Germany
Abstract
1. Introduction
2. Materials and Methods
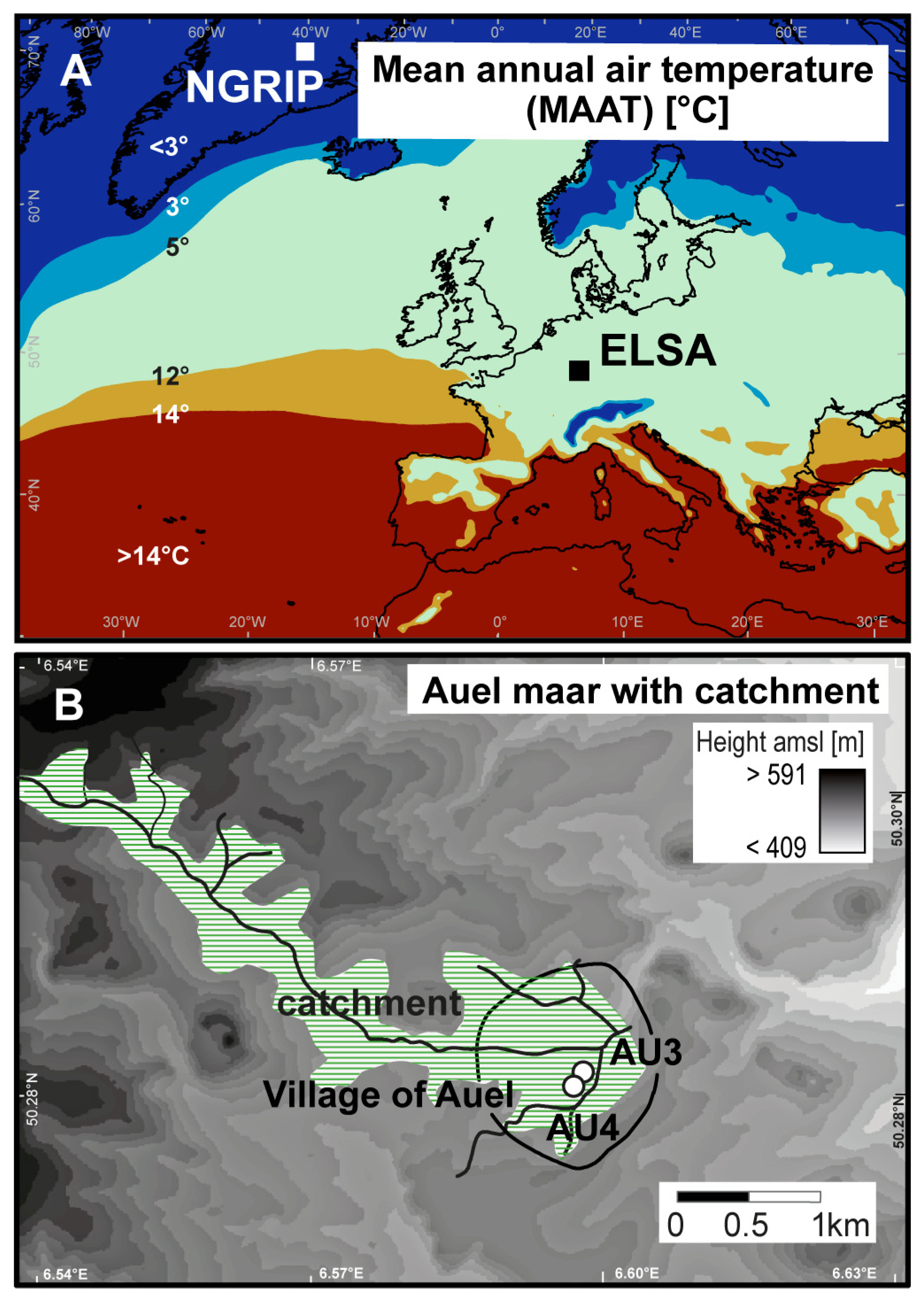
2.1. Coring Site
2.2. Organic Carbon, µ-XRF
2.3. Pollen and Spore Sample Preparation
3. Results
3.1. Age Model
3.2. Pollen and Spores
- LPAZ 1 (41,800–41,520 yr b2k): Alnus-Salix-Ulmus-zone
- LPAZ 2 (41,520–41,220 yr b2k): Carpinus-zone
- LPAZ 3 (41,220–40,420 yr b2k): Alnus-Ulmus-zone
- LPAZ 4 (40,420–39,600 yr b2k): Quercus-Salix-zone
- Salix, Poaceae and total spores increase. During zone 4a, Salix and Quercus are representatives of temperate cold-deciduous trees, whereas main taxa are boreal species Betula and Pinus. Picea is not continuously present.
