Current Phylogeographic Structure of Anemone altaica (Ranunculaceae) on the Khamar-Daban Ridge Reflects Quaternary Climate Change in Baikal Siberia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Isolation, PCR, and Sequencing
2.3. Sequence Alignment and Phylogenetic Analysis
3. Results
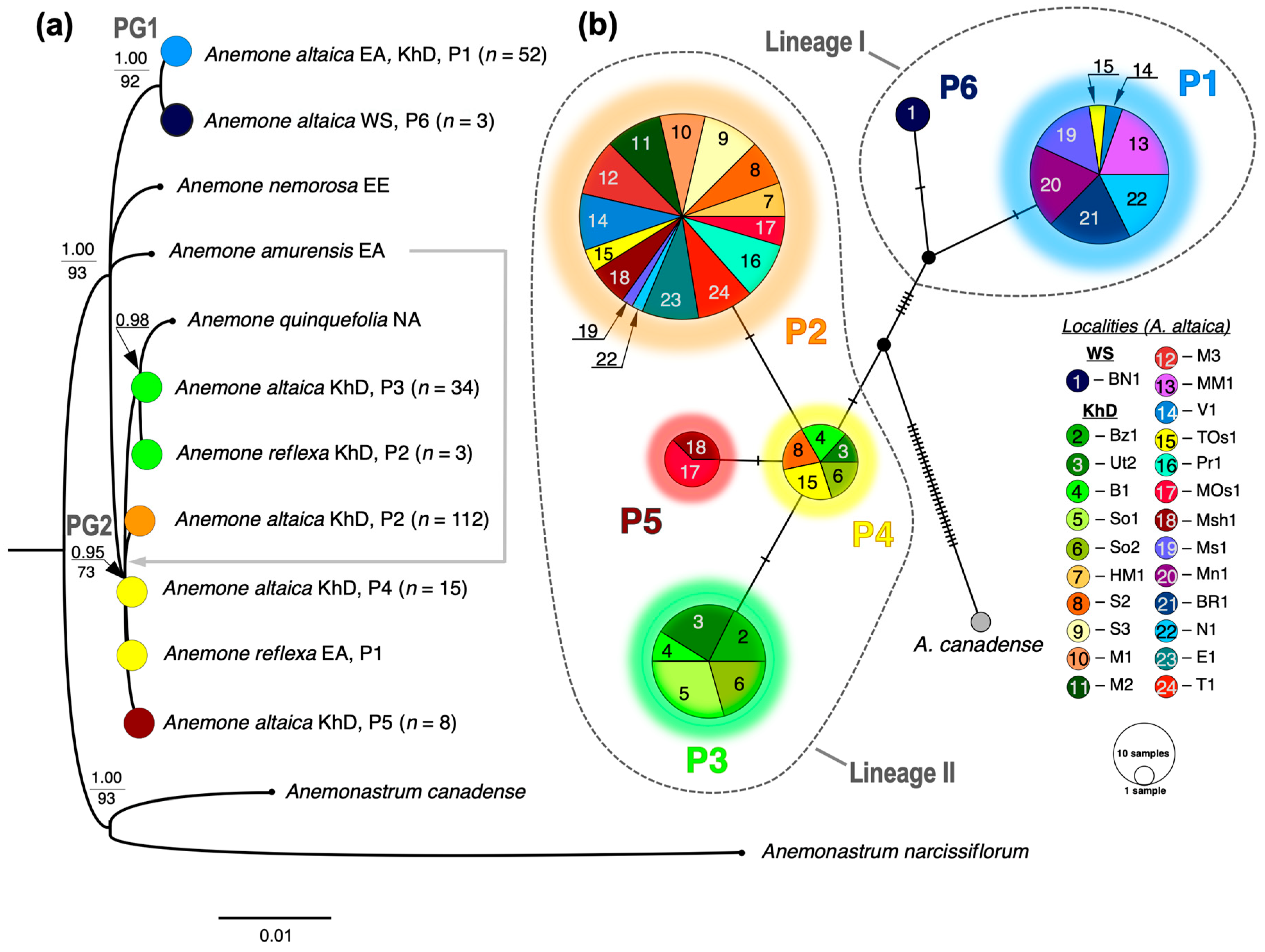
3.1. Phylogenetic Relationships Between Anemone altaica and Close Related Species
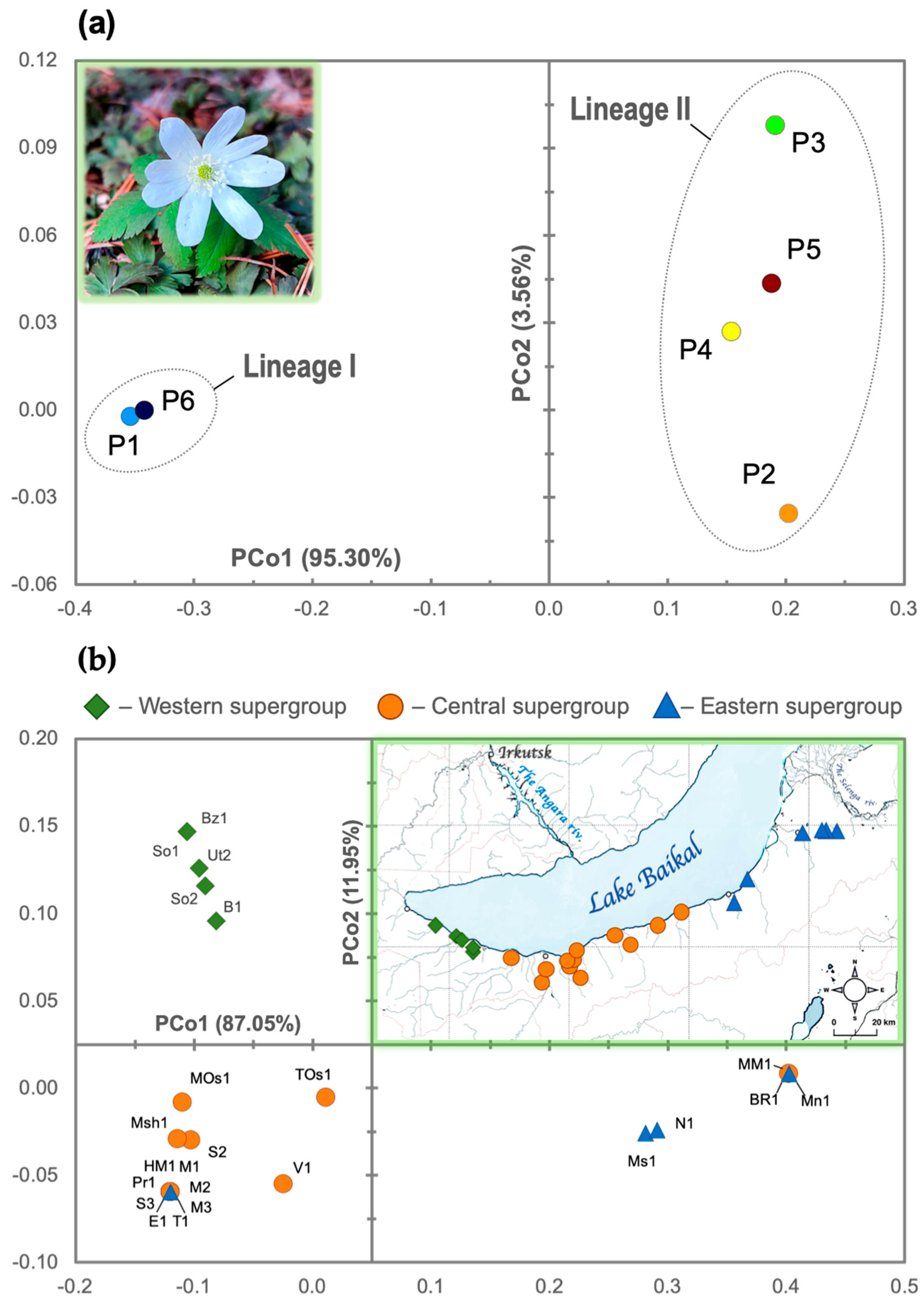
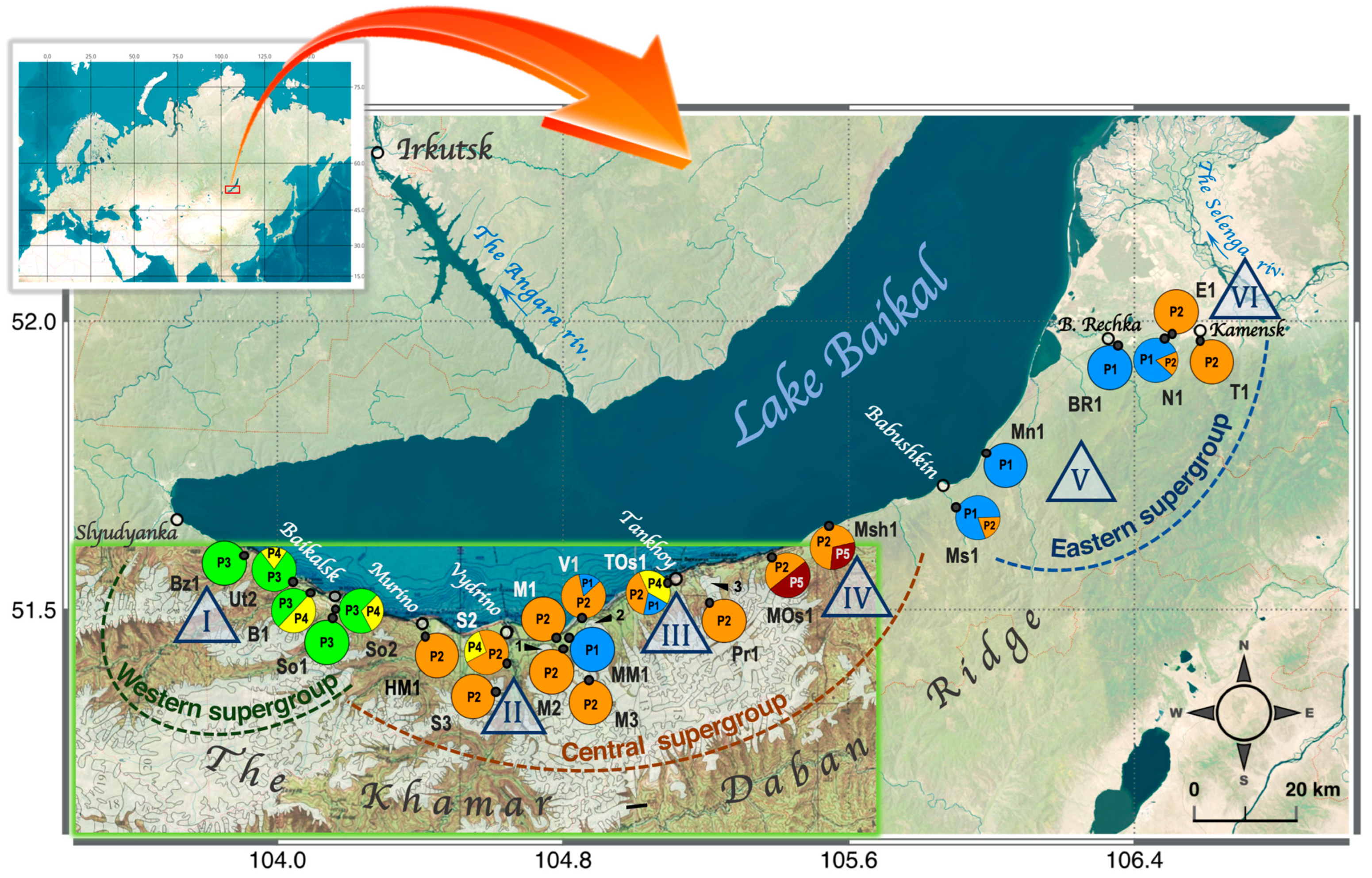
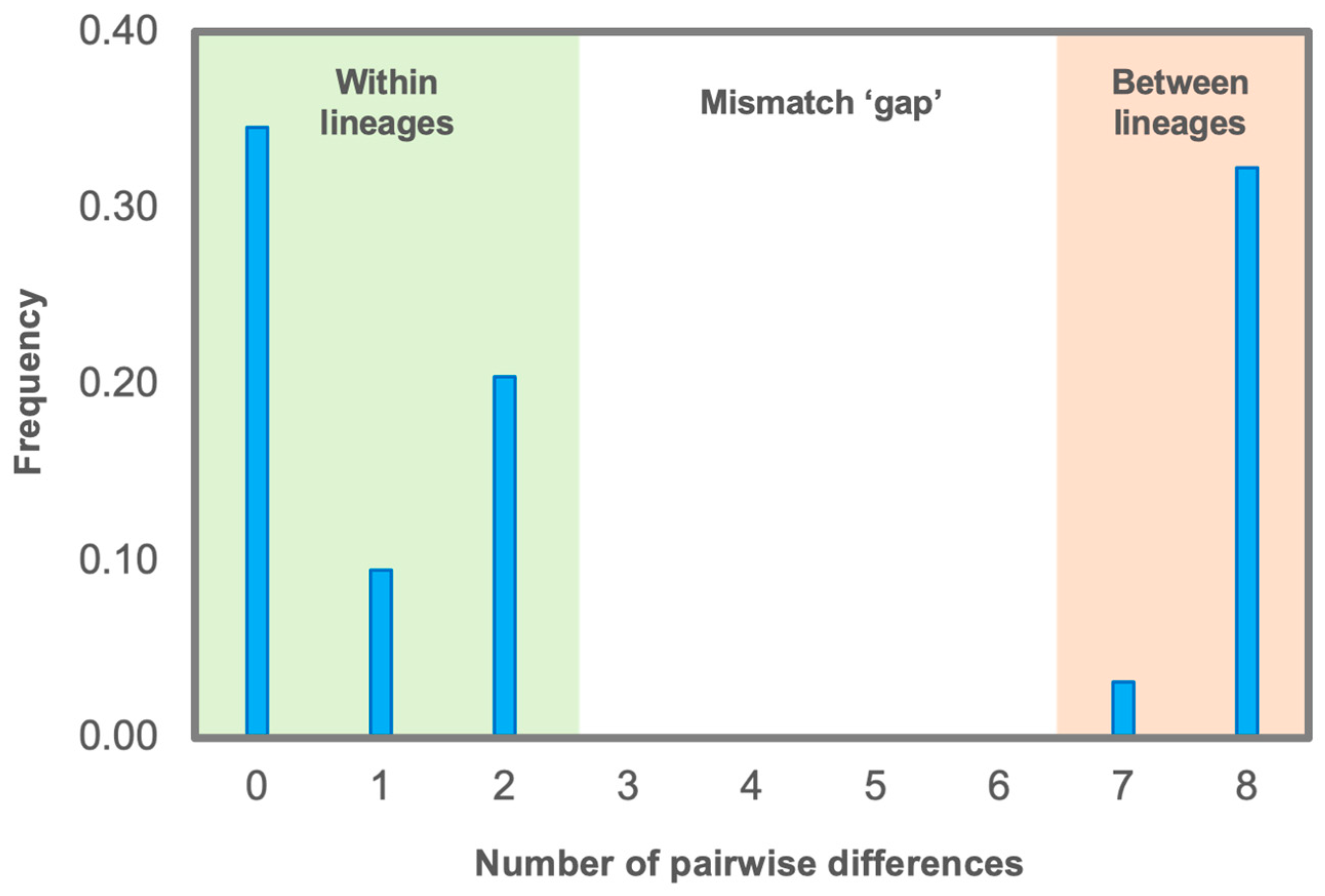
3.2. Phylogeographical Patterns of A. altaica on the Khamar-Daban Ridge Based on ptDNA Data
- Microrefugium I: Bz1, Ut2, B1, So1, and So2 localities
- Microrefugium II: HM1, S2, S3, M1, M2, and M3 localities
- Microrefugium III: MM1, V1, and TOs1 localities
- Microrefugium IV: Pr1, MOs1, and Msh1 localities
- Microrefugium V: Ms1, Mn1, Br1, and N1 localities
- Microrefugium VI: E1 and T1 localities
4. Discussion
4.1. Reevaluating the Phylogenetic Relationships of Anemone altaica and Closely Related Species
4.2. Present Phylogeographic Patterns of Anemone altaica on the Khamar-Daban Ridge and Potentional Pleistocene Distribution Scenarios
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krestov, P.V.; Omelko, A.M.; Nakamura, Y. Phytogeography of Higher Units of Forests and Krummholz in North Asia and Formation of Vegetation Complex in the Holocene. Phytocoenologia 2010, 40, 41–56. [Google Scholar] [CrossRef]
- Krestov, P.V.; Barkalov, V.Y.; Omelko, A.M.; Yakubov, V.V.; Nakamura, Y.; Sato, K. Relic Vegetation Complexes in the Modern Refugia of Northeast Asia. Komar. Chtenia [V. L. Komar. Meml. Lect.] 2009, 56, 5–63. (In Russian) [Google Scholar]