- Single Tilia pollen were found at the transition from zone 4a to 4b, when Ulmus and Alnus return to the pollen spectrum. During zone 4b, all SCF increase to maximum values and are frequently present throughout the analyzed samples. Picea pollen concentration is low from 39,800 yr b2k on.
- LPAZ 5 (39,600–38,860 yr b2k): open boreal forest
- LPAZ 6 (38,860–37,980 yr b2k): Alnus-Quercus-Ulmus-Salix-zone
- With the onset of zone 6, Picea pollen percentages are reduced to values below 5%, while Alnus, Salix, Quercus, and Ulmus pollen reappear, followed by Carpinus about 38,450 yr b2k. Grasses and forbs make up to 50% of the total pollen sum until 38,220 yr b2k when forests of boreal and cold-deciduous taxa dominated by Betula pollen become established.
- From 38,300–37,980 b2k, vegetation is dominated by Pinus, Betula, and Poaceae, with minor components of other boreal taxa (Larix, Populus). Broad-leaved temperate trees are Alnus, Quercus, Carpinus, and Salix.
- LPAZ 7 (37,960–36,400 yr b2k): Open mixed forest with increased Betula
- During earliest zone 7, total pollen amount increases. The main taxa are Betula, Pinus, and Poaceae. Steppe herbs and forbs are reduced. Except for Salix, tree taxa of the flood plains (Ulmus, Alnus) are reduced to minimum values.
- At about 37,400 yr b2k, Betula pollen amounts decrease by 40%. Poaceae are the dominant taxon, whereas trees such as Salix and Carpinus show small maxima before they newly decrease at 37,000 yr b2k.
- Similar to zone 7a, Betula become the dominant species with about 50% of the total pollen at about 37,000 yr b2k.
4. Discussion of Factors Influencing the Vegetation-Succession in the Eifel from 41,800 to 36,400 yr b2k
4.1. The Effect of the Laschamp Magnetic Excursion, Volcanic Eruptions, and Heinrich Stadial 4 on the Vegetation Succession
4.2. The Effect of Initial Warming during Early GI8 on the Terrestrial Ecosystem

4.3. The Role of Plant Succession and Megaherbivores for the Shift in Human Species and Culture
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmussen, S.O.; Bigler, M.; Blockley, S.P.; Blunier, T.; Buchardt, S.L.; Clausen, H.B.; Cvijanovic, I.; Dahl-Jensen, D.; Johnsen, S.J.; Fischer, H.; et al. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: Refining and extending the INTIMATE event stratigraphy. Quat. Sci. Rev. 2014, 106, 14–28. [Google Scholar] [CrossRef]
- Sirocko, F.; Albert, J.; Britzius, S.; Dreher, F.; Martínez-García, A.; Dosseto, A.; Burger, J.; Terberger, T.; Haug, G. Thresholds for the presence of Glacial megafauna in central Europe during the last 60,000 years. Sci. Rep. 2022, 12, 20055. [Google Scholar] [CrossRef] [PubMed]
- Sirocko, F.; Martínez-García, A.; Mudelsee, M.; Albert, J.; Britzius, S.; Christl, M.; Diehl, D.; Diensberg, B.; Friedrich, R.; Fuhrmann, F.; et al. Muted multidecadal climate variability in central Europe during cold stadial periods. Nat. Geosci. 2021, 14, 651–658. [Google Scholar] [CrossRef]
- Sirocko, F.; Knapp, H.; Dreher, F.; Förster, M.W.; Albert, J.; Brunck, H.; Veres, D.; Dietrich, S.; Zech, M.; Hambach, U.; et al. The ELSA-Vegetation-Stack: Reconstruction of Landscape Evolution Zones (LEZ) from laminated Eifel maar sediments of the last 60,000 years. Glob. Planet. Chang. 2016, 142, 108–135. [Google Scholar] [CrossRef]
- Fleitmann, D.; Cheng, H.; Badertscher, S.; Edwards, R.L.; Mudelsee, M.; Göktürk, O.M.; Fankhauser, A.; Pickering, R.; Raible, C.C.; Matter, A.; et al. Timing and climatic impact of Greenland Interstadials recorded in stalagmites from Northern Turkey. Geophys. Res. Lett. 2009, 36, L19707. [Google Scholar] [CrossRef]
- Wang, Y.J.; Cheng, H.; Edwards, R.L.; An, Z.S.; Wu, J.Y.; Shen, C.-C.; Dorale, J.A. A high-resolution absolute-dated Late Pleistocene monsoon record from Hulu Cave, China. Science 2001, 294, 2345–2348. [Google Scholar] [CrossRef]
- Martrat, B.; Grimalt, J.O.; Shackleton, N.J.; de Abreu, L.; Hutterli, M.A.; Stocker, T.F. Four climate cycles of recurring deep and surface water destabilizations on the Iberian margin. Science 2007, 317, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, N.J.; Hall, M.A.; Vincent, E. Phase relationships between millennial-scale events 64,000–24,000 years ago. Paleooceanography 2000, 15, 565–569. [Google Scholar] [CrossRef]
- Förster, M.W.; Sirocko, F. The ELSA tephra stack: Volcanic activity in the Eifel during the last 500,000 years. Glob. Planet. Chang. 2016, 142, 100–107. [Google Scholar] [CrossRef]
- Albert, J.; Sirocko, F. Evidence for an extreme cooling event prior to the Laschamp geomagnetic excursion in Eifel maar sediments. Quaternary 2023, 6, 14. [Google Scholar] [CrossRef]
- Markova, A.K.; Puzachenko, A.Y.; van Kolfschoten, T.; van der Plicht, J.; Ponomarev, D.V. New data on changes in the European distribution of the mammoth and the woolly rhinoceros during the second half of the Late Pleistocene and the Early Holocene. Quat. Int. 2013, 292, 4–14. [Google Scholar] [CrossRef]
- Kahlke, R.-D. The origin of Eurasian mammoth faunas (Mammuthus–Coelodonta faunal complex). Quat. Sci. Rev. 2014, 96, 32–49. [Google Scholar] [CrossRef]
- Fletcher, W.J.; Sánchez Goñi, M.F.; Allen, J.R.M.; Cheddadi, R.; Combourieu-Nebout, N.; Huntley, B.; Lawson, I.; Londeix, L.; Magri, D.; Margari, V.; et al. Millennial-scale variability during the Last Glacial in vegetation records from Europe. Quat. Sci. Rev. 2010, 29, 2839–2864. [Google Scholar] [CrossRef]
- Lorenzen, E.D.; Nogués-Bravo, D.; Orlando, L.; Weinstock, J.; Binladen, J.; Marske, K.A.; Ugan, A.; Borregaard, M.K.; Gilbert, M.T.P.; Nielsen, R.; et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 2011, 479, 359–364. [Google Scholar] [CrossRef]
- Morin, E. Evidence for declines in human population densities during the Early Upper Paleolithic in Western Europe. Proc. Natl. Acad. Sci. USA 2008, 105, 48–53. [Google Scholar] [CrossRef]