- Malyshev, L.I.; Peshkova, G.A. Osobennosti I Genezis Flory Sibiri (Predbaikalye I Zabaikalye) [Particularities and Genesis of the Flora of Siberia (Prebaikalia and Transbaikalia)]; Nauka: Novosibirsk, Russia, 1984. (In Russian) [Google Scholar]
- Polozii, A.V.; Krapivkina, E.D. Relikty Tretichnyh Shirokolistvennyh Lesov vo Flore Sibiri [Relics of Tertiary Deciduous Forests in the Flora of Siberia]; Tomsk University Press: Tomsk, Russia, 1985. (In Russian) [Google Scholar]
- Chepinoga, V.V.; Protopopova, M.V.; Pavlichenko, V.V. Detection of the Most Probable Pleistocene Microrefugia on the Northern Macroslope of the Khamar-Daban Ridge (Southern Prebaikalia). Contemp. Probl. Ecol. 2017, 10, 38–42. [Google Scholar] [CrossRef]
- Epova, N.A. Relics of Tertiary Broad-Leaved Forests in the Siberian Fir Taiga of the Khamar-Daban Ridge. In Izvestiia Biologo-Geograficheskogo Nauchno-Issledovatel’skogo Instituta pri Irkutskom Gosudarstvennom Universitete Imeni A. A. Zhdanova; Institute of Biology and Geography, A.A. Zhdanov Irkutsk State University: Irkutsk, Russia, 1956; Volume 16, pp. 47–61. (In Russian) [Google Scholar]
- Belov, A.V.; Bezrukova, E.V.; Sokolova, L.P.; Abzayeva, A.A.; Letunova, P.P.; Fisher, E.E.; Orlova, L.A. Vegetation of the Baikal Region as an Indicator of Global and Regional Changes in Natural Conditions of North Asia in the Late Cainozoic. Geogr. Nat. Resour. 2006, 6, 5–18. (In Russian) [Google Scholar]