- Jöris, O.; Street, M. At the End of the 14C-Scale: Scenarios at the Transition from the Middle to the Upper Palaeolithic. J. Hum. Evol. 2008, 55, 782–802. [Google Scholar] [CrossRef] [PubMed]
- Von Berg, A.; Condemi, S.; Frechen, M. Die Schädelkalotte des Neanderthalers von Ochtendung/Osteifel—Archäologie, Paläoanthropologie und Geologie. Eiszeitalt. Ggw. 2000, 50, 56–68. [Google Scholar] [CrossRef]
- Baales, M. Kartstein bei Mechernich/Eifel. Ein Naturkundlich-Archäologischer Rundgang; Forschungsbereich Altsteinzeit des RGZM Mainz; Rheinland-Verlag: Mechernich, Germany, 2001. [Google Scholar]
- Hahn, J. Aurignacien. Das Ältere Jungpaläolithikum in Mittel- und Osteuropa; Fundamenta A 9. Köln; Böhlau-Verlag: Mechernich, Germany, 1977. [Google Scholar]
- Rein, B.; Sirocko, F. In-situ reflectance spectroscopy—Analysing Techniques for high-resolution pigment logging in sediment cores. Int. J. Earth Sci. 2002, 91, 950–954. [Google Scholar] [CrossRef]
- Berglund, B.E.; Ralska-Jasiewiczowa, M. Pollen analysis and pollen diagrams. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley and Sons: Chichester, UK, 1986; pp. 455–484. [Google Scholar]
- Fægri, K.; Iversen, J. Textbook of Pollen Analysis; John Wiley and Sons: Chichester, UK, 1989. [Google Scholar]
- Stockmarr, J. Tablets with spores used in absolute pollen analysis. Pollen Spores 1971, 13, 615–621. [Google Scholar]
- Etienne, D.; Jouffroy-Bapicot, I. Optimal counting limit for fungal spore abundance estimation using Sporormiella as a case study. Veg. Hist. Archaeobot. 2014, 23, 743–749. [Google Scholar] [CrossRef]
- Schenk, F.; Hambach, U.; Britzius, S.; Sirocko, F. First evidence of Campanian Ignimbrite ash airfall in central Europe. Quaternary 2023. in preparation. [Google Scholar]
- Laj, C.; Kissel, C.; Beer, J. High resolution global paleointensity stack since 75 kyr (GLOPIS-75) calibrated to absolute values. In Timescales of the Geomagnetic Field; Channell, J.E.T., Kent, D.V., Lowrie, W., Meert, J.G., Eds.; American Geophysical Union: Washington, DC, USA, 2004; pp. 255–265. [Google Scholar] [CrossRef]
- Cooper, A.; Turney, C.; Hughen, K.A.; Brook, B.W.; McDonald, H.G.; Bradshaw, C.J.A. Abrupt warming events drove Late Pleistocene holarctic megafaunal turnover. Science 2015, 349, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Zieger, B.; Glassmeier, K.-H.; Stadelmann, A.; Kallenrode, M.-B.; Sinnhuber, M.; Winkler, H. Energetic particles in the paleomagnetosphere: Reduced dipole configurations and quadrupolar contributions. J. Geophys. Res. 2007, 112, A062146. [Google Scholar] [CrossRef]
- Winkler, H.; Sinnhuber, M.; Notholt, J.; Kallenrode, M.-B.; Steinhilber, F.; Vogt, J.; Zieger, B.; Glassmeier, K.-H.; Stadelmann, A. Modeling impacts of geomagnetic field variations on middle atmospheric ozone responses to solar proton events on long timescales. J. Geophys. Res. 2008, 113, D02302. [Google Scholar] [CrossRef]
- Fuhrmann, F.; Seelos, K.; Sirocko, F. Eolian sedimentation in central European Auel dry maar from 60 to 13 ka. Quat. Res. 2021, 101, 4–12. [Google Scholar] [CrossRef]