- Krasnopevtseva, V.M.; Namzalov, B.B.; Krasnopevtseva, A.S. 3. Peculiarity of the Biology and Rhythm Flowering Ephemeral Plants of Khamar-Daban Mountain Range (Southn Prebaikalye). Bull. Buryat State Univ. 2008, 4, 107–111. (In Russian) [Google Scholar]
- Gamova, N.S.; Protopopova, M.V.; Pavlichenko, V.V.; Korotkov, Y.N. First Reliable Finding of Galium odoratum (L.) Scop. And New Findings of Galium Paradoxum Maxim. In the Republic of Buryatia. Trans. Karelian Res. Cent. RAS 2024, 1, 1–10. (In Russian) [Google Scholar] [CrossRef]
- Enikeev, F. Paleogeography of the Sartan Glaciation of the Hamar-Daban Ridge (Southern Baikal Region). Transbaikal State Univ. J. 2020, 26, 17–32. [Google Scholar] [CrossRef]
- Mel’nikov, Y.I. Pleistocene Gaps in the Areas of Birds of Eastern Siberia and Their Filling in the Modern Period of Climate Warming. IOP Conf. Ser. Earth Environ. Sci. 2023, 1154, 012065. [Google Scholar] [CrossRef]
- Chepinoga, V.V.; Protopopova, M.V.; Pavlichenko, V.V.; Dudov, S.V. Habitat Distribution Patterns of Nemoral Relict Plant Species on the Khamar-Daban Ridge (the South of Eastern Siberia) according to Grid Mapping Data. Russ. J. Ecol. 2021, 52, 212–222. [Google Scholar] [CrossRef]
- Krasnoborov, I.M. Flora Alpina Montium Sajanesium Occidentalium; Nauka: Novosibirsk, Russia, 1976. (In Russian) [Google Scholar]
- Krasnopevtseva, V.M.; Krasnopevtseva, A.S. Relict Plants of the Tertiary Nemoral Complex in Southern Transbaikalia. Tr. Tigirekskogo Zapov. [Proc. Tigirek State Nat. Reserve] 2010, 3, 194–196. (In Russian) [Google Scholar] [CrossRef]