- Barberi, F.; Innocenti, F.; Lirer, L.; Munno, R.; Pescatore, T.; Santacroce, R. The Campanian Ignimbrite: A major prehistoric eruption in the Neapolitan area (Italy). Bull. Volcanol. 1978, 41, 10–31. [Google Scholar] [CrossRef]
- Fitzsimmons, K.E.; Hambach, U.; Veres, D.; Iovita, R. The Campanian Ignimbrite eruption: New data on volcanic ash dispersal and its potential impact on human evolution. PLoS ONE 2013, 8, e65839. [Google Scholar] [CrossRef]
- Lindauer, S.; Döppes, D.; Britzius, S.; Knapp, H.; Rosendahl, W.; Sirocko, F. 14C dated mammal bones from MIS 3 recovered from Upper Rhine Graben gravel pits. Quaternary 2023. in preparation. [Google Scholar]
- Guillevic, M.; Bazin, L.; Landais, A.; Stowasser, C.; Masson-Delmotte, V.; Blunier, T.; Eynaud, F.; Falourd, S.; Michel, E.; Minster, B.; et al. Evidence for a three-phase sequence during Heinrich Stadial 4 using a multiproxy approach based on Greenland ice core records. Clim. Past 2014, 10, 2115–2133. [Google Scholar] [CrossRef]
- Fuhrmann, F.; Diensberg, B.; Gong, X.; Lohmann, G.; Sirocko, F. Aridity synthesis for eight selected key regions of the global climate system during the Last 60,000 years. Clim. Past 2020, 16, 2221–2238. [Google Scholar] [CrossRef]
- Flückiger, J.; Knutti, R.; White, J.W.C.; Renssen, H. Modeled seasonality of glacial abrupt climate events. Clim. Dyn. 2008, 31, 633–645. [Google Scholar] [CrossRef]
- Hublin, J.-J.; Talamo, S.; Julien, M.; David, F.; Connet, N.; Bodu, P.; Vandermeersch, B.; Richards, M.P. Radiocarbon dates from the Grotte du Renne and Saint-Césaire support a Neandertal Origin for the Châtelperronian. Proc. Natl. Acad. Sci. USA 2012, 109, 18743–18748. [Google Scholar] [CrossRef] [PubMed]
- Semal, P.; Rougier, H.; Crevecoeur, I.; Jungels, C.; Flas, D.; Hauzeur, A.; Maureille, B.; Germonpré, M.; Bocherens, H.; Pirson, S.; et al. New data on the Late Neandertals: Direct dating of the Belgian Spy fossils. Am. J. Phys. Anthropol. 2009, 138, 421–428. [Google Scholar] [CrossRef]
- Higham, T.; Basell, L.; Jacobi, R.; Wood, R.; Bronk Ramsey, C.; Conard, N.J. Τesting models for the beginnings of the Aurignacian and the advent of figurative art and music the radiocarbon chronology of Geißenklösterle. J. Hum. Evol. 2012, 62, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Mellars, P. Neanderthals and the modern human colonization of Europe. Nature 2004, 432, 461–465. [Google Scholar] [CrossRef]
- Germonpré, M.; Udrescu, M.; Fiers, E. The fossil mammals of Spy. Anthropol. Praehist. 2012, 123, 289–327. [Google Scholar]
- Staubwasser, M.; Drăgușin, V.; Onac, B.P.; Assonov, S.; Ersek, V.; Hoffmann, D.L.; Veres, D. Impact of climate change on the transition of Neanderthals to modern humans in Europe. Proc. Natl. Acad. Sci. USA 2018, 115, 9116–9121. [Google Scholar] [CrossRef] [PubMed]