- Chepinoga, V.V.; Mishina, A.V.; Protopopova, M.V.; Pavlichenko, V.V.; Bystrov, S.O.; Vilor, M.A. New Data on Distribution of Several Nemoral Relict Plant Species on the Foothills of the Khamar-Daban Ridge (Southern Baikal). Bot. Zhurnal 2015, 100, 478–489. (In Russian) [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Protopopova, M.V.; Pavlichenko, V.V. Eranthis salisb (Ranunculaceae) in South Siberia: Insights into Phylogeography and Taxonomy. Diversity 2022, 14, 779. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal Primers for Amplification of Three Non-Coding Regions of Chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, N. Direct Submission in GenBank. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OK267274.1 (accessed on 18 July 2024).
- Lee, C.S.; Lee, N.S.; Yeau, S.H. Molecular Phylogenetic Study of Anemone pendulisepala (Ranunculaceae). Korean J. Plant Taxon. 2006, 36, 263–277. [Google Scholar] [CrossRef]
- Zhang, N.; Lu, Y.; Zhang, Z. The Complete Chloroplast Genome Sequence of Anemone reflexa (Ranunculaceae). Mitochondrial DNA Part B Resour. 2021, 6, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Senni, K. Phylogeography of Japanese Alpine Plants: Biogeographic Importance of Alpine Region of Central Honshu in Japan. Taxon 2006, 55, 43–52. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the Human-Ape Splitting by a Molecular Clock of Mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary Trees from DNA Sequences: A Maximum Likelihood Approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, Version 1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 July 2022).
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A Cladistic Analysis of Phenotypic Associations with Haplotypes Inferred from Restriction Endonuclease Mapping and DNA Sequence Data. III. Cladogram Estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-Feature Software for Haplotype Network Construction. Med. Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Isolation by Distance. Genetics 1943, 28, 114–138. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Huff, D.R.; Peakall, R.; Smouse, P.E. RAPD Variation within and among Natural Populations of Outcrossing Buffalograss [Buchloë Dactyloides (Nutt.) Engelm.]. Theor. Appl. Genet. 1993, 86, 927–934. [Google Scholar] [CrossRef]
- Nei, M. Genetic Distance between Populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Smouse, P.E.; Long, J.C.; Sokal, R.R. Multiple Regression and Correlation Extensions of the Mantel Test of Matrix Correspondence. Syst. Zool. 1986, 35, 627–632. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R. Spatial Autocorrelation Analysis of Individual Multiallele and Multilocus Genetic Structure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Smouse, P.E.; Peakall, R.; Gonzales, E. A Heterogeneity Test for Fine-Scale Genetic Structure. Mol. Ecol. 2008, 17, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Double, M.C.; Peakall, R.; Beck, N.R.; Cockburn, A. Dispersal, Philopatry and Infidelity: Dissecting Local Genetic Structure in Superb Fairy-Wrens (Malurus cyaneus). Evolution 2005, 59, 625–635. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel. Available online: https://office.microsoft.com/excel (accessed on 31 December 2016).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 6 April 2024).
- Fu, W.; Zhao, K.; Zhang, C.; Tunney, H. Using Moran’s I and Geostatistics to Identify Spatial Patterns of Soil Nutrients in Two Different Long-Term Phosphorus-Application Plots. J. Plant Nutr. Soil. Sci. 2011, 174, 785–798. [Google Scholar] [CrossRef]
- Dutton, B.E.; Keener, C.S.; Ford, B.A. Anemone quinquefolia Linnaeus. In 1993+ Flora of North America North of Mexico; 25+ volumes, volume 3; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 1997; Available online: http://floranorthamerica.org/Anemone_quinquefolia (accessed on 11 July 2024).
- Tamura, M. Angiospermae: Ordnung Ranunculales, Fam. Ranunculaceae, Anemoneae. In Die Natürlichen Pflanzenfamilien; Hiepko, P., Ed.; Duncker and Homblot: Berlin, Germany, 1995; Volume 17a IV, pp. 324–349. [Google Scholar]
- Ziman, S.N.; Bulakh, E.V.; Kadota, Y.; Keener, C.S. Modern View on the Taxonomy of the Genus Anemone L. Sensu Stricto (Ranunculaceae). J. Jpn. Bot. 2008, 83, 127–155. [Google Scholar]
- Hoot, S.B.; Meyer, K.M.; Manning, J.C. Phylogeny and Reclassification of Anemone (Ranunculaceae), with an Emphasis on Austral Species. Syst. Bot. 2012, 37, 139–152. [Google Scholar] [CrossRef]
- Hu, S.; Shi, W.; Huang, Y.; Zhang, Z.; Lin, Q.; Shi, C. Comparative Analysis of Complete Chloroplast Genomes of Five Anemone Species and Phylogenetic Analysis within Tribe Anemoneae (Ranunculaceae). J. Plant Biochem. Biotechnol. 2024, 33, 271–287. [Google Scholar] [CrossRef]
- Yuzepchuk, S.V. Anemone L. In Flora of the USSR; Komarov, V.L., Ed.; Academy of Sciences of the USSR: Moscow-Leningrad, Russia, 1937; Volume 7, pp. 236–282. (In Russian) [Google Scholar]
- Popov, M.G. Flora Sredney Sibiri [Flora of Central Siberia]; Academy of Sciences of the USSR: Moscow-Leningrad, Russia, 1957; Volume 1. (In Russian) [Google Scholar]
- Boyd, O.F.; Philips, T.K.; Johnson, J.R.; Nixon, J.J. Geographically Structured Genetic Diversity in the Cave Beetle Darlingtonea Kentuckensis Valentine, 1952 (Coleoptera, Carabidae, Trechini, Trechina). Subterr. Biol. 2020, 34, 1–23. [Google Scholar] [CrossRef]
- Velichko, V.V. Oledeneniya Chetvertichnogo Perioda [the Quaternary Glaciations]. Great Russian Encyclopedia. Electronic Version 2019. Available online: https://old.bigenc.ru/geology/text/5556825 (accessed on 11 May 2024). (In Russian).