- Nigst, P.R.; Haesaerts, P.; Damblon, F.; Frank-Fellner, C.; Mallol, C.; Viola, B.; Götzinger, M.; Niven, L.; Trnka, G.; Hublin, J.-J. Early modern human settlement of Europe north of the Alps occurred 43,500 years ago in a cold steppe-type environment. Proc. Natl. Acad. Sci. USA 2014, 111, 14394–14399. [Google Scholar] [CrossRef]
- Hublin, J.-J. The modern human colonization of western Eurasia: When and where? Quat. Sci. Rev. 2015, 118, 194–210. [Google Scholar] [CrossRef]
- Ocobock, C.; Lacy, S.; Niclou, A. Between a rock and a cold place: Neanderthal biocultural cold adaptations. Evol. Anthropol. 2021, 30, 262–279. [Google Scholar] [CrossRef]
- Pomeroy, E. Review: The different adaptive trajectories in Neanderthals and Homo sapiens and their implications for contemporary human physiological variation. Comp. Biochem. Physiol. Part A Mol. 2023, 280, 111420. [Google Scholar] [CrossRef]
- Dibble, H.L.; Abodolahzadeh, A.; Aldeias, V.; Goldberg, P.; McPherron, S.P.; Sandgathe, D.M. How did hominins adapt to Ice Age Europe without fire? Curr. Anthropol. 2017, 58, S278. [Google Scholar] [CrossRef]
- Goldfield, A.E.; Booton, R.; Marston, J.M. Modeling the role of fire and cooking in the competitive exclusion of Neanderthals. J. Hum. Evol. 2018, 124, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Fedele, F.G.; Giaccio, B.; Isaia, R.; Orsi, G. The Campanian Ignimbrite eruption, Heinrich Event 4, and palaeolithic change in Europe: A high-resolution investigation. In Volcanism and Earth’s Atmosphere; Robock, A., Oppenheimer, C., Eds.; American Geophysical Union: Washington, DC, USA, 2003; pp. 301–325. [Google Scholar] [CrossRef]
- Obreht, I.; Hambach, U.; Veres, D.; Zeeden, C.; Bösken, J.; Stevens, T.; Marković, S.B.; Klasen, N.; Brill, D.; Burow, C.; et al. Shift of large-scale atmospheric systems over Europe during Late MIS 3 and implications for modern human dispersal. Sci. Rep. 2017, 7, 5848. [Google Scholar] [CrossRef] [PubMed]
- Valet, J.-P.; Valladas, H. The Laschamp-Mono Lake geomagnetic events and the extinction of Neanderthal: A causal link or a coincidence? Quat. Sci. Rev. 2010, 29, 3887–3893. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L. The role of large mammals as vitamin C sources for MIS 3 hominins. Quaternary 2023, 6, 20. [Google Scholar] [CrossRef]
- Banks, W.E.; d’Errico, F.; Peterson, A.T.; Kageyama, M.; Sima, A.; Sánchez-Goñi, M.-F. Neanderthal extinction by competitive exclusion. PLoS ONE 2008, 3, e3972. [Google Scholar] [CrossRef]
- Timmermann, A. Quantifying the potential causes of Neanderthal extinction: Abrupt climate change versus competition and interbreeding. Quat. Sci. Rev. 2020, 238, 106331. [Google Scholar] [CrossRef]
- Discamps, E.; Jaubert, J.; Bachellerie, F. Human choices and environmental constraints: Deciphering the variability of large game procurement from Mousterian to Aurignacian times (MIS 5-3) in southwestern France. Quat. Sci. Rev. 2011, 30, 2755–2775. [Google Scholar] [CrossRef]