- Gorchakovsii, P.L.; Shurova, E.A. Redkie I Ischezayushchie Rastenia Urala I Priural’ya Rare and Endangered Plants of the Ural and Cis-Ural Region; Nauka: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Chen, T.; Wang, X.; Tang, H.; Chen, Q.; Huang, X.; Chen, J. Genetic Diversity and Population Structure of Chinese Cherry Revealed by Chloroplast DNA TrnQ-Rps16 Intergenic Spacers Variation. Genet. Resour. Crop Evol. 2013, 60, 1859–1871. [Google Scholar] [CrossRef]


| Locality Abbr. | Voucher Information 1 | Coordinates, Altitude 2 |
|---|---|---|
| Anemone altaica | ||
| B1 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Babkha Riv., 6 June 2018, V. Chepinoga, A. Skornyakova, A. Yudintseva (IRKU094289) | 51.52832° N |
| 104.09334° E | ||
| 493 m alt. | ||
| BN1 | Russia, South Siberia, Krasnoyarskiy Kray, Ermakovskiy Rayon, the Western Sayan Mountains, Nizhnyaya Buyba Riv., 11 June 2018, V. Pavlichenko, V. Chepinoga, M. Protopopova (IRKU042130–31) | 52.824383° N |
| 93.309001° E | ||
| 1313 m alt. | ||
| BR1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Bol’shaya Rechka Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094254) | 51.95788° N |
| 106.35214° E | ||
| 469 m alt. | ||
| Bz1 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Bezymyannaya Riv., 3 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094248) | 51.59373° N |
| 103.90829° E | ||
| 461 m alt. | ||
| E1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Elovka Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094252) | 51.975154° N |
| 106.504712° E | ||
| 519 m alt. | ||
| HM1 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Khara-Murin Riv., 3 June 2022, M. Protopopova, V. Pavlichenko (IRKU094247) | 51.455189° N |
| 104.413624° E | ||
| 479 m alt. | ||
| M1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Bol’shoy Mamay Riv., 3 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094246) | 51.44989° N |
| 104.78263° E | ||
| 472 m alt. | ||
| M2 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Bol’shoy Mamay Riv., 27 June 2023, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU IRKU094271) | 51.433755° N |
| 104.798493° E | ||
| 518 m alt. | ||
| M3 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Bol’shoy Mamay Riv., 27 June 2023, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU IRKU094272–74) | 51.376564° N |
| 104.868684° E | ||
| 1208 m alt. | ||
| MM1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Maliy Mamay Riv., 2 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094257) | 51.450789° N |
| 104.813297° E | ||
| 466 m alt. | ||
| Mn1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Manturikha Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094242) | 51.771462° N |
| 105.984607° E | ||
| 451 m alt. | ||
| MOs1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Osinovka Riv. (near Mishikha Riv.), 2 June 2024, M. Protopopova, P. Nelyubina, V. Pavlichenko (IRKU094278-80) | 51.591755° N |
| 105.384154° E | ||
| 474 m alt. | ||
| Ms1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Mysovka Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094253) | 51.67841° N |
| 105.89572° E | ||
| 513 m alt. | ||
| Msh1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Mishikha Riv., 1 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094255) | 51.645528° N |
| 105.542409° E | ||
| 461 m alt. | ||
| N1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Nikitkina Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094243) | 51.971705° N |
| 106.487322° E | ||
| 487 m alt. | ||
| Pr1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Pereemnaya Riv., 1 June 2024, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094275-77) | 51.513294° N |
| 105.207800° E | ||
| 474 m alt. | ||
| S2 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Snezhnaya Riv., 2 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094251) | 51.406766° N |
| 104.642759° E | ||
| 473 m alt. | ||
| S3 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Snezhnaya Riv., 29 June 2023, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094261-63) | 51.35570° N |
| 104.61346° E | ||
| 936 m alt. | ||
| So1 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Solzan Riv., 3 June 2024, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094281-83,87) | 51.488595° N |
| 104.157478° E | ||
| 534 m alt. | ||
| So2 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Solzan Riv., 3 June 2024, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094284-86) | 51.498915° N |
| 104.158083 E | ||
| 513 m alt. | ||
| T1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Timlyuy Riv., 31 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094244) | 51.965865° N |
| 106.581702° E | ||
| 516 m alt. | ||
| TOs1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Osinovka (near Tankhoy setl.) Riv., 1 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094256) | 51.549228° N |
| 105.094652° E | ||
| 469 m alt. | ||
| Ut2 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Utulik Riv., 30 May 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094250) | 51.54594° N |
| 104.04675° E | ||
| 453 m alt. | ||
| V1 | Russia, South Siberia, the Republic of Buryatia, Kabanskiy Rayon, the Khamar-Daban Ridge, the Vydrinaya Riv., 1 June 2022, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094245) | 51.485538° N |
| 104.848959° E | ||
| 457 m alt. | ||
| Anemone nemorosa | ||
| Republic of Belarus, Eastern Europe, Minskaya Oblast’, Minskiy Rayon, 16 May 1979, G.A. Kim (MSK-V388) | Unknown | |
| Anemone reflexa | ||
| Bz1 | Russia, South Siberia, Irkutskaya Oblast’, Slyudyanskiy Rayon, the Khamar-Daban Ridge, the Bezymyannaya Riv., 30 June 2023, M. Protopopova, V. Pavlichenko, P. Nelyubina (IRKU094288) | 51.59373° N |
| 103.90829° E | ||
| 461 m alt. | ||
| Anemone quinquefolia | ||
| – | USA, North America, Massachusetts, Belchertown, 14 May 1989, C.R. Lombardi (VLA) | Unknown |
| Taxon Name | Region 1 | Locality | Microrefugium 2 | Supergroup 2 | Plastotype 3 | n 4 | GenBank Accession Numbers | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| trnL | trnL-trnF | ||||||||
| Anemone altaica | EA * | – | – | – | P1 | 1 | OK267274.1 | OK267274.1 | [20] |
| Anemone altaica | KhD | B1 | I | W | P3 | 3 | PQ660085 | PQ660123 | curr. |
| Anemone altaica | KhD | B1 | I | W | P4 | 3 | PQ660086 | PQ660124 | curr. |
| Anemone altaica | KhD | BR1 | V | E | P1 | 10 | PQ660087 | PQ660125 | curr. |
| Anemone altaica | KhD | Bz1 | I | W | P3 | 6 | PQ660088 | PQ660126 | curr. |
| Anemone altaica | KhD | E1 | VI | E | P2 | 10 | PQ660089 | PQ660127 | curr. |
| Anemone altaica | KhD | HM1 | II | C | P2 | 6 | PQ660090 | PQ660128 | curr. |
| Anemone altaica | KhD | M1 | II | C | P2 | 8 | PQ660091 | PQ660129 | curr. |
| Anemone altaica | KhD | M2 | II | C | P2 | 10 | PQ660092 | PQ660130 | curr. |
| Anemone altaica | KhD | M3 | II | C | P2 | 10 | PQ660093 | PQ660131 | curr. |
| Anemone altaica | KhD | MM1 | III | C | P1 | 10 | PQ660094 | PQ660132 | curr. |
| Anemone altaica | KhD | Mn1 | V | E | P1 | 10 | PQ660095 | PQ660133 | curr. |
| Anemone altaica | KhD | MOs1 | IV | C | P2 | 5 | PQ660096 | PQ660134 | curr. |
| Anemone altaica | KhD | MOs1 | IV | C | P5 | 5 | PQ660097 | PQ660135 | curr. |
| Anemone altaica | KhD | Ms1 | V | E | P1 | 8 | PQ660098 | PQ660136 | curr. |
| Anemone altaica | KhD | Ms1 | V | E | P2 | 2 | PQ660099 | PQ660137 | curr. |
| Anemone altaica | KhD | Msh1 | IV | C | P2 | 7 | PQ660100 | PQ660138 | curr. |
| Anemone altaica | KhD | Msh1 | IV | C | P5 | 3 | PQ660101 | PQ660139 | curr. |
| Anemone altaica | KhD | N1 | V | E | P1 | 9 | PQ660102 | PQ660140 | curr. |
| Anemone altaica | KhD | N1 | V | E | P2 | 2 | PQ660103 | PQ660141 | curr. |
| Anemone altaica | KhD | Pr1 | IV(III) | C | P2 | 10 | PQ660104 | PQ660142 | curr. |
| Anemone altaica | KhD | S2 | II | C | P2 | 8 | PQ660105 | PQ660143 | curr. |
| Anemone altaica | KhD | S2 | II | C | P4 | 3 | PQ660106 | PQ660144 | curr. |
| Anemone altaica | KhD | S3 | II | C | P2 | 10 | PQ660107 | PQ660145 | curr. |
| Anemone altaica | KhD | So1 | I | W | P3 | 10 | PQ660108 | PQ660146 | curr. |
| Anemone altaica | KhD | So2 | I | W | P3 | 7 | PQ660109 | PQ660147 | curr. |
| Anemone altaica | KhD | So2 | I | W | P4 | 3 | PQ660110 | PQ660148 | curr. |
| Anemone altaica | KhD | T1 | VI | E | P2 | 10 | PQ660111 | PQ660149 | curr. |
| Anemone altaica | KhD | TOs1 | III | C | P1 | 2 | PQ660112 | PQ660150 | curr. |
| Anemone altaica | KhD | TOs1 | III | C | P2 | 4 | PQ660113 | PQ660151 | curr. |
| Anemone altaica | KhD | TOs1 | III | C | P4 | 4 | PQ660114 | PQ660152 | curr. |
| Anemone altaica | KhD | Ut2 | I | W | P3 | 8 | PQ660115 | PQ660153 | curr. |
| Anemone altaica | KhD | Ut2 | I | W | P4 | 2 | PQ660116 | PQ660154 | curr. |
| Anemone altaica | KhD | V1 | III | C | P1 | 2 | PQ660117 | PQ660155 | curr. |
| Anemone altaica | KhD | V1 | III | C | P2 | 10 | PQ660118 | PQ660156 | curr. |
| Anemone altaica | WS | BN1 | – | – | P6 | 3 | PQ660119 | PQ660157 | curr. |
| Anemone amurensis | EA | – | – | – | – | 1 | EF139341.1 | EF139341.1 | [21] |
| Anemone nemorosa | EE | – | – | – | – | 1 | PQ660120 | PQ660158 | curr. |
| Anemone reflexa | EA | – | – | – | P1 | 1 | MW043774 | MW043774 | [22] |
| Anemone reflexa | KhD | Bz1 | – | – | P2 | 3 | PQ660121 | PQ660159 | curr. |
| Anemone quinquefolia | NA | – | – | – | – | 1 | PQ660122 | PQ660160 | curr. |
| Anemonastrum canadense (L.) Mosyakin | – | – | – | – | – | 1 | AB248018.1 | AB248018.1 | [23] |
| Anemonastrum narcissiflora L. | – | – | – | – | – | 1 | AB248017.1 | AB248017.1 | [23] |
| Source of Variation | d.f. | Sum of Squares | Variation, % | Ф-Statistics 1 | p-Value 2 |
|---|---|---|---|---|---|
| Hypothesis 1: 23 distinct populations (without grouping) | |||||
| Among populations | 22 | 2124.583 | 81.16 | ФST = 0.81161 | <0.001 |
| Within populations | 197 | 431.576 | 18.84 | ||
| Hypothesis 2: Population supergroups: Western, Central, and Eastern | |||||
| Among groups | 2 | 811.977 | 36.69 | ФCT = 0.36693 | =0.006 |
| Ф’CT = 0.81955 | =0.001 | ||||
| Among populations within groups | 20 | 1312.607 | 47.61 | ФSC = 0.75199 | <0.001 |
| Ф’SC = 0.68006 | =0.001 | ||||