- Wißing, C.; Rougier, H.; Crevecoeur, I.; Germonpré, M.; Naito, Y.I.; Semal, P.; Bocherens, H. Isotopic evidence for dietary ecology of late Neandertals in north-western Europe. Quat. Int. 2016, 411, 327–345. [Google Scholar] [CrossRef]
- Richards, M.P.; Trinkaus, E. Isotopic evidence for the diets of European Neanderthals and early modern humans. Proc. Natl. Acad. Sci. USA 2009, 106, 16034–16039. [Google Scholar] [CrossRef]
- Naito, Y.I.; Chikaraishi, Y.; Drucker, D.G.; Ohkouchi, N.; Semal, P.; Wißing, C.; Bocherens, H. Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. J. Hum. Evol. 2016, 93, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Rendu, W.; Renou, S.; Soulier, M.-C.; Rigaud, S.; Roussel, M.; Soressi, M. Subsistence strategy changes during the Middle to Upper Paleolithic Transition reveals specific adaptations of human populations to their environment. Sci. Rep. 2019, 9, 15817. [Google Scholar] [CrossRef] [PubMed]







| Age [yr b2k] | Vegetation | LPAZ | LEZ | GS/GI | MIS | |||
|---|---|---|---|---|---|---|---|---|
37,000 | ↑Betula | Open mixed forest | 7 | c | ↑ 6 Forest - steppe | GI8 | Marine Isotope Stage 3 | |
37,400 | ↓Poaceae ↑Poaceae, Carpinus | b | 7 Cold temperate forest | |||||
37,960 | ↓Betula ↑Tot. pollen | a | ||||||
| ↓Alnus, SCFs, herbs, forbs ↑Carpinus single Tilia | 6 | b | ||||||
38,500 | GS9 | |||||||
38,860 | ↑Alnus, Quercus, Salix, Ulmus | a | ||||||
39,700 | ↓Picea ↑Artemisia | Open boreal forest | 5 |  | ||||
| ↓Alnus, Quercus, Salix, Ulmus ↑Alnus, Ulmus, single Tilia | Open mixed forest | 4 | b | CI | ||||
40,130 | GI9 | |||||||
↑Quercus, Salix, Poaceae, Chenopodiaceae | a | |||||||
40,420 | DWT GS10 | |||||||
41,220 | ↓Ericaceae, Ulmus, Alnus, Picea, Betula ↑Pinus, Alnus, Ulmus, single Tilia | 3 | ||||||
| ↓Carpinus, Poaceae ↑Betula, Carpinus, Poaceae | 2 | GI10 | ||||||
41,520 | GS11 | |||||||
| ↓Pinus, Picea, Alnus, Ulmus, Salix | 1 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britzius, S.; Sirocko, F. Vegetation Dynamics and Megaherbivore Presence of MIS 3 Stadials and Interstadials 10–8 Obtained from a Sediment Core from Auel Infilled Maar, Eifel, Germany. Quaternary 2023, 6, 44. https://doi.org/10.3390/quat6030044
Britzius S, Sirocko F. Vegetation Dynamics and Megaherbivore Presence of MIS 3 Stadials and Interstadials 10–8 Obtained from a Sediment Core from Auel Infilled Maar, Eifel, Germany. Quaternary. 2023; 6(3):44. https://doi.org/10.3390/quat6030044
Chicago/Turabian StyleBritzius, Sarah, and Frank Sirocko. 2023. "Vegetation Dynamics and Megaherbivore Presence of MIS 3 Stadials and Interstadials 10–8 Obtained from a Sediment Core from Auel Infilled Maar, Eifel, Germany" Quaternary 6, no. 3: 44. https://doi.org/10.3390/quat6030044
APA StyleBritzius, S., & Sirocko, F. (2023). Vegetation Dynamics and Megaherbivore Presence of MIS 3 Stadials and Interstadials 10–8 Obtained from a Sediment Core from Auel Infilled Maar, Eifel, Germany. Quaternary, 6(3), 44. https://doi.org/10.3390/quat6030044