| Within populations | 197 | 431.576 | 15.70 | ФST = 0.84299 | <0.001 |
| Hypothesis 3: Population groups associated with 4 microrefugia according to [5] | |||||
| Among groups | 3 | 493.425 | 12.08 | ФCT = 0.12082 | =0.138 |
| Among populations within groups | 19 | 1631.158 | 70.36 | ФSC = 0.80028 | <0.001 |
| Within populations | 197 | 431.576 | 17.56 | ФST = 0.82441 | <0.001 |
| Hypothesis 4: Population groups associated with 5 microrefugia: I, II, III, IV, and V + VI | |||||
| Among groups | 4 | 1112.208 | 39.22 | ФCT = 0.39223 | =0.011 |
| Ф’CT = 0.54290 | =0.001 | ||||
| Among populations within groups | 18 | 1012.376 | 44.00 | ФSC = 0.72231 | <0.001 |
| Ф’SC =0.69092 | =0.001 | ||||
| Within populations | 197 | 431.576 | 16.88 | ФST = 0.83123 | <0.001 |
| Hypothesis 5: Population groups associated with 6 microrefugia (main): I, II, III, IV, V, and VI | |||||
| Among groups | 5 | 1812.855 | 70.98 | ФCT = 0.70983 | <0.001 |
| Ф’CT = 0.86459 | =0.001 | ||||
| Among populations within groups | 17 | 311.728 | 12.71 | ФSC = 0.43817 | <0.001 |
| Ф’SC = 0.36053 | =0.001 | ||||
| Within populations | 197 | 431.576 | 16.30 | ФST = 0.83698 | <0.001 |
| Hypothesis 6: Population groups associated with 6 microrefugia (alternative): I, II, III, IV, V, and VI | |||||
| Among groups | 5 | 1717.003 | 66.45 | ФCT = 0.66449 | <0.001 |
| Ф’CT = 0.85700 | =0.001 | ||||
| Among populations within groups | 17 | 407.580 | 17.93 | ФSC = 0.51245 | <0.001 |
| Ф′SC = 0.39447 | =0.001 | ||||
| Within populations | 197 | 431.576 | 16.36 | ФST = 0.83642 | <0.001 |
| Within (super)groups | |||||
| Western supergroup (W): | |||||
| Among populations | 4 | 1.276 | 10.79 | ФST = 0.10791 | =0.058 |
| Within populations | 37 | 5.200 | 89.21 | ||
| Central supergroup (C): | |||||
| Among populations | 11 | 586.786 | 69.77 | ФST = 0.69769 | <0.001 |
| Within populations | 105 | 219.248 | 30.23 | ||
| Eastern supergroup (E): | |||||
| Among populations | 5 | 724.545 | 75.44 | ФST = 0.75442 | <0.001 |
| Within populations | 55 | 207.127 | 24.56 | ||
| Microrefurium (group) I: | |||||
| Among populations | 4 | 1.276 | 10.79 | ФST = 0.10791 | =0.058 |
| Within populations | 37 | 5.200 | 89.21 | ||
| Microrefurium (group) II: | |||||
| Among populations | 5 | 0.655 | 14.99 | ФST = 0.14992 | =0.020 |
| Within populations | 49 | 2.182 | 85.01 | ||
| Microrefurium (group) III: | |||||
| Among populations | 2 | 280.833 | 54.92 | ФST = 0.54926 | <0.001 |
| Within populations | 29 | 198.667 | 45.07 | ||
| Microrefurium (group) IV: | |||||
| Among populations | 2 | 5.067 | 15.34 | ФST = 0.15337 | =0.019 |
| Within populations | 27 | 18.400 | 84.66 | ||
| Microrefurium (group) V: | |||||
| Among populations | 3 | 23.897 | 3.00 | ФST = 0.03002 | =0.227 |
| Within populations | 37 | 207.127 | 97.00 | ||
| Microrefurium (group) VI: | |||||
| Among populations | 1 | 0 | NaN | NaN | <0.001 |
| Within populations | 18 | 0 | NaN | ||
| Group/Supergroup | Npop | P | h | Ne | I | S | π | Tajima’s D (p-Value) |
|---|---|---|---|---|---|---|---|---|
| Western supergroup (W) | 5 | 2 | 0.316 | 1.446 | 0.487 | 1 | 0.00040 | 0.5060 (0.305) |
| Central supergroup (C) | 12 | 4 | 0.415 | 1.700 | 0.820 | 9 | 0.00256 | 0.4412 (0.321) |
| Eastern supergroup (E) | 6 | 2 | 0.485 | 1.913 | 0.670 | 8 | 0.00498 | 3.3435 (0.000) |
| Microrefurium (group) I | 5 | 2 | 0.316 | 1.446 | 0.487 | 1 | 0.00040 | 0.5060 (0.305) |
| Microrefurium (group) II | 6 | 2 | 0.105 | 1.115 | 0.212 | 1 | 0.00013 | −0.6810 (0.735) |
| Microrefurium (group) III | 3 | 3 | 0.621 | 2.510 | 0.983 | 8 | 0.00522 | 3.1312 (0.000) |
| Microrefurium (group) IV | 3 | 2 | 0.405 | 1.642 | 0.580 | 2 | 0.00103 | 1.2349 (0.119) |
| Microrefurium (group) V | 4 | 2 | 0.180 | 1.214 | 0.320 | 8 | 0.00185 | −0.6464 (0.722) |
| Microrefurium (group) VI | 2 | 1 | 0.000 | 1.000 | 0.000 | 0 | 0.00000 | 0.0000 (0.000) |
| The general population | 23 | 5 | 0.660 | 2.917 | 1.275 | 10 | 0.00429 | 2.2914 (0.017) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopopova, M.; Nelyubina, P.; Pavlichenko, V. Current Phylogeographic Structure of Anemone altaica (Ranunculaceae) on the Khamar-Daban Ridge Reflects Quaternary Climate Change in Baikal Siberia. Quaternary 2025, 8, 20. https://doi.org/10.3390/quat8020020
Protopopova M, Nelyubina P, Pavlichenko V. Current Phylogeographic Structure of Anemone altaica (Ranunculaceae) on the Khamar-Daban Ridge Reflects Quaternary Climate Change in Baikal Siberia. Quaternary. 2025; 8(2):20. https://doi.org/10.3390/quat8020020
Chicago/Turabian StyleProtopopova, Marina, Polina Nelyubina, and Vasiliy Pavlichenko. 2025. "Current Phylogeographic Structure of Anemone altaica (Ranunculaceae) on the Khamar-Daban Ridge Reflects Quaternary Climate Change in Baikal Siberia" Quaternary 8, no. 2: 20. https://doi.org/10.3390/quat8020020
APA StyleProtopopova, M., Nelyubina, P., & Pavlichenko, V. (2025). Current Phylogeographic Structure of Anemone altaica (Ranunculaceae) on the Khamar-Daban Ridge Reflects Quaternary Climate Change in Baikal Siberia. Quaternary, 8(2), 20. https://doi.org/10.3390/quat8020020







